Abstract
The term ‘translational research’ was coined 20 years ago and has become a guiding influence in biomedical research. It refers to a process by which the findings of basic research are extended to the clinical research setting (bench to bedside) and then to clinical practice and eventually health policy (bedside to community). It is a dynamic, multidisciplinary research approach. The concept of translational physiology applies the translational research model to the physiological sciences. It differs from the traditional areas of integrative and clinical physiology by its broad investigative scope of basic research to community health. Translational physiology offers exciting opportunities, but presently is under-developed and -utilized. A key challenge will be to expand physiological research by extending investigations to communities of patients and healthy (or at risk) individuals. This will allow bidirectional physiological investigation throughout the translational continuum: basic research observations can be studied up to the population level, and mechanisms can be assessed by ‘reverse translation’ in clinical research settings and preclinical models based on initial observations made in populations. Examples of translational physiology questions, experimental approaches, roadblocks and strategies for promotion are discussed. Translational physiology provides a novel framework for physiology programs and an investigational platform for physiologists to study function from molecular events to public health. It holds promise for enhancing the completeness and societal impact of our work, while further solidifying the critical role of physiology in the biomedical research enterprise.
 |
Douglas Seals obtained his PhD from the University of Wisconsin-Madison and performed postdoctoral training at Washington University School of Medicine in St. Louis, Missouri. His initial faculty position was at the University of Arizona in Tucson. In 1992, he moved to the University of Colorado Boulder, where he is presently a College Professor of Distinction in the Department of Integrative Physiology. His research focuses on the integrative physiology of ageing. For the past decade, his laboratory has used translational experimental approaches to investigate the physiology and pathophysiology of vascular ageing, particularly the underlying physiological mechanisms and efficacy of treatments.
There has been much commentary about the future of physiology in recent years. This discussion has focused largely on issues concerning the domain of physiology and use of high-throughput molecular approaches in physiological research (Strange, 2005; Noble, 2008; Joyner, 2011; Joyner & Pedersen, 2011; Kuster et al. 2011; Pitt et al. 2011). Although the importance of physiological investigation up to the clinical and population levels has been noted (Joyner, 2011; Pitt et al. 2011; Wagner & Paterson, 2011; Granger et al. 2012), a more in-depth discussion of the entire scope of physiology may be useful.
In this perspective, the concept of translational physiology is emphasized as a model for physiological investigation from molecular and cellular physiology to population physiology and public health. A brief review and update of translational research is presented first to lay the groundwork for a subsequent, more detailed discussion of translational physiology per se. In the latter, the concept of translational physiology is described, followed by a representative example of a physiological question that has been studied in its full translational breadth. Sections follow that address investigative opportunities in population physiology, discuss translational experimental approaches, and emphasize translational models for studying the efficacy of interventions aimed at enhancing physiological function. Finally, barriers to and strategies for promoting translational physiological research are identified.
It is hoped that the overall discussion will broadly inform student trainees and early career stage postdoctoral investigators, while emphasizing translational opportunities for physiologists presently engaged solely in basic research. Although physiologists currently utilizing translational approaches will find much of the content familiar, suggestions for extending their work to community health applications or, alternatively, towards greater mechanistic depth, may prove helpful.
Translational research
The term ‘translational research’ is familiar to any investigator working in the biomedical sciences. Traditionally, translational research has referred to the process by which basic science observations are ‘translated’ into knowledge and products (new therapies, guidelines, etc.) that benefit clinical practice and community health. Although research based on this approach has been performed throughout the history of medicine, the label translational research was coined by the National Cancer Institute in the U.S. in 1993 to describe the research process needed to link cancer risk to predisposing genes. Use of the phrase and its underlying concept has increased dramatically over the last decade, such that translational research is now a fixture in the biomedical research lexicon and a guiding influence in programmatic directives and the missions of funding agencies worldwide (Woolf, 2008; Homer-Vanniasinkam & Tsui, 2012; van der Laan & Boenink, 2012). Indeed, more than 1500 articles have been published with translational research (or a related term) in the title and scientific journals have been established to feature research on this topic. Recently, specific programs and centres have been established in the U.S. and E.U. to facilitate translational research, driven by concerns that basic discoveries are not being advanced to improved health outcomes at an appropriate rate (Homer-Vanniasinkam & Tsui, 2012; van der Laan & Boenink, 2012).
The overall process of translational research is defined by a series of steps or phases within a continuum (Woolf, 2008; Homer-Vanniasinkam & Tsui, 2012) (Fig. 1). ‘T1’ refers to research aimed at translation of observations from basic research to the clinical research setting (bench to bedside), whereas investigations extending insight from clinical research to medical practice and community health initially were described as ‘T2’ (bedside to community). More recently, T2 has been further subdivided into T2 (clinical science to clinical practice) and ‘T3’ (clinical practice to community or population health) (Homer-Vanniasinkam & Tsui, 2012), and other descriptions of the process advance alternative and broader ranges of the phases involved (Khoury et al. 2010). Such differences aside, translational research can be viewed more narrowly (T1) or broadly (T1–T3). In concept, it often is presented in the broadest sense (basic science to medical practice and public health), whereas in practice it is typically performed within a particular phase (van der Laan & Boenink, 2012). The latter is due, at least in part, to the fact that the various steps of translational research require very different investigative skills and infrastructure (Woolf, 2008). T1 research requires basic to clinical science skills and laboratory facilities. T2 and T3 research requires skills in epidemiology, behavioural science, public policy and other fields that are needed for synthesizing (via systematic reviews, meta-analyses and medical guidelines development) and then disseminating and implementing observations made at the clinical research level to population health outcomes in settings of clinical practice and the community (schools, assisted living centres, etc.).
Figure 1. Translational research continuum.
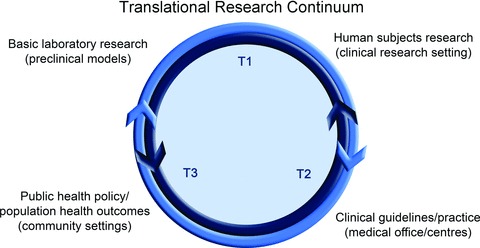
Basic science observations are translated initially to the clinical research setting (T1) with subsequent translation for implementation into clinical practice (T2) and public health policy (T3). The process is bidirectional in that observations made initially at higher levels of translation can be studied for underlying mechanisms or other features at the clinical and preclinical levels.
A few additional features of translational research serve to inform the present discussion. The process, when approached with full theoretical intent, is dynamic, with knowledge on a topic expanding across multiple levels of observation and application. This dynamic expansion of information should be bidirectional. Although conventionally viewed as translation of basic science to clinical application, at its conceptual best, observations made in medical practice and community health settings also should stimulate investigation at the clinical and preclinical research levels to delineate mechanisms of action (‘reverse translation’ or ‘bench to bedside and back to bench’) (Marincola, 2003; Homer-Vanniasinkam & Tsui, 2012; Liebman & Marincola, 2012; van der Laan & Boenink, 2012). Mechanistic evidence can then lead to new diagnostic and/or treatment options, which can then be tested in preclinical models and advanced in the translational process for eventual implementation as new health practices. These features of the translational research process require multidisciplinary investigational approaches.
Concept of translational physiology
As an integrative life science, historically, physiological research has been conducted using many of the same principles that guide translational research. Physiology is studied at multiple levels of observation and the influences of higher and lower levels of organization on function are determined (Noble, 2008; Joyner, 2011; Kuster et al. 2011). However, it seems reasonable to state that, at least to date, most physiological research has been conducted within a limited range of the T1 phase, i.e. from cells to small groups of human subjects.
Although practiced from the beginning of the discipline, the term translational physiology was first introduced in a brief editorial by John Hall in 2002 (Hall, 2002). Among several key points, he emphasized that translational physiology could make important contributions by both confirming basic science findings in humans, and testing hypotheses in preclinical models based on observations made initially at the clinical level. Over the last few years there has been additional useful commentary related to the potential role of physiology in translational research (Head, 2010; Joyner, 2011; Wagner & Paterson, 2011; Granger et al. 2012).
So, how does translational physiology differ in concept or practice from the traditional tenets of integrative or clinical physiology? Perhaps the primary difference is in the scope and spirit of translational physiology. Integrative physiology aims to connect observations from subcellular events to the whole organism, and clinical physiology typically focuses on specific groups of patients and/or healthy controls. Translational physiology can be viewed as the study of physiological function from the molecular/cellular level to populations of humans with application to public health (T1–T3) (Fig. 2). In this interpretation, integrative and clinical physiology remain as foundational components of translational physiology, but become part of a broader vision with a more direct path to population health status. The far-reaching translational endpoint becomes optimal physiological function of populations operating within their environment. This has important health implications because physiological dysfunction is a common gateway to clinical disease. Thus, the ultimate goal of translational physiology is the attainment and preservation of optimal societal function, health and quality of life.
Figure 2. Translational physiology.
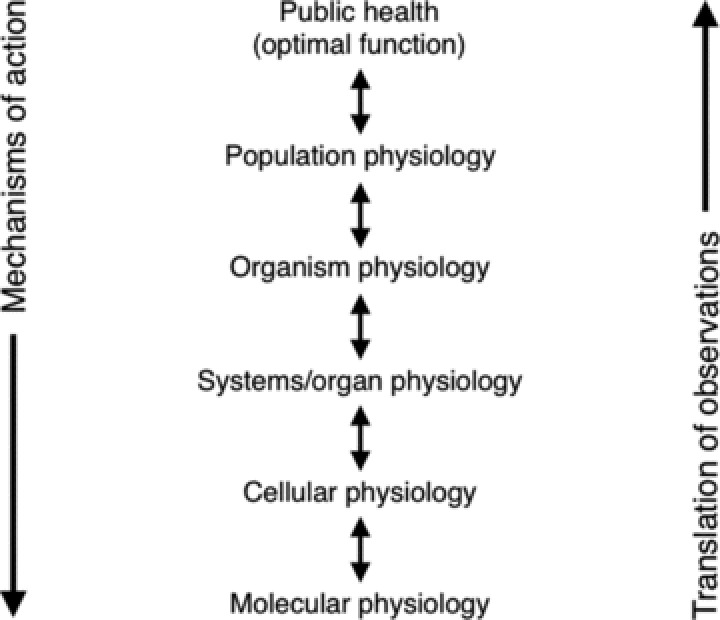
Physiological observations are made at the molecular and cellular levels and translated towards eventual application to public health. Mechanisms underlying differences in health outcomes or population function are studied at progressively lower levels of observation. The ultimate goal is optimal societal physiological function, health and quality of life.
Translational physiology in practice: sodium intake and blood pressure
One example of translational physiology in its full expression is the investigation of the influence of dietary sodium intake on arterial blood pressure. From initial discovery reports of a possible role for excess sodium intake in essential hypertension in the early 1960's (Dahl, 1961), insight into the relation between sodium and blood pressure has been extended bi-directionally (Fig. 3). ‘Forward translation’ involved association studies that confirmed the relation, followed by small-scale prospective studies in humans in which sodium intake was manipulated and blood pressure found to change in a predictable manner (MacGregor et al. 1989). These results lead to efficacy studies on larger groups of subjects showing that dietary sodium restriction could reduce blood pressure (Kumanyika et al. 1993), followed by effectiveness trials confirming the response and establishing subject compliance (Kumanyika et al. 2005). Reverse translation has included studies in human subjects, animal models and cell culture aimed at determining the mechanisms by which sodium influences blood pressure and its renal-cardiovascular physiological determinants (Dahl et al. 1974; Gu et al. 1998; Gates et al. 2004; Jablonski et al. 2013). With this collective information, position stands and policy statements were developed (Appel et al. 2011), and, working with both industry and public health programs, several countries including Japan, Finland and the UK have instituted nation-wide sodium reduction policies (Mohan et al. 2009). Importantly, physiologists have played a prominent role in this overall effort up through the highest levels of translation (T3) (Appel et al. 2011). Other examples of physiology contributing to the development of clinical guidelines and public health policy have been emphasized previously (Joyner, 2011).
Figure 3. Sodium intake and blood pressure.
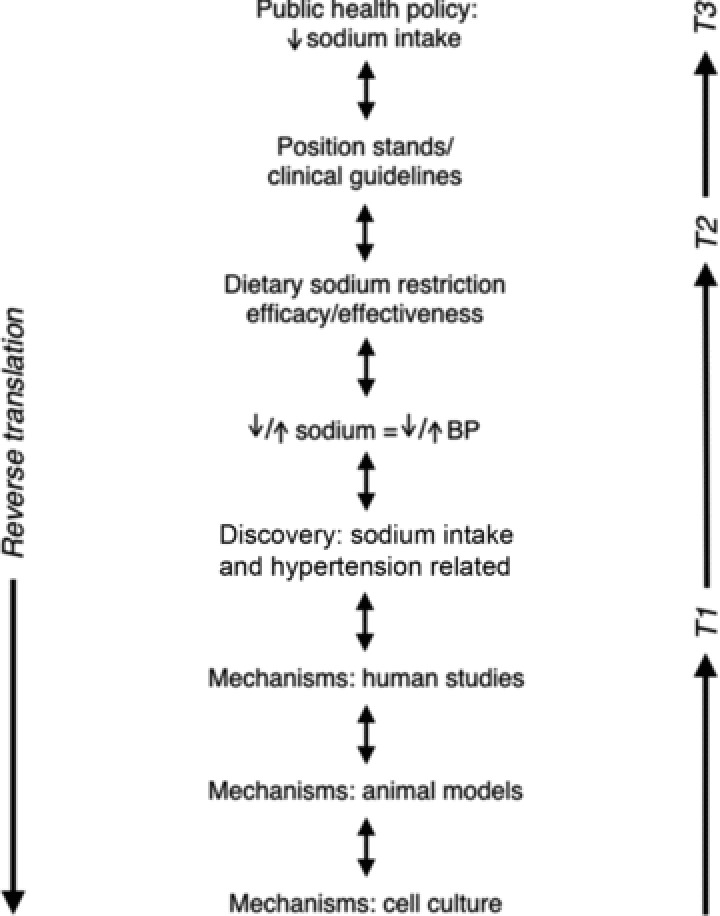
Mid-to-upper: Discovery observation that arterial blood pressure (BP) is related to dietary sodium intake is translated by experimental studies manipulating sodium intake and assessing BP, followed by dietary sodium restriction trials, to clinical guidelines, and finally to public health policies for reducing population sodium intake. Mid-to-lower: The mechanisms underlying the discovery observation are studied in humans in the clinical research setting and in preclinical models. Modified from Jablonski, K.L. (personal communication).
Getting there from here
Despite these notable examples, translational physiology has not been a guiding principle in our discipline. To establish it as such, more emphasis will be required to extend the current strengths of traditional physiology (i.e. integrative studies of function from the molecular/cellular to organism level) to population physiology and public health. The latter end of the spectrum is in large part where the greatest developmental challenges lie, but reaching that end will be necessary to achieve the full potential of translational physiology.
A more uniform extension (‘fleshing out’) of physiology to the population level will allow us to directly link physiological function/dysfunction of groups of patients and communities of healthy individuals to the mechanisms of action underlying their functional status (Fig. 2). Such efforts will generate new information on how higher levels of function and organization influence lower levels, and vice versa. This approach also will provide a means to determine how physiological function of populations is related to long-term health outcomes. A more comprehensive effort in population phenotyping will provide greater insight into functional variability among individuals, how individuals adapt to stress and interventions, and a better understanding of the connections between physiological and genomic variation, with important implications for personalized medicine. These issues cannot be fully addressed with preclinical models or in small groups of human subjects, i.e. with the conventional physiological approaches of the past.
Greater efforts also will be required to expand T2-T3 translational physiological research. This will necessitate greater use of the tools of synthesizing existing evidence on physiological function obtained from basic, clinical and population studies (e.g. using systematic reviews and meta-analyses) for developing guidelines on how to optimize and preserve physiological function in different populations and settings. Dissemination and implementation research will be needed to combine information derived from physiology-based investigations with insight from behavioural and social sciences, education and communication theory, biostatistics/bioinformatics, health care delivery, public policy and other areas (Woolf, 2008) to establish evidence-based ‘best practices’ for optimizing physiological function in individuals and populations.
Physiological epidemiology
One novel area of translational and population physiology relates to network medicine and ‘diseasomes’, i.e. clustering of diseases and major risk factors or processes for disease not previously recognized to be associated (Barabasi, 2007; Chan & Loscalzo, 2012). As an example, recently a diseaseome of physical inactivity associated with type II diabetes, cardiovascular disease, cancer and brain diseases was recognized (Pedersen, 2009). Although the association among diseases within human populations has long been the purview of clinical epidemiology, translational physiologists can play an important role in identifying the integrative physiological and pathophysiological mechanisms that connect these clinical disorders (Pedersen, 2009).
Moreover, if one simply substitutes the physiological dysregulation underlying the disease process in question (Fig. 4), an observational research model is created that could be termed physiological epidemiology or the association (clustering) of physiological functions/dysfunction. This represents another opportunity to extend the scope of traditional physiology to the population level and public health. With our ability to obtain high-resolution phenotypes, physiologists can play an important role in epidemiological research. Indeed, there is a well-established history of physiological assessments in longitudinal observational studies in medicine (Braun et al. 1979) and more recent generations of community-based epidemiological investigations like the Framingham Heart Study in the U.S. have added technically demanding measurements of physiological function (e.g. brachial artery flow-mediated dilation) that are performed on large samples of human subjects.
Figure 4. Physiological epidemiology.
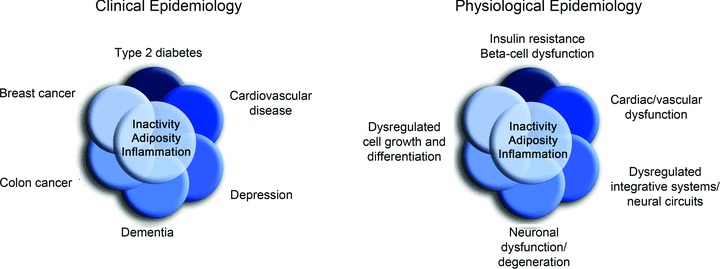
The clinical and physiological epidemiology of physical inactivity. In complementary fashion to clinical epidemiology, physiological epidemiology studies the associations of function and dysfunction within/between populations, and the physiological mechanisms that explain these associations. Modified from Pedersen (2009).
There are many other opportunities for translational physiology in population research. For example, in addition to genetic influences, a number of social-demographic (e.g. social networks, education, income), behavioural, cultural, race/ethnic and other environmental factors are related to physical function, disability and chronic disease (Link & Phelan, 1995; Fried & Guralnik, 1997; Koukouli et al. 2002; Banks et al. 2006; Barabasi, 2007; Turrell et al. 2007) (Fig. 5), in many cases independent of conventional risk factors (Banks et al. 2006). The possible effects of these factors on physiological function and the underlying biological mechanisms are largely unknown, and physiologists are needed to help fill in these knowledge gaps. Such information also may provide valuable insight into the physiological basis for differences in health outcomes within/across societies (Banks et al. 2006). Importantly, some of the social and environmental modulatory influences on physiological function can be studied using preclinical models, as is being done so effectively in the behavioural sciences (Huhman, 2006).
Figure 5. Population physiology.
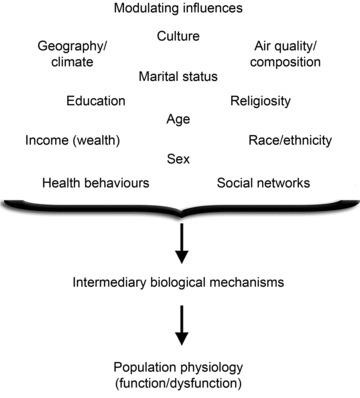
Social, economic, cultural, environmental and other factors influencing physiological function and dysfunction in populations. In most cases, the intermediary biological mechanisms are unknown and represent novel investigative opportunities for translational physiology.
Population ageing represents another opportunity for translational physiology (Kirkland, 2013). Advancing age is the primary risk factor for most chronic diseases, and worldwide ageing patterns are inducing an ever-increasing burden on our health care systems and insurance programs. The key challenge will be to understand the mechanisms by which ageing leads to physiological dysfunction and increased risk of disability and disease, and to establish effective prevention strategies that act to preserve function, independence and overall health status as long as possible during natural life (‘compression of dysfunction and disability’). As the current ‘baby boomer’ generation continues to age, they will be seeking insight into approaches for maintaining physiological function, while remaining physically and cognitively active and productive in the workforce. Physiologists working at all levels of the translational continuum will be needed to meet this growing demand. Translation of basic research models and observations to establish interventions that can retard primary ageing processes and prevent the development of clinical diseases in humans is among the most important future opportunities in this area (Kirkland, 2013).
Lastly, several types of clinical disorders that affect large segments of the population can benefit from translational physiological approaches. For example, highly prevalent mood disorders such as depression may be driven by dysregulation of integrated physiological systems and neural circuits, rather than simply due to neurochemical imbalances in the brain (Drevets et al. 2008; Hale et al. 2013). Moreover, novel treatments may include translational application of long-studied physiological regulatory processes such as thermoregulation (Hale et al. 2013). Translational physiologists also can help explain issues such as how stress leads to epigenetic changes linked to higher prevalence rates of anxiety and depression.
Experimental approaches
A variety of experimental approaches are available to advance translational physiology, and many of these are well established in physiological research. The most conventional approach has been to study phenotypes or mechanisms initially in cell culture, then translating to non-mammalian and mammalian animal models and, if the observations hold, to humans (Fig. 2). Reverse translation in which function is assessed initially in populations or small groups of humans and mechanisms of action are investigated using preclinical models is equally powerful. Combining results on the same functional outcomes from different physiological models within a single study can provide a compelling story with unique ‘internal validity’ of observations (Donato et al. 2011; LaRocca et al. 2012; Pedersen & Febbraio, 2012).
Numerous physiological phenotypes can be studied using such multi-level translational approaches. For example, aortic pulse wave velocity, the gold standard measure of large elastic artery stiffness, initially can be shown to be a strong independent predictor of incident cardiovascular events in a large population sample from an epidemiological (observational) study (Mitchell et al. 2010) and in clinical trials (Mitchell et al. 2007). Modulating factors and mechanisms can be studied in smaller groups of human subjects in a clinical research setting (Tanaka et al. 1998) and in rodents (Sindler et al. 2011), with the ability to further assess intrinsic mechanical properties in isolated arteries (Fleenor et al. 2012) and individual vascular cells (Qiu et al. 2010). Measurement of aortic pulse wave velocity likely will be incorporated into medical practice in the near future, further illustrating how functional assessments can eventually influence clinical guidelines and public health. Such multi-level translational approaches are being used in several areas of physiology including skeletal muscle function (Pedersen & Febbraio, 2012).
Continued development and greater utilization of innovative ex vivo experimental techniques will be essential if the full potential of translational physiology is to be realized. An unprecedented variety of fluid (plasma, serum, saliva, sweat, urine, cerebral spinal fluid, etc.) and cell/tissue (blood and vascular cells, muscle, fat, skin, feces, bone, etc.) samples can be obtained from human subjects, allowing bidirectional translation (Fig. 6). Using either conventional or newer high-throughput techniques, samples can be analysed for signalling molecules (e.g. superoxide), metabolites, gene and microRNA expression, epigenetic modifications, protein concentrations, microbiomes (oral, gut, skin, etc.) and other mechanistic biomarkers, which can then be related to physiological phenotypes of interest across preclinical models up to populations of humans (Casado-Vela et al. 2011; Donato et al. 2011; Shaw & Brettman, 2011; LaRocca et al. 2012; Pedersen & Febbraio, 2012; Jablonski et al. 2013; Kujala et al. 2013). Samples from animal models or human subjects can be studied ex vivo after incubation with stressors or pharmacological/genetic modulators of mechanistic pathways of interest (Shenouda et al. 2011; Fleenor et al. 2012), or studied in culture (Shaw & Brettman, 2011; Pedersen & Febbraio, 2012). If a humoral (circulating) factor is suspected, cells or tissues of interest can be treated with serum samples from experimental and control groups (animals or humans) to isolate the mechanism (Barua et al. 2001; Lopez-Lluch et al. 2006).
Figure 6. Human samples for translational physiology.
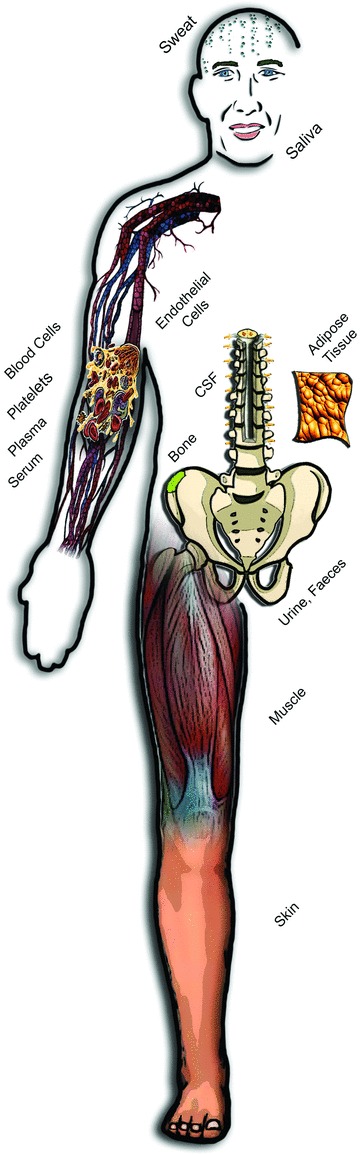
Translational physiological research can be facilitated by ex vivo analysis of fluid, cell and tissue samples obtained from individual, groups and even populations of human subjects.
New in vivo approaches also continue to be developed, including a growing list of compounds that can be safely administered to human subjects to activate or inhibit (‘pharmaco-dissect’) specific signalling pathways influencing physiological function (Crecelius et al. 2012; Jablonski et al. 2013). Rapid developments in imaging provide unprecedented opportunities for physiologists engaged in basic research to translate their observations, especially for investigations on the brain and other tissues that cannot be directly accessed in humans. In some cases, the biochemical properties of tissues can be assessed with creative use of non-invasive technology (de Vos et al. 2013).
Finally, access to large databases and technical advances in biological and behavioural monitoring provide new opportunities to assess physiological function in both healthy and clinical populations. Creative analysis of world records for different types of athletic performance can provide unique insight into the limits of physiological function and the effects of modulating influences such as age and sex (Tanaka & Seals, 2008). The development of commercially available devices that can monitor variables such as blood oxygenation, glucose concentrations, ECG, blood pressure, temperature, physical activity and energy expenditure are creating large population data sets that can used to assess physiological function in a variety of settings, including longitudinally (Nose et al. 2009). Clinically, remote monitoring of physiological function in patients with chronic diseases continues to be explored with the goal of improving medical management and reducing mortality, hospitalizations and health care costs (Koehler et al. 2011). Harnessing the potential of such information to address questions of biological and biomedical importance represents another novel opportunity (and a big challenge) for translational physiology.
Interventions and treatments
Presently there is great interest in establishing new prevention strategies and treatments for disease. Here too, translational physiologists will be invaluable assets for accurate assessments of functional outcomes and knowledge of integrative physiological mechanisms. Translational approaches to interventions/treatments, particularly those involving experimental agents, initially may test efficacy (i.e. improvement in function) in cells or experimental animals, then seek to confirm the results in human trials. Alternatively, treatments already shown to be safe and effective in preclinical models can be directly assessed in individuals with baseline dysfunction or risk factors, or in patients with clinical disease. After establishing functional improvements, translational approaches can be used to determine the physiological mechanisms through which the treatment exerts its beneficial effects. Mechanisms can be investigated using pharmaco-dissection and/or ex vivo analysis of samples in the human subjects undergoing treatment, in subsequent preclinical studies, or both (Jablonski et al. 2013). Research on omega-3 fatty acids was sparked by epidemiological findings in native Eskimo populations, and translational physiological approaches have since been used to establish benefits to function and mechanisms of action at multiple levels of observation in several populations (Mozaffarian & Wu, 2011).
Assessing the potential efficacy of pharmaceutical or nutraceutical compounds translationally in cells, experimental animals and groups of human subjects is a well-established process, but lifestyle-based interventions also can be assessed using such an approach. Effects of exercise are routinely investigated in human cohorts, from the community to the clinical research setting, and in rodents. However, exercise also can be studied in situ (e.g. electrical stimulation of intact muscle) and in vitro in cells (e.g. stimulation of myocytes in culture or periodic increases in shear stress [blood flow] in vascular endothelial cell culture) (Broholm et al. 2011) (Fig. 7). Energy restriction can be studied in humans and experimental animals via well-established protocols, but also in cell culture using glucose deprivation, caloric restriction mimetics and serum incubation techniques (Lopez-Lluch et al. 2006). Indeed, ‘serum treatment’ models can be used to assess the effects of a variety of lifestyle behaviours on cellular and tissue function and the mechanisms involved (Roberts et al. 2006; Marin et al. 2012).
Figure 7. Translational study of stressors and interventions.
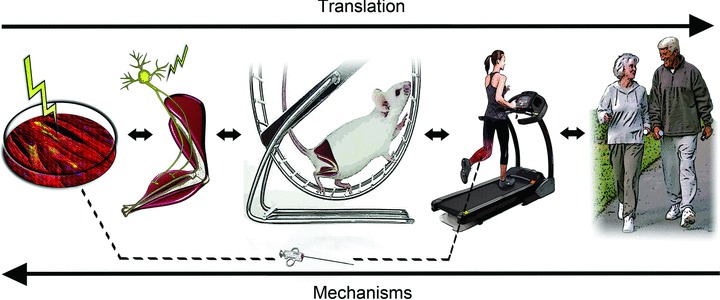
Acute and chronic stressors and interventions can be studied using translational physiological approaches. Effects of exercise on skeletal muscle can be studied (R to L) in community settings, clinical research centres, and in laboratories using in vivo (e.g. rodent), in situ (nerve stimulation) and in vitro (electrical stimulation of muscle cells in culture) preclinical models.
The combination of translational thinking and new technology will increasingly allow physiologists to extend the reach of prevention and therapeutic trials on function to population levels. For example, remote assessment of automated measurements of physiological variables now allow the effectiveness of interventions to be studied over wide geographical regions without the need for subjects to repeatedly travel to centralized clinical research facilities for assessment and staff support (Nose et al. 2009).
The inability to advance promising treatments from the preclinical to clinical stages has been referred to as the ‘Valley of Death’ (Butler, 2008; Homer-Vanniasinkam & Tsui, 2012). Translational physiology can play an important role in bridging this gap, while providing novel insight into the integrative mechanisms of action (Hall, 2002; Head, 2010; Granger et al. 2012).
Roadblocks
There are several potential roadblocks for advancing translational physiology (Table 1), many of which are shared with translational research in general (Deschamps & Eng, 2012; Homer-Vanniasinkam & Tsui, 2012; van der Laan & Boenink, 2012). Differences in education and research training can contribute to scientific cultures and produce ‘cognitive firewalls’ that are not conducive for promoting translational physiology. Lack of knowledge (of the literature, experimental process or research environment), technical skills and access to major equipment, facilities and other resources can be an impediment to basic scientists with interest in translating their work to humans, as well as for clinical and population physiologists interested in pursuing mechanisms using preclinical models and biochemical/molecular techniques. For physiologists working with cell culture or animal models, the additional time, higher costs and daunting regulatory burden of human subjects research can be prohibitive. Moreover, with regard to prestige, publication opportunities in high impact scientific journals, and competitiveness for tenure-track positions and promotion and tenure, much of the current system in biological and biomedical science rewards ‘discovery’ research. Collectively, these factors can serve as strong disincentive to basic scientists contemplating translational efforts.
Table 1.
Roadblocks (left) and strategies (right) for advancement of translational physiology research
| Roadblocks | Strategies |
|---|---|
| Academic/research culture | Education (classes, research rotations, journal clubs, etc.) |
| Lack of knowledge and/or technical skills | “Translational” research career development proposals |
| Access to equipment/facilities | Featured meeting symposia and highlighted journal series |
| Resources to extend research to other levels of observation | Multidisciplinary collaboration |
| Time, costs and regulatory burden of human research | Broaden hiring practices |
| Disincentives (e.g. prestige/rewards of discovery research) | Investigators expand scope |
| Developing/sustaining successful collaborations | Preclinical data as preliminary results for initial human study |
| Expanding present techniques | Strategic use of core labs and clinical research centres |
Strategies for promoting translational physiology
For translational physiology to be successful, these obstacles must be offset by advantages, be they altruistic (greater contribution to societal health and well being), scientific (ability to study and confirm function or mechanisms at different levels of observation) or monetary (related to access to/competitiveness for extramural funding or commercialization of products).
One strategy should focus on reducing the size of the barriers (Table 1). Educational experiences involving graduate seminars, research rotations and journal clubs in which students are exposed to basic, clinical and population based research approaches (and their respective strengths and limitations) can help establish an informed perspective about translational physiology. Individual and institutional research career development grants featuring translational physiology-oriented research training plans, projects, mentoring teams and environments should be considered where appropriate, and peer reviewers must look for opportunities to support worthy proposals emphasizing truly translational training. Featured symposia in our meetings and highlighted series in our journals should be used to promote translational physiology, and this already is occurring in some cases. Greater interaction with both physiologists conducting research at other levels of observation and biomedical investigators in other disciplines, including T2/3-related fields such as biostatistics, epidemiology, public health, sociology and behavioural psychology, will be important.
Hiring strategies in physiology units likely will have a major impact on the development of translational physiology. The long-standing trend has been to seek talented investigators who employ reductionist and, particularly recently, high-throughput molecular and systems biology approaches. Clearly there is demand for such expertise in physiology, as emphasized previously (Strange, 2005; Noble, 2008; Head, 2010; Kuster et al. 2011). However, there also is a compelling need (and an opportunity) for investigators with skills in areas such as clinical physiology, epidemiology, biostatistics and bioinformatics in order to extend physiological research to the population level with the attendant potential for direct impact on public health. Indeed, investigators trained in the emerging areas of translational epidemiology and translational bioinformatics (Khoury et al. 2010) would provide unique synergism for physiology departments interested in increasing their ability to engage in translational research and network medicine (Barabasi, 2007; Chan & Loscalzo, 2012). Such an approach would serve to expand and diversify the research portfolio of physiology programs, creating new scientific and funding opportunities.
For full development and implementation of translational physiology, however, more investigators presently working in human/clinical physiology must explore opportunities to either extend the scope of their work towards populations (T2/3), to perform reverse translational investigation of cellular and molecular mechanisms of action, or both. Similarly, more physiologists performing basic research must look for opportunities to translate their findings to the organism and population levels. One advantage of such efforts is that observations from preclinical models can be used effectively as preliminary data for proposing initial studies in humans, while requiring only a fraction of the time, labor and expense involved with obtaining preliminary results on human subjects. In the same manner, initial studies establishing a clear phenotype of interest in humans can serve as a convincing preliminary step for proposing experiments aimed at identifying mechanisms of action using basic science models. In either case, many peer reviewers find a translational foundation to proposed research projects appealing.
Pragmatically, how can such extension of our research be accomplished? Much of the challenge lies in the multidisciplinary nature of contemporary biomedical science and our limited range of expertise as individual investigators. The most obvious approach is collaboration. Collaborators can contribute their knowledge, facilities, equipment, technical skills and personnel to the effort, avoiding the need to replicate those resources. The challenges include identifying reliable partners, potential loss of control over time lines and the quality of the data, intellectual property issues, authorship disputes and possible concerns with independence when early career investigators are involved. Establishing the necessary expertise and infrastructure in your own group eliminates these problems, but can be unfeasibly time-consuming and expensive. A ‘mixed model’ strategy using a combination of collaborations, core laboratory facilities and establishment of new techniques within the home laboratory may be most efficient (and successful). Many campuses have clinical research centres with staff that can assist basic scientists with approval procedures for using human subjects, experimental agents, etc., as well as other administrative tasks, while providing the facilities, equipment, personnel and technical skills required for conducting protocols on human subjects.
Conclusions
It has been stated that translational research is not for the faint hearted (Homer-Vanniasinkam & Tsui, 2012), and certainly the same can be said for translational physiology. It is, in its entirety, a challenging proposition. However, as an old adage suggests, ‘with change comes opportunity’. Translational physiology provides both a framework and a platform for physiologists and their departments that emphasizes the study of function from molecular events to populations, clinical practice and public health (Fig. 8). For investigators, translational physiology holds the potential for enhancing the completeness and societal impact of our science, and the competitiveness of our grant proposals. If established across the broadest possible scope, translational physiology can serve to further highlight and cement the critical role of physiology in the biomedical research enterprise. Importantly, translational physiology is a ‘big tent’ model: investigators and approaches from basic science to population physiology are welcome (and needed) to achieve its full transformative promise.
Figure 8. Summary: translational physiology.
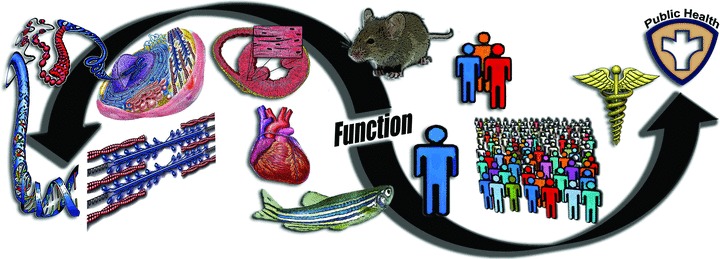
Physiological function is studied bidirectionally over a continuum from molecular events, to sub-cellular, cellular, tissue and organ function, at the organism level using preclinical (non-mammalian and mammalian) models, in individual and small groups of humans, and in populations of humans, with direct implications for clinical practice and public health.
Acknowledgments
The author recognizes Forrest Brooks for his extensive research assistance, Dr Amy Sindler for her technical support, Drs Kristen Jablonski, Thomas LaRocca, Christopher Lowry and Matthew McQueen for content suggestions, and Dr Jamie Justice for graphics contributions.
Additional information
Competing interests
None.
Funding
The author is supported by grant awards from the National Institute on Aging and the National Heart, Lung and Blood Institute of the National Institutes of Health in the U.S.
References
- Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC, Jr, Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123:1138–1143. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- Banks J, Marmot M, Oldfield Z, Smith JP. Disease and disadvantage in the United States and in England. JAMA. 2006;295:2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Barabasi AL. Network medicine–from obesity to the “diseasome”. N Engl J Med. 2007;357:404–407. doi: 10.1056/NEJMe078114. [DOI] [PubMed] [Google Scholar]
- Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation. 2001;104:1905–1910. doi: 10.1161/hc4101.097525. [DOI] [PubMed] [Google Scholar]
- Braun SR, doPico GA, Tsiatis A, Horvath E, Dickie HA, Rankin J. Farmer's lung disease: long-term clinical and physiologic outcome. Am Rev Respir Dis. 1979;119:185–191. doi: 10.1164/arrd.1979.119.2.185. [DOI] [PubMed] [Google Scholar]
- Broholm C, Laye MJ, Brandt C, Vadalasetty R, Pilegaard H, Pedersen BK, Scheele C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J Appl Physiol. 2011;111:251–259. doi: 10.1152/japplphysiol.01399.2010. [DOI] [PubMed] [Google Scholar]
- Butler D. Translational research: crossing the valley of death. Nature. 2008;453:840–842. doi: 10.1038/453840a. [DOI] [PubMed] [Google Scholar]
- Casado-Vela J, Cebrian A, Gomez del Pulgar MT, Lacal JC. Approaches for the study of cancer: towards the integration of genomics, proteomics and metabolomics. Clin Transl Oncol. 2011;13:617–628. doi: 10.1007/s12094-011-0707-9. [DOI] [PubMed] [Google Scholar]
- Chan SY, Loscalzo J. The emerging paradigm of network medicine in the study of human disease. Circ Res. 2012;111:359–374. doi: 10.1161/CIRCRESAHA.111.258541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. ATP-mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans. J Physiol. 2012;590:5349–5359. doi: 10.1113/jphysiol.2012.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl LK. Possible role of chronic excess salt consumption in the pathogenesis of essential hypertension. Am J Cardiol. 1961;8:571–575. doi: 10.1016/0002-9149(61)90137-0. [DOI] [PubMed] [Google Scholar]
- Dahl LK, Heine M, Thompson K. Genetic influence of the kidneys on blood pressure. Evidence from chronic renal homografts in rats with opposite predispositions to hypertension. Circ Res. 1974;40:94–101. doi: 10.1161/01.res.40.4.94. [DOI] [PubMed] [Google Scholar]
- de Vos LC, Noordzij MJ, Mulder DJ, Smit AJ, Lutgers HL, Dullaart RP, Kamphuisen PW, Zeebregts CJ, Lefrandt JD. Skin autofluorescence as a measure of advanced glycation end products deposition is elevated in peripheral artery disease. Arterioscler Thromb Vasc Biol. 2013;33:131–138. doi: 10.1161/ATVBAHA.112.300016. [DOI] [PubMed] [Google Scholar]
- Deschamps AM, Eng C. Engaging basic scientists in translational research. Endocr Relat Cancer. 2012;19:E1–3. doi: 10.1530/ERC-12-0106. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol. 2012;47:588–594. doi: 10.1016/j.exger.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- Granger JP, Barman SM, Barrett KE. Promoting physiology as an essential element in translational research. Physiology (Bethesda) 2012;27:326. doi: 10.1152/physiol.00045.2012. [DOI] [PubMed] [Google Scholar]
- Gu JW, Anand V, Shek EW, Moore MC, Brady AL, Kelly WC, Adair TH. Sodium induces hypertrophy of cultured myocardial myoblasts and vascular smooth muscle cells. Hypertension. 1998;31:1083–1087. doi: 10.1161/01.hyp.31.5.1083. [DOI] [PubMed] [Google Scholar]
- Hale MW, Raison CL, Lowry CA. Integrative physiology of depression and antidepressant drug action: Implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacol Ther. 2013;137:108–118. doi: 10.1016/j.pharmthera.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Hall JE. The promise of translational physiology. Am J Physiol Lung Cell Mol Physiol. 2002;283:L235–236. doi: 10.1152/ajplung.00098.2002. [DOI] [PubMed] [Google Scholar]
- Head GA. Exciting challenges ahead for integrative physiology. Front Physiol. 2010;1:127. doi: 10.3389/fphys.2010.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer-Vanniasinkam S, Tsui J. The continuing challenges of translational research: clinician-scientists’ perspective. Cardiol Res Pract. 2012;2012:246710. doi: 10.1155/2012/246710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology. Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ. Physiology: alone at the bottom, alone at the top. J Physiol. 2011;589:1005. doi: 10.1113/jphysiol.2010.203893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ, Pedersen BK. Ten questions about systems biology. J Physiol. 2011;589:1017–1030. doi: 10.1113/jphysiol.2010.201509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury MJ, Gwinn M, Ioannidis JP. The emergence of translational epidemiology: from scientific discovery to population health impact. Am J Epidemiol. 2010;172:517–524. doi: 10.1093/aje/kwq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol. 2013;48:1–5. doi: 10.1016/j.exger.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD, Telemedical Interventional Monitoring in Heart Failure I Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123:1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- Koukouli S, Vlachonikolis IG, Philalithis A. Socio-demographic factors and self-reported functional status: the significance of social support. BMC Health Serv Res. 2002;2:20. doi: 10.1186/1472-6963-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala UM, Makinen VP, Heinonen I, Soininen P, Kangas AJ, Leskinen TH, Rahkila P, Wurtz P, Kovanen V, Cheng S, Sipila S, Hirvensalo M, Telama R, Tammelin T, Savolainen MJ, Pouta A, O’Reilly PF, Mantyselka P, Viikari J, Kahonen M, Lehtimaki T, Elliott P, Vanhala MJ, Raitakari OT, Jarvelin MR, Kaprio J, Kainulainen H, Ala-Korpela M. Long-term leisure-time physical activity and serum metabolome. Circulation. 2013;127:340–348. doi: 10.1161/CIRCULATIONAHA.112.105551. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK, Cook NR, Cutler JA, Belden L, Brewer A, Cohen JD, Hebert PR, Lasser VI, Raines J, Raczynski J, Shepek L, Diller L, Whelton PK, Yamamoto M, Trials of Hypertension Prevention Collaborative Research G Sodium reduction for hypertension prevention in overweight adults: further results from the Trials of Hypertension Prevention Phase II. J Hum Hypertens. 2005;19:33–45. doi: 10.1038/sj.jhh.1001774. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK, Hebert PR, Cutler JA, Lasser VI, Sugars CP, Steffen-Batey L, Brewer AA, Cameron M, Shepek LD, Cook NR, et al. Feasibility and efficacy of sodium reduction in the Trials of Hypertension Prevention, phase I. Trials of Hypertension Prevention Collaborative Research Group. Hypertension. 1993;22:502–512. doi: 10.1161/01.hyp.22.4.502. [DOI] [PubMed] [Google Scholar]
- Kuster DW, Merkus D, van der Velden J, Verhoeven AJ, Duncker DJ. ‘Integrative Physiology 2.0’: integration of systems biology into physiology and its application to cardiovascular homeostasis. J Physiol. 2011;589:1037–1045. doi: 10.1113/jphysiol.2010.201533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–3316. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman MN, Marincola FM. Expanding the perspective of translational medicine: the value of observational data. J Transl Med. 2012;10:61. doi: 10.1186/1479-5876-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;Spec No:80–94. [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet. 1989;2:1244–1247. doi: 10.1016/s0140-6736(89)91852-7. [DOI] [PubMed] [Google Scholar]
- Marin C, Delgado-Lista J, Ramirez R, Carracedo J, Caballero J, Perez-Martinez P, Gutierrez-Mariscal FM, Garcia-Rios A, Delgado-Casado N, Cruz-Teno C, Yubero-Serrano EM, Tinahones F, Malagon Mdel M, Perez-Jimenez F, Lopez-Miranda J. Mediterranean diet reduces senescence-associated stress in endothelial cells. Age (Dordr) 2012;34:1309–1316. doi: 10.1007/s11357-011-9305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincola FM. Translational Medicine: A two-way road. J Transl Med. 2003;1:1. doi: 10.1186/1479-5876-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Dunlap ME, Warnica W, Ducharme A, Arnold JM, Tardif JC, Solomon SD, Domanski MJ, Jablonski KA, Rice MM, Pfeffer MA, Prevention of Events With Angiotensin-Converting Enzyme Inhibition I Long-term trandolapril treatment is associated with reduced aortic stiffness: the prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension. 2007;49:1271–1277. doi: 10.1161/HYPERTENSIONAHA.106.085738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Campbell NR, Willis K. Effective population-wide public health interventions to promote sodium reduction. CMAJ. 2009;181:605–609. doi: 10.1503/cmaj.090361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- Noble D. Claude Bernard, the first systems biologist, and the future of physiology. Exp Physiol. 2008;93:16–26. doi: 10.1113/expphysiol.2007.038695. [DOI] [PubMed] [Google Scholar]
- Nose H, Morikawa M, Yamazaki T, Nemoto K, Okazaki K, Masuki S, Kamijo Y, Gen-No H. Beyond epidemiology: field studies and the physiology laboratory as the whole world. J Physiol. 2009;587:5569–5575. doi: 10.1113/jphysiol.2009.179499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol. 2009;587:5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Pitt BR, Christman JW, Gunst SJ, Matthay MA, Stevens T, Ware LB. Physiology, reductionism, and translational medicine: the right mix. Am J Physiol Lung Cell Mol Physiol. 2011;301:L389–390. doi: 10.1152/ajplung.00270.2011. [DOI] [PubMed] [Google Scholar]
- Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CK, Won D, Pruthi S, Kurtovic S, Sindhu RK, Vaziri ND, Barnard RJ. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol. 2006;100:1657–1665. doi: 10.1152/japplphysiol.01292.2005. [DOI] [PubMed] [Google Scholar]
- Shaw SY, Brettman AD. Phenotyping patient-derived cells for translational studies in cardiovascular disease. Circulation. 2011;124:2444–2455. doi: 10.1161/CIRCULATIONAHA.111.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K. The end of “naive reductionism”: rise of systems biology or renaissance of physiology. Am J Physiol Cell Physiol. 2005;288:C968–974. doi: 10.1152/ajpcell.00598.2004. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Seals DR. Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol. 2008;586:55–63. doi: 10.1113/jphysiol.2007.141879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrell G, Lynch JW, Leite C, Raghunathan T, Kaplan GA. Socioeconomic disadvantage in childhood and across the life course and all-cause mortality and physical function in adulthood: evidence from the Alameda County Study. J Epidemiol Community Health. 2007;61:723–730. doi: 10.1136/jech.2006.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan AL, Boenink M. Beyond Bench and Bedside: Disentangling the Concept of Translational Research. Health Care Anal. 2012 doi: 10.1007/s10728-012-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD, Paterson DJ. Physiology: found in translation. J Physiol. 2011;589:3899–3900. doi: 10.1113/jphysiol.2011.215079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299:211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]


