Abstract
During muscle fatigue, firing of small-diameter muscle afferents can decrease voluntary activation of the fatigued muscle. However, these afferents may have a more widespread effect on other muscles in the exercising limb. We examined if the firing of fatigue-sensitive afferents from elbow extensor muscles in the same arm reduces torque production and voluntary activation of elbow flexors. In nine subjects we examined voluntary activation of elbow flexors by measuring changes in superimposed twitches evoked by transcranial magnetic stimulation of the motor cortex during brief (2–3 s) maximal voluntary contractions (MVC). Inflation of a blood pressure cuff following a 2-min sustained MVC blocked blood flow to the fatigued muscle and maintained firing of small-diameter afferents. After a fatiguing elbow flexion contraction, maximal flexion torque was lower (26.0 ± 4.4%versus 67.9 ± 5.2% of initial maximal torque; means ±s.d.; P < 0.001) and superimposed twitches were larger (4.1 ± 1.1%versus 1.8 ± 0.2% ongoing MVC, P= 0.01) with than without ischaemia. After a fatiguing elbow extensor contraction, maximal flexion torque was also reduced (82.2 ± 4.9%versus 91.4 ± 2.3% of initial maximal torque; P= 0.007), superimposed twitches were larger (2.7 ± 0.7%versus 1.3 ± 0.2% ongoing MVC; P= 0.02) and voluntary activation lower (81.6 ± 8.2%versus 95.5 ± 6.9%; P= 0.04) with than without ischaemia. After a fatiguing contraction, voluntary drive to the fatigued muscles is reduced with continued input from small-diameter muscle afferents. Furthermore, fatigue of the elbow extensor muscles decreases voluntary drive to unfatigued elbow flexors of the same arm. Therefore, firing of small-diameter muscle afferents from one muscle can affect voluntary activation and hence torque generation of another muscle in the same limb.
Key points
Maintained firing of fatigue-sensitive small-diameter muscle afferents is reported to reduce voluntary activation of the homonymous (fatigued) muscle.
Our study determined if firing of fatigue-sensitive afferents from elbow extensor muscles reduces voluntary activation and torque of the non-fatigued elbow flexors.
We examined voluntary activation of the elbow flexors by measuring changes in superimposed twitches evoked by magnetic cortical stimulation during maximal voluntary contractions.
Following a fatiguing contraction of elbow extensors, the voluntary drive to unfatigued flexor muscles was reduced with continued activation of small-diameter muscle afferents produced by a blood pressure cuff inflated to maintain muscle ischaemia.
Continued discharge of small-diameter muscle afferents from one muscle can decrease voluntary drive to another muscle in the same limb and can reduce its maximal voluntary torque.
Introduction
Exercise performance is impaired by fatigue. While much of the decrease in exercise performance from fatigue is due to processes within the muscle (Allen et al. 2008), central fatigue also contributes significantly to the loss of force (Taylor & Gandevia, 2008). Central fatigue is defined as a progressive exercise-related decline in voluntary activation (Gandevia, 2001), that is, a decline in the ability of the nervous system to drive the muscle to generate maximal force. Thus, despite a subject's maximal effort, some motor units are not firing or not firing fast enough to produce fused contractions. A number of mechanisms may contribute to this essential feature of central fatigue. One possibility is feedback from small-diameter muscle afferents (Bigland-Ritchie et al. 1986; Woods et al. 1987; see also Garland & Kaufman, 1995). Blocking of these fatigue-sensitive afferents through anaesthesia or analgesia during high-intensity cycling results in increased voluntary drive. This emphasises the importance of small-diameter muscle afferent feedback on central fatigue from the exercising muscle (Amann et al. 2009, 2011). It has been suggested that the action of small-diameter muscle afferents to reduce voluntary activation to the fatigued muscle occurs at the level of the cortex but does not directly affect motor cortical output cells (Gandevia et al. 1996; Taylor et al. 1996). However, this has only been demonstrated in a single experiment with a small sample size.
Small-diameter muscle afferents are a heterogeneous group of sensory fibres that respond to a range of mechanical and metabolic inputs (e.g. Paintal, 1960; Kniffki et al. 1978; Kaufman et al. 1984; Rotto & Kaufman, 1988; Adreani et al. 1997; Light et al. 2008). They are a subset of flexor reflex afferents and exert a range of spinal reflex effects (Kniffki et al. 1981; Hayward et al. 1991; Schomburg et al. 1999, 2012; Schomburg & Steffens, 2002). One method to activate these receptors is to inject the muscle with hypertonic saline, which induces muscle pain (e.g. Kellgren, 1938; Laursen et al. 1999; Graven-Nielsen et al. 2003; Henderson et al. 2006). Another experimental method to activate muscle nociceptors is to maintain muscle ischaemia during or after exercise. As early as 1931, Lewis and colleagues concluded that some product that accumulates after fatiguing contractions produces pain and that this can be maintained with the occlusion of blood flow to the muscle. Subsequently, ligation or inflation of a blood pressure cuff around the limb to maintain muscle ischaemia has been widely used to investigate the effects of small-diameter muscle afferents on motor output (e.g. Mills et al. 1982; Wiles & Edwards, 1982; Kaufman et al. 1984; Bigland-Ritchie et al. 1986; Woods et al. 1987; Gandevia et al. 1996; Martin et al. 2006) and on cardiorespiratory responses to exercise (e.g. Alam & Smirk, 1937; Coote et al. 1971; Kaufman et al. 1983; Ichinose et al. 2011).
Recently, we investigated the effect of muscle pain induced by intramuscular hypertonic saline on voluntary activation of the elbow flexors (Khan et al. 2011). While there was a slight reduction in maximal voluntary torque, there was no effect on voluntary activation. By contrast, voluntary activation decreases during a fatiguing contraction and remains depressed when ischaemia is used to prevent recovery of the muscle (Gandevia et al. 1996). Thus, activation of small-diameter muscle afferents in association with fatiguing contractions reduces voluntary drive, but drive is not affected during non-ischaemic muscle pain. One possible explanation for these different actions on voluntary activation is that different classes of afferents are activated by the two stimuli (Graven-Nielsen et al. 2003). Another possible explanation is that neurones driving motor output to the contracting muscle are in a different state after repetitive activation during a fatiguing voluntary contraction (McNeil et al. 2009, 2011b). If so, fatigue-related firing of small-diameter muscle afferents may only impair voluntary activation of a fatigued muscle group.
In humans, fatigue-sensitive small-diameter muscle afferents exert reflex effects at the spinal level beyond the homonymous muscle. Continued discharge of muscle afferents in either the elbow flexor or extensor muscles through maintained ischaemia following a fatiguing contraction both inhibits motoneurones of the elbow extensors and facilitates motoneurones of the elbow flexors (Martin et al. 2006), as expected for a nociceptive flexor reflex (e.g. Creed et al. 1932). However, it is not known whether impairment of voluntary activation by fatigue-sensitive afferents is confined to the fatigued muscles or is more widespread.
The current study investigated the novel question of whether fatigue-sensitive small-diameter muscle afferents from the elbow extensor muscles impair voluntary activation of the unfatigued elbow flexor muscles of the same limb. In addition, the study confirmed for the elbow flexor muscles that maintained firing of fatigue-sensitive muscle afferents reduces voluntary activation of the homonymous fatigued muscle.
Methods
Three studies were carried out to determine whether firing of fatigue-sensitive muscle afferents in the elbow flexor or extensor muscles altered voluntary activation of the elbow flexor muscles. Nine healthy subjects (age: 21–57 years, mean 38 years; seven men, two women) attended the laboratory on five separate days. They performed a sustained 2-min maximal voluntary contraction (MVC) of the elbow flexors on 2 days, of the elbow extensors on 2 days, and undertook one control session. The Human Research Ethics Committee at the University of New South Wales approved the study, and written informed consent to the experimental protocol was obtained. The study was conducted according to the Declaration of Helsinki.
The subject was seated at a table with the right arm held in supination, 90° of shoulder flexion, and 70° of elbow flexion (180° is full extension). The arm was fixed at the wrist to a stationary arm bar designed to measure torque about the elbow via a force transducer (XTran, Melbourne, Vic., Australia: linear to 1 kN). For familiarisation, subjects performed three brief (2–3 s) MVC with 90-s rests. Surface EMG was acquired from the biceps brachii, triceps brachii and brachioradialis muscles of the right arm through Ag-AgCl electrodes (Conmed ClearTrace ECG Sensor Electrodes Utica, NY, USA). For biceps brachii and brachioradialis, electrodes were placed at the midpoint of the muscle belly (likely overlapping both heads for biceps) and over the distal tendon. For triceps brachii, electrodes were fixed over the belly of the medial head and over the distal tendon. EMG signals were amplified (× 100) and band-pass filtered (16–1000 Hz) using CED 1902 amplifiers (Cambridge Electronic Design, Cambridge, UK). Torque was sampled at 1000 Hz and EMG data at 2000 Hz and recorded to a computer with a 12-bit A/D converter (CED 1401 Plus) and Spike2 software (v 6.06, CED). Visual feedback of torque was also provided with an array of LEDs.
Brachial plexus stimulation
To determine the maximal compound muscle action potential (Mmax), a constant -current stimulator (model DS7AH, Digitimer, Welwyn Garden City, UK) was used (200 μs pulse width). The cathode was placed in the supraclavicular fossa over Erb's point and the anode over the acromion. Single stimuli were delivered with increasing intensity until the peak-to-peak amplitude of M waves from biceps and triceps showed no further increase.
Motor point stimulation
Electrical stimulation was used to activate the intramuscular nerve fibres of biceps brachii and brachialis to evoke maximal twitches. The cathode was placed just proximal and medial to the midpoint of biceps with the anode ∼6 cm distal near the biceps tendon. Single stimuli (200 μs duration) were delivered to the resting muscle with increasing intensity until the amplitude of the evoked twitch showed no further increase. The intensity used for subsequent stimulation (88–264 mA) was 110% of that which evoked a maximal twitch.
Transcranial magnetic stimulation
TMS was delivered using a round coil (13.5 cm outside diameter; Magstim 200; Magstim, Whitland, UK) over the vertex oriented to preferentially activate the left motor cortex and muscles of the right arm. The main aim of TMS was to elicit superimposed twitches from the elbow flexor muscles as described by Todd and colleagues (2003). Hence, TMS intensity was set to elicit the largest possible responses from the elbow flexor muscles while ensuring that the elbow extensor responses remained small. TMS intensity was set such that during an elbow flexion contraction of 50% MVC the amplitude of the motor evoked potential (MEP) in biceps was 70% Mmax or greater and during an elbow flexor MVC, the triceps MEP was below 15% Mmax (Todd et al. 2003). The same intensity of stimulation (40–65% stimulator output) was used for each session.
Experimental procedures
Experiment 1: 2-min maximal voluntary contraction of elbow flexors with or without maintained ischaemia
Subjects attended for 2 days at least 2 days apart. The same protocol was carried out on the 2 days except that on 1 day a sphygmomanometer cuff was used to block blood flow to and from the arm (Fig. 1). Subjects were randomly assigned to perform the ‘cuff’ or ‘no-cuff’ day first. The cuff was 72 mm wide and positioned as proximal as possible to minimise compression of the belly of biceps brachii. When required, the cuff was inflated to 300 mmHg using compressed air, which ensured inflation in ∼1 s.
Figure 1. Experimental protocol.
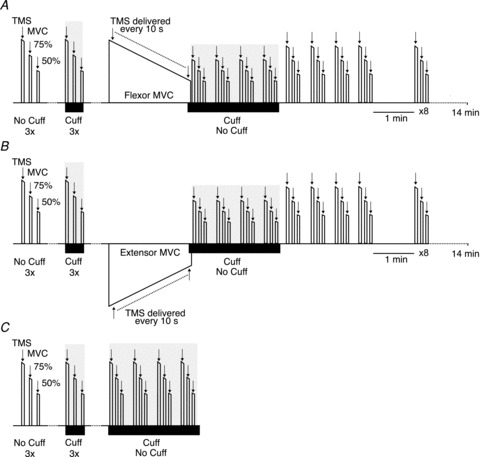
In Experiments 1 (A) and 2 (B) subjects attended on 2 days. On each day they initially performed six control sets of elbow flexor contractions. Each set consisted of an MVC followed by two submaximal contractions at 75% MVC and 50% MVC. On one day all sets were performed without inflation of a cuff around the upper arm. On the other day, three sets were performed with and three without the cuff (black horizontal bar represents the period with the cuff inflated). Subsequently, in Experiment 1 (A) subjects performed a 2-min sustained elbow flexor contraction and in Experiment 2 (B) subjects performed a 2-min sustained elbow extensor contraction. After the sustained MVC, subjects performed four elbow flexor contractions sets with and without a cuff on the different days. Each experiment concluded with further contraction sets with 10-s rests between sets for 4 min after the end of the sustained MVC and then 1 min apart until 14 min. TMS of the motor cortex was delivered during each brief contraction and during the 2-min sustained contraction. In Experiment 3 (C) subjects performed control contraction sets as in Experiments 1 and 2. Subjects then performed four contractions sets over 2 min with or without a cuff. Following a 15-min break, they repeated the four contraction sets with the complementary condition. Arrows indicate stimuli. MVC, maximal voluntary contraction; TMS, transcranial magnetic stimulation.
Subjects began the experiment with sets of control contractions. Each contraction set began with a brief (2–3 s) MVC followed by a motor point stimulus with the muscle at rest and then two brief submaximal (75% and 50% MVC) contractions with 5 s between contractions. Rests of 90 s in between sets minimised fatigue. TMS was delivered during each contraction. The target torques for the submaximal contractions were always derived from the MVC of that set. On the cuff day, the cuff was inflated for the second, fourth and sixth contraction sets. It was inflated ∼5 s before the start of each of these sets and was deflated immediately after the end of the set. Subjects then performed a 2-min sustained MVC of the elbow flexor muscles and TMS was delivered every 10 s. All subjects were given verbal encouragement and visual feedback to provide maximal effort. On the cuff day, the cuff was inflated 5 s before the end of the 2-min sustained contraction. The cuff remained inflated for 2 min while the subject performed four of the same contraction sets as described above but with only 10-s rests between sets. The condensed schedule was chosen to maximise the number of data points collected during the critical 2 min of ischaemia even though the multiple brief contractions in a short time was itself likely to delay recovery following the sustained MVC. After 2 min, the cuff was deflated and subjects performed a further four contraction sets over the next 2 min. Subjects then performed another eight contraction sets with 1-min rests in between sets. On the ‘no-cuff’ day the procedure was the same but the cuff was not inflated for any of the sets of control contractions or following the 2-min fatiguing contraction.
Experiment 2: 2-min maximal voluntary contraction of elbow extensors with or without maintained ischaemia
Procedures for Experiment 2 were the same as for Experiment 1, but instead of a 2-min sustained elbow flexor contraction subjects performed a 2-min sustained elbow extensor contraction. After the sustained extensor MVC, subjects performed multiple, brief contractions sets of the unfatigued elbow flexors. In the text, we refer to the elbow flexors during this period as unfatigued, though we recognise that the multiple contractions in this condensed schedule may themselves have caused slight fatigue. Again, subjects were randomly assigned to complete the ‘cuff’ or ‘no-cuff’ day first. Typical superimposed twitches are shown in Fig. 2.
Figure 2. Raw traces of superimposed twitches from Experiment 2 in a typical subject.
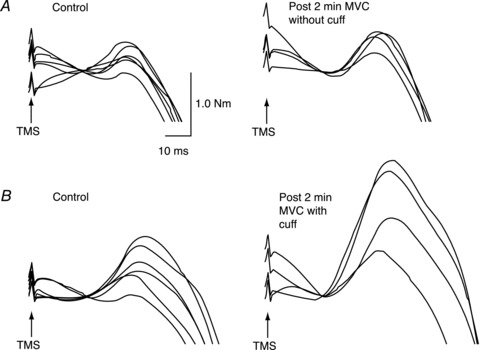
A, superimposed twitches of a single subject evoked during brief MVC of the elbow flexors before (left panel) and after (right panel) a sustained elbow extensor MVC. Traces are overlaid and baseline torque is offset for the illustration. B, superimposed twitches for the same subject recorded on a different day. Twitches in the left panel were recorded during brief control MVCs with (three) and without (three) the cuff inflated. The right panel shows twitches recorded during brief MVCs following the sustained elbow extensor MVC with maintained ischaemia. MVC, maximal voluntary contraction; TMS, transcranial magnetic stimulation.
Experiment 3: Brief maximal voluntary contractions with or without an ischaemic cuff
To examine the effect of 2 min of ischaemia on brief elbow flexion MVCs in the absence of a sustained fatiguing MVC, subjects returned on a fifth day. Subjects performed the same control procedures as in Experiments 1 and 2 and then performed six sets of control contractions, three with and three without an inflated cuff, as described previously. Subjects then performed two series of four contraction sets. Each series lasted 2 min with a 15-min rest between them. Before one series the cuff was inflated and the arm was held ischaemic through the 2-min series.
Data analysis and statistics
All measures were analysed off-line using Signal software (v. 4.06; CED). Mean torque and root mean square EMG were measured over the 100 ms before TMS for each MVC. The maximal amplitude of the superimposed twitches evoked by TMS were measured and normalised to peak MVC torque in the control sets. MEP peak-to-peak amplitude was measured between cursors set from the initial deflection from baseline to the second crossing of the horizontal axis and normalised to the amplitude of resting Mmax recorded during set-up. EMG responses for brachioradialis behaved similarly to those of biceps brachii and are not reported. Voluntary activation was calculated using the equation: voluntary activation (%) =[1 – (superimposed twitch/estimated resting twitch)]× 100, where the estimated resting twitch amplitude is the y-intercept of the linear regression between the size of the evoked twitch and the voluntary torque at MVC, 75% and 50% of that MVC (Todd et al. 2003, 2004). In Experiment 1, after the fatiguing contraction, the relationship between the evoked twitch and voluntary torque was often not linear (r < 0.90) so voluntary activation could not be quantified using this method. Hence, the size of the superimposed twitch was expressed as a percentage of MVC torque just before stimulation to reflect failure of voluntary activation (Gandevia et al. 1996).
Two-way repeated measures ANOVAs were used to examine the effect of ischaemia on voluntary activation, MVC torque and MEP amplitude during the 2 min following the sustained MVC in Experiments 1 and 2. One-way repeated measures ANOVAs were used to examine differences within a single session on the ‘cuff’ and ‘no-cuff’ days. Pairwise multiple comparisons were made using a Tukey's HSD test. Data are given in the text as means ±s.d. and shown in the figures as means ±s.e.m. Significance was set at P < 0.05.
Results
Experiment 1: 2-min maximal voluntary contraction of elbow flexors with or without maintained ischaemia
Subjects performed brief elbow flexion MVCs with and without maintained ischaemia of the arm after a 2-min MVC of the elbow flexors. Before the sustained contraction, MVC torque did not differ on the 2 days (‘cuff’, 58.9 ± 13.8 N m; ‘no-cuff’, 58.6 ± 14.0 N m; P= 0.72). Voluntary activation, measured using the method of Todd and colleagues (2003) (see Methods), did not differ on the 2 days (95.0 ± 6%, 95.2 ± 5.1%; P= 0.64), nor did the potentiated resting twitch and estimated resting twitch (see Table 1). During the 2-min MVC, elbow flexor torque fell a similar amount (67.5 ± 7.0% and 66.4 ± 5.4%; P= 0.11) on the ‘cuff’ and ‘no-cuff’ days, respectively (Fig. 3A). The superimposed twitch expressed as a percentage of the ongoing MVC during the sustained contraction increased from 1.1 ± 1.2% to 11.1 ± 7.0% on the ‘cuff’ day and from 1.4 ± 1.5% to 11.2 ± 8.4% on the ‘no-cuff’ day (P= 0.4).
Table 1.
Resting twitches of elbow flexors produced by motor point stimulation and estimated resting twitches calculated from superimposed twitches produced by cortical stimulation
| No cuff day Mean ± SD | Cuff day Mean ± SD | P | |
|---|---|---|---|
| Motor point resting twitch (% peak MVC) | |||
| Experiment 1 | |||
| Control | 9.3 ± 3.0% | 10.2 ± 3.1% | 0.2 |
| 2-min no ischaemia/ischaemia | 4.1 ± 1.3% | 0.8 ± 0.6% | <0.001 |
| Experiment 2 | |||
| Control | 9.7 ± 2.9% | 8.8 ± 2.6% | 0.06 |
| 2-min no ischaemia/ischaemia | 10.6 ± 3.5% | 9.3 ± 3.6% | 0.02 |
| Experiment 3 | |||
| Control | 10.1 ± 2.3% | 9.8 ± 2.6% | 0.31 |
| 2-min no ischaemia/ischaemia | 10.4 ± 2.5% | 9.7 ± 2.8% | 0.1 |
| Estimated resting twitch (% peak MVC) | |||
| Experiment 1 | |||
| Control | 16.5 ± 4.1% | 16.0 ± 2.9% | 0.71 |
| 2-min no ischaemia/ischaemia | † | † | † |
| Experiment 2 | |||
| Control | 16.6 ± 4.2% | 15.7 ± 3.4% | 0.27 |
| 2-min no ischaemia/ischaemia | 16.0 ± 5.1% | 12.7 ± 3.2% | 0.02 |
| Experiment 3 | |||
| Control | 15.7 ± 4.4% | 14.3 ± 3.4% | 0.09 |
| 2-min no ischaemia/ischaemia | 16.3 ± 4.9% | 13.2 ± 3.7% | 0.005 |
MVC, maximal voluntary contraction. † Data not available, see text.
Figure 3. Group data for elbow flexor torque and SIT for the study with a sustained flexor MVC (Experiment 1).
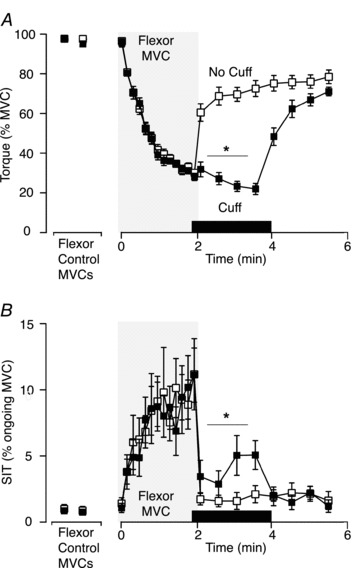
A, elbow flexor maximal voluntary torque normalised to the control MVC. Maximal voluntary torque was less when ischaemia was maintained after the 2-min MVC (filled squares; ‘cuff’ condition) on one day versus a separate day without ischaemia (open squares; P < 0.001). B, amplitude of the SIT as a percentage of ongoing MVC. SIT was larger when ischaemia was maintained after the 2-min flexor MVC (filled squares; ‘cuff’ condition) on one day compared with a separate day without ischaemia (open squares; P= 0.01). Asterisk indicates statistically significant difference between the four contractions performed with the cuff inflated and corresponding contractions in the ‘no-cuff’ condition. In this and subsequent figures, data are shown as mean ± SEM (n= 9). MVC, maximal voluntary contraction; SIT, superimposed twitch.
The first four sets of contractions after the end of the 2-min contraction were performed during ischaemic (‘cuff’ day) or non-ischaemic (‘no-cuff’ day) conditions. Two-way repeated measures ANOVA showed significant differences between maximal voluntary torque on the 2 days (F1,24 139.05, P < 0.001). Elbow flexor torque in the brief MVCs was lower during ischaemia (26.0 ± 4.4%versus 67.9 ± 5.2% of initial maximal torque; Fig. 3A) with a significant interaction between ‘cuff’ and ‘no-cuff’ days and time (F1,24 33.12, P < 0.001). During ischaemia, torque decreased for the last two MVCs compared to the initial MVC (P < 0.001). The opposite occurred in the absence of ischaemia, where the last two MVCs increased compared to the first MVC after the 2-min contraction (P < 0.001). Resting twitches were also decreased during ischaemia compared to the matching period on the ‘no-cuff’ day (Table 1).
In ∼80% of the first four contraction sets after the 2-min flexor MVC, the usual inverse relationship between the superimposed twitch and voluntary torque was no longer linear (r < 0.9). Therefore, estimated resting twitches could not be calculated reliably and voluntary activation could not be quantified as described by Todd and colleagues (2003). Consequently, to compare the failure of activation between the contraction sets with and without ischaemia, the superimposed twitch was expressed as a percentage of the ongoing MVC (e.g. Gandevia et al. 1996; Beltman et al. 2004; Martin et al. 2005). Before the 2-min MVC, the superimposed twitch averaged 0.81 ± 0.7% MVC and 0.86 ± 1.0% MVC on the ‘cuff’ and ‘no-cuff’ days, respectively. In the contraction sets at the end of the 2-min MVC, the superimposed twitch averaged 4.1 ± 1.1% MVC during ischaemia and 1.8 ± 0.2% MVC without ischaemia. Superimposed twitches were significantly greater during ischaemia than in its absence (F1,24 11.44, P= 0.01; Fig. 3B).
The amplitude of biceps brachii MEP increased during the 2-min sustained elbow flexor MVC on both the ‘cuff’ and ‘no-cuff’ days (see Table 2 for detailed MEP data). Immediately after the sustained MVC, the MEP amplitudes decreased towards baseline and there was no difference between biceps MEPs elicited in the four brief MVCs during ischaemia versus those with no ischaemia (P= 0.28). Triceps MEPs were also not different between the two conditions (P= 0.13).
Table 2.
Biceps and triceps brachii motor evoked potential (MEP) amplitude as % Mmax
| Biceps MEP | Triceps MEP | |||
|---|---|---|---|---|
| No cuff Mean ± SD | Cuff Mean ± SD | No cuff Mean ± SD | Cuff Mean ± SD | |
| Experiment 1 | ||||
| Control | 67.8 ± 23.6 | 60.7 ± 15.6 | 7.7 ± 4.7 | 6.4 ± 3.1 |
| Start 2-min MVC | 66.3 ± 25.2 | 55.5 ± 17.1 | 6.9 ± 4.2 | 5.8 ± 3.6 |
| 1st min of 2-min MVC | 97.6 ± 47.7 | 96.6 ± 43.4 | 9.6 ± 6.1 | 8.5 ± 5.7 |
| 2nd min of 2-min MVC | 95.4 ± 42.1 | 94.4 ± 39.3 | 9.5 ± 5.7 | 7.2 ± 5.1 |
| 2-min no ischaemia/ischaemia | 72.4 ± 37.1 | 63.7 ± 32.4 | 7.1 ± 4.5 | 6.1 ± 4.7 |
| Experiment 2 | ||||
| Control | 67.2 ± 20.5 | 65.4 ± 16.6 | 7.4 ± 4.4 | 6.1 ± 3.5 |
| Start 2-min MVC | 31.7 ± 31.4 | 23.5 ±16.7 | 37.4 ± 13.7 | 46.3 ± 13.4 |
| 1st min of 2-min MVC | 15.2 ± 13.1 | 17.1 ± 15.4 | 51.7 ± 27.0 | 51.4 ± 27.1 |
| 2nd min of 2-min MVC | 7.2 ± 4.7 | 8.7 ± 7.2 | 51.4 ± 30.3 | 47.9 ± 29.1 |
| 2-min no ischaemia/ischaemia | 70.0 ± 19.6 | 76.4 ± 30.2 | 6.7 ± 3.3 | 6.8 ± 5.1 |
| Experiment 3 | ||||
| Control | 52.6 ± 10.9 | 68.3 ± 23.6 | 5.8 ± 2.5 | 5.9 ± 3.3 |
| 2-min no ischaemia/ischaemia | 58.3 ± 19.1 | 64.8 ± 26.1 | 6.7 ± 2.3 | 6.7 ± 2.6 |
Experiment 2: 2-min maximal voluntary contraction of elbow extensors with or without maintained ischaemia
Subjects performed brief elbow flexion MVCs with and without maintained ischaemia of the arm after a 2-min MVC of the elbow extensors. Before the sustained contraction, elbow flexion torque (57.4 ± 12.8 N m, 57.8 ± 11.9 N m; P= 0.84) and voluntary activation (95.0 ± 3.6%, 94.1 ± 3.7%; P= 0.51) were not different on the 2 days. Resting twitches and estimated resting twitches also did not differ between days (Table 1). During the 2-min elbow extensor MVC, extensor torque decreased a similar amount (57.5 ± 4.3% and 51.2 ± 7.4%; P= 0.47) during the ‘cuff’ and ‘no-cuff’ days, respectively. After the sustained extensor contraction, flexor torque produced in the first four brief elbow flexor MVCs was significantly less with sustained ischaemia compared to no ischaemia (82.2 ± 4.9%versus 91.4 ± 2.3% of initial maximal torque; F1,24 13.05, P= 0.007, Fig. 4A). Both the resting twitch and estimated resting twitch were significantly smaller during ischaemia than in the matching period without ischaemia (9.3 ± 3.6% of peak MVC versus 10.6 ± 3.5% of peak MVC; P= 0.02 and 12.7 ± 3.2% of peak MVC versus 16.0 ± 5.1%; P= 0.02, respectively) (see Table 1).
Figure 4. Group data for elbow flexor torque, SIT and voluntary activation for the study with a sustained extensor MVC (Experiment 2).
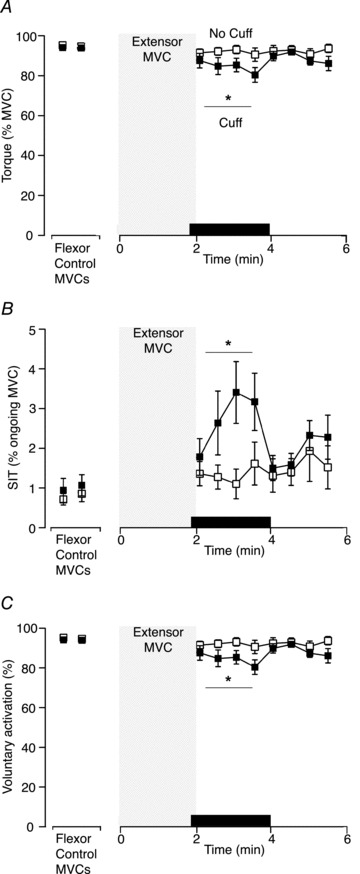
A, elbow flexor maximal voluntary torque normalised to the control MVC following 2-min MVC of elbow extensors. Maximal voluntary torque was less when ischaemia was maintained after the 2-min MVC (filled squares; ‘cuff’ condition) on one day versus a separate day without ischaemia (open squares; P= 0.007). B, amplitude of the SIT as a percentage of ongoing MVC. The SIT was larger when ischaemia was maintained after the 2-min extensor MVC (filled squares; ‘cuff’ condition) on one day compared with a separate day without ischaemia (open squares; P= 0.02). C, voluntary activation for elbow flexors following a 2-min MVC of elbow extensors. Voluntary activation was lower with 2 min of sustained ischaemia (filled squares; ‘cuff’ condition), compared to no ischaemia, (open squares; P < 0.05). Asterisk indicates statistically significant difference between the four contractions in the ‘cuff’ and ‘no-cuff’ condition. MVC, maximal voluntary contraction; SIT, superimposed twitch.
To allow comparison with Experiment 1, the superimposed twitch of the elbow flexors was also expressed as a percentage of the ongoing MVC. Before the 2-min MVC, the superimposed twitch averaged 1.0 ± 0.1% MVC and 0.8 ± 0.1% MVC on the ‘cuff’ and ‘no-cuff’ days, respectively. During the elbow flexor contraction sets after the 2-min extensor MVC, superimposed twitches of the elbow flexors were greater during maintained ischaemia than in its absence (2.8 ± 0.7% MVC versus 1.3 ± 0.2% MVC; F1,24 7.461, P= 0.03; Fig. 2 and 4B).
In the 2 min after the sustained extensor MVC, estimated resting twitches for the elbow flexors could be calculated reliably (r≥ 0.9) and were used to calculate voluntary activation. Mean voluntary activation of elbow flexors was lower with (81.6 ± 8.2%) the cuff inflated than without (95.5 ± 6.9%) the cuff inflated (F1,24 5.72, P= 0.04; Fig. 4C). A separate one-way repeated measures ANOVA for the ‘cuff’ day showed decreases in voluntary activation in the second, third and fourth of the four brief flexor MVCs performed during ongoing ischaemia when compared to control (F1,32 9.23, P < 0.001). On the ‘no cuff’ day, mean voluntary activation of the elbow flexors following the sustained extensor MVC was not different from the control value (F1,32 0.88, P= 0.49).
The amplitude of biceps brachii MEPs decreased during the 2-min sustained elbow extensor MVC on both days, while the amplitude of triceps brachii MEPs increased (Table 2). All MEPs returned to baseline during brief elbow flexor MVCs at the termination of the extensor MVC. There were no differences between the biceps brachii MEP amplitudes in brief MVCs during sustained ischaemia versus no ischaemia after the 2-min elbow extensor MVC (P= 0.22), nor did the triceps brachii MEPs differ (P= 0.91) (Table 2).
Experiment 3: Brief maximal voluntary contractions with or without an ischaemic cuff
There were no differences in torque (‘cuff’ 54.1 ± 12.7 N m, ‘no-cuff’, 57.6 ± 14.0 N m; P= 0.14), voluntary activation (‘cuff’ 93.0 ± 5.0%, ‘no-cuff’ 93.9 ± 4.1%; P= 0.97) or the size of the superimposed twitch expressed as a percentage of ongoing MVC torque (‘cuff’, 0.87 ± 0.93%; ‘no-cuff’, 0.94 ± 0.89%; P= 0.63) between brief MVCs of elbow flexors during the control period with the cuff briefly inflated or not inflated. In addition, there were no differences in the resting twitches or estimated resting twitches in the two conditions (Table 1).
The mean torque was lower with the cuff inflated (87.7 ± 8.0% of initial maximal torque) than without the cuff inflated (93.0 ± 3.9%) for the four MVCs during the 2 min of maintained ischaemia (F1,21 11.57, P= 0.01; Fig. 5A) and a significant interaction showed that torque decreased more over time with ischaemia compared to no ischaemia (F1,21 6.88, P= 0.002). In contrast, there were no significant changes in voluntary activation over the 2 min and no differences during sustained ischaemia versus no ischaemia (F1,21 0.03, P= 0.87; Fig. 5B). Likewise, there were no differences in the size of the superimposed twitch expressed as a percentage of ongoing MVC (F1,21 0.05, P= 0.83). The potentiated resting twitches were not different during the period of ischaemia and the matching non-ischaemic period. However, the estimated resting twitches were smaller during maintained ischaemia (see Table 1). Additionally, there were no differences in biceps or triceps MEP amplitude between the ‘cuff’ and ‘no-cuff’ days (F1,24 1.16, P= 0.31; F1,24 0.01, P= 0.91, respectively, Table 2).
Figure 5. Group data for torque and voluntary activation for the study without a sustained MVC (Experiment 3).
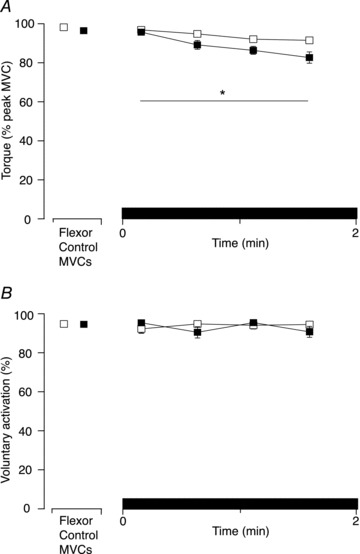
A, maximal voluntary torque (normalised to the control MVC) for brief MVCs of elbow flexors during 2-min of either sustained ischaemia (closed squares; ‘cuff’ condition) or no ischaemia (open squares) performed on the same day. Maximal voluntary torque was less when ischaemia was maintained under the cuff compared to no ischaemia (P < 0.01). B, voluntary activation for brief MVCs of elbow flexors during 2 min of sustained ischaemia (closed squares) or no ischaemia (open squares). There were no differences in voluntary activation during sustained ischaemia under the cuff compared to no ischaemia (P= 0.87). Asterisk indicates statistically significant difference between the four contractions in the ‘cuff’ and ‘no-cuff’ condition. MVC, maximal voluntary contraction.
Discussion
The novel finding of this study is that feedback from small-diameter afferents from fatigued elbow extensor muscles contributes to a decrease in voluntary activation and torque of the elbow flexors when these muscles have not been engaged in the fatiguing sustained contraction. Additionally, this study confirms that maintained firing of small-diameter muscle afferents of fatigued elbow flexor muscles contributes to the decrease in voluntary activation and torque of those muscles. These results suggest that central fatigue is in part mediated by small-diameter muscle afferents that are sensitive to muscle metabolites generated during fatiguing contractions and that this effect is not confined to the fatigued muscle.
During a maximal effort, the delivery of TMS over the motor cortex commonly evokes additional force from the contracting muscle. A decrease in voluntary drive to the muscle is suggested when this increment (superimposed twitch) increases (Gandevia et al. 1996). Furthermore, because the superimposed twitch is evoked by stimulation of the motor cortex, an increase suggests that output from the motor cortex has become less than optimal to produce maximal force with the muscle. In the present study the superimposed twitch evoked by TMS increased for both fatigued and unfatigued elbow flexors when ongoing ischaemia maintained the firing of small-diameter muscle afferents from a fatigued muscle. The change in superimposed twitch for unfatigued muscles suggests that the firing of fatigue-sensitive muscle afferents does not just act to impair activation of the homonymous fatigued muscle, but has broader effects. As the superimposed twitches were evoked by cortical stimulation, the likely site of action of the afferents is at or above motor cortical output neurones.
Gandevia and colleagues (1996) demonstrated that after a 2-min sustained maximal elbow flexion contraction, voluntary activation of the elbow flexor muscles remained low if the muscle was held ischaemic by inflation of a cuff around the upper arm. The study proposed that continued firing of fatigue-sensitive small-diameter muscle afferents contributed to supraspinal fatigue. Increases in the superimposed twitch to ∼15% of MVC were reported in brief MVCs during the maintained ischaemia. In contrast, changes in the size of the superimposed twitch in the current study were more modest. Although individual superimposed twitches ranged up to ∼15%, the average size was 4% of ongoing MVC. It is likely that the smaller change in the current study is more accurate given the relatively small sample size (n= 4) in the previous study. Other methodological differences include use of a narrow cuff that was placed more proximally on the arm compared to a standard sphygmomanometer cuff used in the previous study. The standard cuff was nearly twice as wide and likely resulted in greater compression of the biceps brachii, and particularly of the underlying brachialis muscle, which originates from the lower two-thirds of the humerus. Lower stimulus intensities for TMS were used here based on the methodological requirement to minimize the size of extensor MEPs while still strongly activating elbow flexors (Todd et al. 2003). The lower intensity of TMS reduces any stimulus-evoked extensor torque, which would counter the elbow flexor twitch torque, and thus gives a more accurate measure of voluntary activation. Nevertheless, despite a less pronounced effect, findings of the current study support a contribution of small-diameter muscle afferents to supraspinal fatigue.
After the fatiguing contraction of the elbow extensors, the superimposed twitches in MVCs of the non-fatigued elbow flexors were approximately double the size when the arm was held ischaemic compared to when the extensor muscles were allowed to recover. This is the first experiment to show attenuation of voluntary activation and torque of non-fatigued muscles due to feedback from afferents of fatigued antagonist muscles. This decrease of voluntary activation in unfatigued elbow flexors was again demonstrated through an increase in the cortically evoked superimposed twitch, which suggests that motor cortical output is insufficient to drive the motor units fully. In addition, previous investigations have shown that cervicomedullary motor evoked potentials (CMEPs) recorded from the elbow flexors increase with input from the fatigue-sensitive muscle afferents of the extensors (Martin et al. 2006). Therefore, although other motoneurone pools may be inhibited, there is a facilitatory rather than direct inhibitory input to the elbow flexor motoneurone pool from these afferents (Butler et al. 2003; Martin et al. 2006). Given that the motoneurone pool is facilitated, the current finding of inadequate drive from the motor cortex is likely to reflect a decrease in motor cortical output rather than a requirement of the motoneurones for more input. Indeed, the reflex spinal facilitatory input from the extensors suggests that the decline in motor cortical drive to the elbow flexors following a fatiguing antagonist contraction may be underestimated by our measure of voluntary activation.
Quantification of voluntary activation of the fatigued elbow flexors was not possible using the estimated resting twitch methodology originally described by Todd and colleagues (2003, 2005), as the linear regression used to estimate the resting twitch was not reliable after the fatiguing contraction. This problem only occurred in the contraction sets after the 2-min flexion MVC; the majority of regressions from all other contraction sets were reliable. Further examination of the data revealed no clear relationship between superimposed twitches and voluntary torque that could explain the failure of linearity. Changes in the size of the superimposed twitches during MVCs were consistent with previous studies (Taylor et al. 1996; Butler et al. 2003; Martin et al. 2008). Furthermore, the sizes of MEPs in biceps and triceps brachii did not differ from those in the control contraction sets, which argues against a central mechanism for the non-linearity.
Although motor cortical output was inadequate to drive the muscle fully during the maintained firing of fatigue-sensitive muscle afferents, we found no decreases in EMG responses to motor cortical stimulation. The time course for changes in the size of biceps brachii MEPs suggested that when tested during maximal efforts corticospinal excitability returned to baseline within 10 s after the fatiguing contraction regardless of continued ischaemia or whether the fatigued muscles were flexors or extensors. The MEPs in the antagonist triceps brachii also returned quickly to baseline during the flexor MVCs after both fatiguing contractions. The influence of changes in the compound muscle fibre action potentials through repetitive activity or ischaemia were not accounted for in the current study. However, our findings are consistent with previous research, which did account for changes in the maximal M wave and demonstrated an initial increase in the size of the MEP (greater motor cortex excitability) during the fatiguing flexor MVC with a plateau at about 1 min, and within 30 s of termination of the contraction, a return to the control level with or without occlusion of blood flow (Taylor et al. 1996, 2000; Butler et al. 2003; Martin et al. 2008). The current data provide no evidence that small-diameter muscle afferents from either elbow flexors or extensors directly inhibit motor cortical output circuits, which generate MEPs, even though voluntary motor cortical output to the elbow flexors has almost certainly decreased. A caveat on this interpretation is that MEPs are influenced by both motor cortex and motoneurone excitability (as well as by muscle fibre membrane), so that decreased motor cortical output in response to stimulation could be masked by increased excitability of the alpha motoneurone pool. Such increases have been demonstrated previously for the elbow flexor motoneurones although not for the elbow extensor motoneurones (Martin et al. 2006).
Understanding the role of small-diameter muscle afferents in central fatigue is complicated by the difference in both motoneurone and motor cortical responses during ongoing sustained contractions compared to the brief contractions tested during ischaemia in the current protocol. Sustained activation appears to produce a profound depression of the responsiveness of the motoneurones that have been active. This depression can be largely counteracted by voluntary drive (McNeil et al. 2009, 2011a,b). In contrast to the motoneurones, motor cortical excitability is increased. Thus, during a sustained elbow flexor MVC, MEPs become larger, while subcortically evoked CMEPs become smaller, despite any facilitatory effect of small-diameter muscle afferents (Taylor et al. 1996; Butler et al. 2003). These changes recover quickly with periods of rest. The high reliance of the responsiveness of the motoneurones during sustained contraction on voluntary drive could amplify the consequences of impairment of drive by the supraspinal effects of small-diameter muscle afferents. In the current study, superimposed twitches were considerably larger near the end of the sustained 2-min flexor MVC than in the subsequent brief MVCs during ischaemia. The greater central fatigue in the sustained effort could reflect the combined effects of repetitive activation of the motoneurones and firing of small-diameter afferents.
It is possible that changes in the superimposed twitch and maximal voluntary torque might occur simply through the use of a blood pressure cuff to maintain 2 min of muscle ischaemia while performing brief contractions rather than through the firing of fatigue-sensitive muscle afferents. However, in the control MVCs, maximal torque and voluntary activation were unaffected by inflation of the cuff. Thus, there were no immediate effects on force production of compression of the proximal part of biceps. Moreover, from Experiment 3, ischaemia lasting for 2 min resulted in no change in voluntary activation or in the biceps brachii MEP, although there was a small but significant decrease in torque. Thus, in the absence of a previous fatiguing contraction, maintained ischaemia for 2 min did not affect central mechanisms of voluntary drive, despite some fatigue produced by the multiple, brief voluntary contractions of the testing protocol. It is most likely that the maintained ischaemia exacerbated impairment of peripheral torque-generating capacity.
In the present study, a sustained fatiguing contraction and subsequent maintenance of the fatigued state decreased both voluntary drive and torque production of the elbow flexors. By contrast, activation of small-diameter muscle afferents by hypertonic saline to induce muscle pain had no significant effect on voluntary activation of these muscles (Khan et al. 2011). For the knee extensor muscles, experimental muscle pain has also been shown to attenuate voluntary force without a clear change in electrically evoked superimposed twitch force and hence, voluntary activation (Graven-Nielsen et al. 2002). One potential reason that firing of small-diameter afferents induced by fatigue might affect voluntary activation differently than that induced by hypertonic saline is that the neurones in the motor pathway may be in different states in the two conditions. However, our study argues against this proposal as voluntary activation of the elbow flexors was reduced by extensor afferent firing with minimal fatigue of the flexors, so that the history of activity of the neurones in the motor pathway would be similar to that in studies that use hypertonic saline. Rather, it may be that there are different spatial and temporal firing of subsets of small-diameter afferents (Graven-Nielsen et al. 2003), or the processing of sensory input is handled differently at supraspinal sites. Animal studies have identified a population of fatigue-sensitive small-diameter muscle afferents activated more during ischaemic contractions than non-ischaemic contractions indicating the presence of subsets of these afferents that respond to different stimuli (Kaufman et al. 1984). Small-diameter muscle afferents may also respond differently to the type and concentration of stimuli. Hypertonic saline may strongly stimulate a small number of group III and IV afferents in the region of injection although its precise mechanism is unknown (Mense, 2009). This could result in the perception of muscle pain but fail to inhibit voluntary drive. In contrast, maximal fatiguing exercise probably activates a greater number of afferents across the whole muscle that respond to both high and low concentrations of metabolites (see Light et al. 2008), and hence may result in decreased voluntary drive.
Comparison of our findings from brief isometric contractions during maintained ischaemia following a strong sustained fatiguing contraction to ongoing rhythmic exercise is difficult. One problem is that it is unknown whether firing of small-diameter afferents is similar during the two conditions. Light and colleagues (2008) propose that there are two sets of chemosensitive muscle afferents, high metabolite-responsive afferents that respond to high levels of metabolites (e.g. ATP) and low pH (7.0–6.6) associated with painful, ischaemic contractions and low metabolite-responsive afferents that respond to the lower levels of metabolites and resting or slightly decreased pH (7.34–7.0) associated more with non-painful, heavy muscle work. In our protocol, it is likely that levels of metabolites are high and pH is low. During the 2-min MVC, the contracting muscle will be ischaemic because of high intramuscular pressure, and during the subsequent period of ischaemia with the cuff inflated, metabolites may have increased further because subjects continued to perform strong, brief intermittent contractions. By comparison, intermittent MVCs performed in ischaemic conditions for 2 min led to the low muscle pH of ∼6.6 (Lanza et al. 2006). This level of acidosis is also possible without experimentally induced ischaemia during high intensity rhythmic exercise of the forearm (pH 6.04; Forbes et al. 2005), or incremental cycling exercise to exhaustion lasting ∼15 min (pH 6.04; Hug et al. 2006). However, moderate intensity rhythmic forearm exercise showed no decreases in muscle pH (7.05; Marsh et al. 1993), nor did longer duration cycling exercise (pH 7.03; Stephens et al. 2002). Thus, our protocol may induce more firing of nociceptive afferents and less firing of other chemosensitive afferents than normally occurs during moderate intensity rhythmic exercise or endurance cycling. However, consideration of only chemically mediated responses of the afferents does not take into account any influence of the mechanical stimulus from contraction, or sensitisation of mechanosensitive afferents by metabolites and ischaemia (e.g. Kaufman et al. 1984; Rotto & Kaufman, 1988; Hayward et al. 1991; Adreani et al. 1997). Empirically, the blocking of small-diameter afferents by injection of lumbar intrathecal fentanyl leads to early increases in EMG and power output in cycling (Amann et al. 2009), which suggests that these afferents can alter submaximal voluntary drive during whole body exercise, as well as impairing maximal voluntary activation as shown here.
In conclusion, after a fatiguing elbow flexor contraction, voluntary drive to the fatigued flexor muscles is reduced with continued activation of small-diameter muscle afferents. Furthermore, fatigue of the antagonist elbow extensor muscles also results in decreased voluntary drive to the elbow flexors and is associated with decreased torque generation by these non-fatigued muscles. Thus, firing of small-diameter muscle afferents from one muscle can affect the exercise performance of another unfatigued muscle in the same limb. The afferents are likely to act at a cortical level but may not act directly on the motor cortical output cells. These results have broader implications for lower limb and whole body exercise in which afferents from fatiguing muscles could limit the central drive to other muscles, thus impairing performance.
Glossary
- CMEP
cervicomedullary motor evoked potential
- EMG
electromyographic activity
- MEP
motor evoked potential
- Mmax
maximal compound muscle action potential
- MVC
maximal voluntary contraction
- SIT
superimposed twitch
- TMS
transcranial magnetic stimulation
Additional information
Competing interests
None.
Author contributions
All authors contributed to all aspects of the study and all approved the final version of the manuscript. All experiments were performed at Neuroscience Research Australia in Sydney, Australia.
Funding
This work was supported by the National Health and Medical Research Council of Australia.
References
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol. 2011;589:5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltman JGM, Sargeant AJ, Van Mechelen W, De Haan A. Voluntary activation level and muscle fibre recruitment of human quadriceps during lengthening contractions. J Appl Physiol. 2004;97:619–626. doi: 10.1152/japplphysiol.01202.2003. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OCJ. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23:10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed RS, Denny-Brown D, Eccles JC, Liddell EGT, Sherrinton CS. Reflex Activity of the Spinal Cord. 1st edn. London: Oxford University Press; 1932. The flexor reflex; pp. 17–46. [Google Scholar]
- Forbes SC, Raymer GH, Kowalchuk JM, Marsh GD. NaHCO3-induced alkalosis reduces the phosphocreatine slow component during heavy-intensity forearm exercise. J Appl Physiol. 2005;99:1668–1675. doi: 10.1152/japplphysiol.01200.2004. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: Evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SJ, Kaufman MP. Role of muscle afferents in the inhibition of motoneurons during fatigue. Adv Exp Med Biol. 1995;384:271–278. doi: 10.1007/978-1-4899-1016-5_21. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T, Jansson Y, Segerdahl M, Kristensen JD, Mense S, Arendt-Nielsen L, Sollevi A. Experimental pain by ischaemic contractions compared with pain by intramuscular infusions of adenosine and hypertonic saline. Eur J Pain. 2003;7:93–102. doi: 10.1016/s1090-3801(02)00069-1. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T, Lund H, Arendt-Nielsen L, Danneskiold-Samsøe B, Bliddal H. Inhibition of maximal voluntary contraction force by experimental muscle pain: A centrally mediated mechanism. Muscle Nerve. 2002;26:708–712. doi: 10.1002/mus.10225. [DOI] [PubMed] [Google Scholar]
- Hayward L, Wesselmann U, Rymer WZ. Effects of muscle fatigue on mechanically sensitive afferents of slow conduction velocity in the cat triceps surae. J Neurophysiol. 1991;65:360–370. doi: 10.1152/jn.1991.65.2.360. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Bandler R, Gandevia SC, Macefield VG. Distinct forebrain activity patterns during deep versus superficial pain. Pain. 2006;120:286–296. doi: 10.1016/j.pain.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Hug F, Grélot L, Le Fur Y, Cozzone PJ, Bendahan D. Recovery kinetics throughout successive bouts of various exercises in elite cyclists. Med Sci Sports Exerc. 2006;38:2151–2158. doi: 10.1249/01.mss.0000235882.86734.9a. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Delliaux S, Watanabe K, Fujii N, Nishiyasu T. Evaluation of muscle metaboreflex function through graded reduction in forearm blood flow during rhythmic handgrip exercise in humans. Am J Physiol Heart Circ Physiol. 2011;301:H609–H616. doi: 10.1152/ajpheart.00076.2011. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol. 1984;57:644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- Kellgren JH. Observation on referred pain arising from muscle. Clin Sci. 1938;3:175–190. [Google Scholar]
- Khan SI, McNeil CJ, Gandevia SC, Taylor JL. Effect of experimental muscle pain on maximal voluntary activation of human biceps brachii muscle. J Appl Physiol. 2011;111:743–750. doi: 10.1152/japplphysiol.00603.2011. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978;31:511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Schomburg ED, Steffens H. Synaptic effects from chemically activated fine muscle afferents upon alpha-motoneurones in decerebrate and spinal cats. Brain Res. 1981;206:361–370. doi: 10.1016/0006-8993(81)90537-0. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol. 2006;577:353–367. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen RJ, Graven-Nielsen T, Jensen TS, Arendt-Nielsen L. The effect of differential and complete nerve block on experimental muscle pain in humans. Muscle Nerve. 1999;22:1564–1570. doi: 10.1002/(sici)1097-4598(199911)22:11<1564::aid-mus12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lewis T, Pickering GW, Rothschild P. Observations on muscular pain in intermittent claudication. Heart. 1931;15:359–383. [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh GD, Paterson DH, Potwarka JJ, Thompson RT. Transient changes in muscle high-energy phosphates during moderate exercise. J Appl Physiol. 1993;75:648–656. doi: 10.1152/jappl.1993.75.2.648. [DOI] [PubMed] [Google Scholar]
- Martin PG, Marino FE, Rattey J, Kay D, Cannon J. Reduced voluntary activation of human skeletal muscle during shortening and lengthening contractions in whole body hyperthermia. Exp Physiol. 2005;90:225–236. doi: 10.1113/expphysiol.2004.028977. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006;26:4796–4802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2008;586:1277–1289. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC, Taylor JL. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol. 2009;587:5601–5612. doi: 10.1113/jphysiol.2009.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol. 2011a;589:3533–3544. doi: 10.1113/jphysiol.2011.207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Khan SI, Gandevia SC, Taylor JL. The reduction in human motoneurone responsiveness during muscle fatigue is not prevented by increased muscle spindle discharge. J Physiol. 2011b;589:3731–3738. doi: 10.1113/jphysiol.2011.210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Algesic agents exciting muscle nociceptors. Exp Brain Res. 2009;196:89–100. doi: 10.1007/s00221-008-1674-4. [DOI] [PubMed] [Google Scholar]
- Mills KR, Newham DJ, Edwards RHT. Force, contraction frequency and energy metabolism as determinants of ischaemic muscle pain. Pain. 1982;14:149–154. doi: 10.1016/0304-3959(82)90095-1. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Functional analysis of Group III afferent fibres of mammalian muscles. J Physiol. 1960;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H. Only minor spinal motor reflex effects from feline group IV muscle nociceptors. Neurosci Res. 2002;44:213–223. doi: 10.1016/s0168-0102(02)00127-x. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H, Kniffki KD. Contribution of group III and IV muscle afferents to multisensorial spinal motor control in cats. Neurosci Res. 1999;33:195–206. doi: 10.1016/s0168-0102(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H, Dibaj P, Sears TA. Major contribution of Aδ and C fibres to increased reflex transmission in the feline spinal cord during acute muscle inflammation. Neurosci Res. 2012;72:155–162. doi: 10.1016/j.neures.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Stephens TJ, McKenna MJ, Canny BJ, Snow RJ, McConell GK. Effect of sodium bicarbonate on muscle metabolism during intense endurance cycling. Med Sci Sports Exerc. 2002;34:614–621. doi: 10.1097/00005768-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol. 2008;104:542–550. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490:519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Gandevia SC. Changes in muscle afferents, motoneurons and motor drive during muscle fatigue. Eur J Appl Physiol. 2000;83:106–115. doi: 10.1007/s004210000269. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol. 2003;551:661–671. doi: 10.1113/jphysiol.2003.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. J Appl Physiol. 2004;97:236–242. doi: 10.1152/japplphysiol.01336.2003. [DOI] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: A failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles CM, Edwards RHT. The effect of temperature, ischaemia and contractile activity on the relaxation rate of human muscle. Clin Physiol. 1982;2:485–497. doi: 10.1111/j.1475-097x.1982.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58:125–137. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]


