Abstract
Objective
Osteoporosis is increasingly common worldwide and there is growing concern that the long-term use of antipsychotic medications increases the risk of this disorder. In this review we consider whether antipsychotics may contribute to the development of osteoporosis through reductions in bone mineral density (BMD), discuss the possible mechanisms involved and consider the clinical implications of such a relationship.
Methods
We searched the literature for studies in this area published between 1966 and 2010 using the Medline and PubMed databases and the following search terms: (schizophrenia OR antipsychotic OR neuroleptic) AND (osteoporosis OR hyperprolactinaemia OR bone mineral density).
Results
The available data indicates that statistically significant reductions in bone mineral density are frequently seen in patients prescribed antipsychotic medications and suggests that there is a higher incidence of clinically significant reductions compared with the normal population.
Conclusions
Clinicians should be aware for the potential negative effects of antipsychotic medications on bone mineral density, particularly in patients with additional risk factors for osteoporosis. Recommendations regarding routine monitoring of bone mineral density for patients prescribed antipsychotic medications cannot be made on the basis of existing evidence and more research is required. Is antipsychotic treatment linked to low bone mineral density and osteoporosis? A review of the evidence and the clinical implications
Keywords: Hyperprolactinaemia, Antipsychotic, Osteoporosis, Schizophrenia, Neuroleptic, Bone mineral density (BMD)
INTRODUCTION
Reduced bone mineral density and osteoporosis
Osteoporosis is a progressive skeletal disease caused by reductions in bone mineral density (BMD), leading to bone fragility, deformity and fracture (Genant et al.,1999). Globally, this condition is increasingly being recognised as a major public health concern. Estimates vary, but it is thought that up to 3 million people in the United Kingdom suffer from osteoporosis (Van Staa et al., 2001). One person suffers a fracture due to osteoporosis every 3 minutes- amounting to around 230,000 fractures per year in the UK (Dennison et al., 2005). The costs are large; osteoporosis is thought to cost the NHS around £1.7 billion annually and hip fractures alone account for more than 20% of orthopaedic bed occupancy in the UK (WHO, 2007).
Many potential factors contribute to reduced BMD and the risk of osteoporosis. Those recognised by the World Health Organisation (WHO) include smoking, low body mass index (BMI), lack of exercise, alcohol consumption, glucocorticoid use, family history and prior fracture (Burge, 2001). The WHO does not currently list antipsychotic medications as a risk factor for osteoporosis. However, it has been suggested that antipsychotic drugs could cause osteoporosis by their effects on the hypothalamic-pituitary-gonadal axis (Howes and Smith, 2002).
Antipsychotics and prolactin
All currently licensed antipsychotic drugs block dopamine receptors. Given that prolactin release by pituitary lactotrophs is under the inhibitory control of dopamine (Freeman et al., 2000), it follows that by blocking dopamine receptors, antipsychotic drugs will reduced the inhibition of prolactin release, thus leading to increased prolactin secretion. Substantial evidence consistently demonstrates a relationship between the use of antipsychotic medications and raised prolactin levels (hyperproalctinemia) (Haddad and Wieck, 2004).
Studies in patients with hyperprolactinaemia secondary to prolactinomas have found high rates of osteopenia and osteoporosis (Vartej et al., 2001; Colao et al., 2000; Kayath et al., 1993). Prolactin regulates the secretion of gonadotrophins, and hence influences the levels of the gonadal hormones oestradiol and testosterone. Hyperprolactinaemia is linked to reduced oestradiol and testosterone levels. Based on this link, and considering evidence that suggests low sex hormone levels are associated with lower BMD (Seeman, 2004) and reduced osteoclast activity (Kaufmann, 2006), it has been proposed that elevated prolactin levels affect bone metabolism by suppressing sex hormone levels (Klibanski et al., 1988). Not all studies have found a link between hyperprolactinemia and hypogonadism, leading to others suggesting that hyperprolactinaemia may affect bone mineral density through alternative mechanisms (Schlechte et al., 1983). Supporting this, there is evidence to suggest that prolactin may have direct effects on bone metabolism (Goffin et al., 2002). Thus, there are 2 potential mechanisms by which hyperprolactinaemia may result in bone mineral density reductions - a direct one in the absence of hypogonadism, and an indirect one mediated though suppression of gonadal hormone levels.
In populations taking antipsychotic medication, rates of prolactin elevation and low gonadal hormone levels have been shown to be high, with rates of hyperprolactinaemia ranging from between 34-75% and hypogonadism between 6-92% (Smith at al., 2002; Howes et al., 2007). It follows from this that people taking antipsychotic drugs are likely to be at increased risk of osteoporosis. Recent animal studies in macaques and rats have found that antipsychotic treatment was associated with reduced bone mineralisation (Sackett et al., 2010; Kunimatsu et al., 2010). In the rat study the significant reductions in femoral BMD were linked to the effects of prolactin elevation and decreased oestradiol levels during antipsychotic treatment (Kunimatsu et al., 2010).
Given the ageing psychiatric population, the effects of the long-term use of antipsychotic medication on BMD are likely to require greater attention. Clinically, the problem may not be immediately apparent to the general psychiatrist, given that patients with fractures and bone deformity are more likely to present to physicians and surgeons rather than psychiatrists. There is, however, evidence that fracture rates are higher in patients taking antipsychotic drugs (Howard et al., 2007). If there is a link between antipsychotic drugs and low BMD, monitoring of BMD through the use of bone scans is cheap and readily available, safe pharmacological treatments exist for osteoporosis and preventive interventions are available, including the use of relatively prolactin-sparing antipsychotic medications and education about lifestyle factors (Haddad and Wieck, 2004).
We conducted a review of published studies in this area, to firstly assess the evidence that antipsychotic treatment is associated with reduced BMD and osteoporosis, and secondly to determine whether the findings are clinically significant. Additionally, we consider the possible mechanisms involved (in particular the role of prolactin-raising medications and lifestyle factors), the clinical implications, and the limitations of existing evidence.
METHOD
Search strategy
Papers were identified through an electronic search using the Medline and PubMed databases. This was supplemented by the hand searching of the reference lists of retrieved reports.
We used the following keywords: (schizophrenia OR antipsychotic OR neuroleptic) AND (osteoporosis OR hyperprolactinaemia OR bone mineral density). We included all studies published in English from 1966 to 2010.
Selection criteria
Studies were only included if they met the following criteria: (1) BMD was measured by DEXA scan (Dual-energy X-ray absorptiometry) at one or more of the anatomical sites studied, (2) BMD data was reported either as a Z-score (standard deviations from reference data for subjects of similar age, ethnicity and gender) or a T-score (standard deviations from reference data for peak bone mass for gender), (3) The study included subjects who were prescribed antipsychotic medications, regardless of diagnosis.
Recorded variables
For each study (Table 1.), basic information was recorded on the year of publication, the study design (including a description of the groups studied and antipsychotics prescribed), the site where BMD was measured and a description of measures of BMD additional to DEXA scanning, sample size, age, gender, diagnoses and menopausal status in females.
Table 1. Summary in chronological order of the methods of identified studies investigating the relationship between antipsychotic use and BMD.
| Study | Study design | Sample size |
Diagnosis | Mean age (sd) | Menopausal status in females |
Site where DEXA BMD measured |
|---|---|---|---|---|---|---|
| Halbreich et al., 1995 | Cross-sectional study | 68 (35 m, 33 f) |
Scz n=33 Dpnn=21 Sczaf n=7 Man n=2 Adj n=5 |
Total: 39.4 (sd 11.8) m: 35.5 (sd 9.7) f: 43.6 (sd 12.6) |
Not specified | L2-L4, L2 & right femoral neck |
| Comparison between normative BMD data and BMD in patient group prescribed unspecified mixed psychotropic medications (antipsychotics, antidepressants and lithium) at time of admission to hospital |
||||||
| Akande et al., 2002 | Cross-sectional study | 9 f | Not specified |
38.6 (sd 7.6) | All premenopausal (all age <48 years) |
L1-L4, femur & left hip |
| Comparison between normative BMD data and BMD in patient group prescribed unspecified antipsychotics, displaying hyperprolactinaemia and amenhorrhoea/oligomennorhoea for at least 12 months |
Note: Additional BMD measure: radial trabecular & cortical BMD using pQCT |
|||||
| Becker et al., 2003 | Cross-sectional study | 26 f | Scz n=26 | Mean not specified |
All premenopausal |
L1-L4 & proximal hip |
| Comparison between normative BMD data and BMD in two patient groups prescribed either risperidone (prolactin-raising) or olanzapine (relatively prolactin- sparing) for at least 24 months |
Range 15-55 | Note: Additional BMD measure: right radius, 3rd finger phalanx & midshaft tibia using QUS |
||||
| Meaney et al., 2004 | Cross-sectional study | 55 (30 m, 25 f) |
Scz n=55 | m: 43.5 (sd 11.4) f: 59 (sd 5.5) |
All postmenopausal |
L1-L4, femoral neck, trochanteric & intertrochanteric regions of the left hip |
| Comparison between normative BMD data and BMD in patient group prescribed various prolactin- raising antipsychotics for over 10 years |
||||||
| Howes et al., 2005 | Cross-sectional study | 102 (54 m, 48 f) |
Not specified |
46.0 (sd 13.1) | 30.4% postmenopausal |
L1-L4 & hip |
| Comparison between normative BMD data and BMD in patient group prescribed various antipsychotics for over 2 years |
||||||
| O’Keane and Meaney, 2005 | Cross- sectional study | 38 f | Scz n=38 | Prolactin-raising group: 32.8 (sd 6.8) Olanzapine group 29.5 (sd 5.7) |
All females premenopausal |
L1-L4, femoral neck, trochanteric & intertrochanteric regions of the left hip |
| Comparison between normative BMD data and BMD data in two patient groups prescribed either various prolactin-raising antipsychotics or olanzapine (relatively prolactin-sparing) for at least 1 year |
||||||
| Kishimoto et al., 2008 | Cross-sectional study | 74 m | Scz n=74 | 58.9 (sd 12.2) | N/A | Distal 1/3 of radial bone of forearm |
| Comparison between normative BMD data and BMD in two patient groups prescribed either ≥1 prolactin-raising antipsychotics or prolactin-sparing antipsychotics at time of study sampling |
Footnotes: BMD=bone mineral density, sd=standard deviation, DEXA=Dual-energy X-ray absorptiometry, Scz=schizophrenia, Dpn=depression,Sczaf=schizoaffective disorder, Man=mania, Adj=adjustment disorder, pQCT=computed tomography, QUS=ultrasound quantified bone speed of sound, L2-L4/L1-L4=lumbar spine, L2=lumbar vertebrae
Statistical analysis
It was not possible to perform a meta-analysis on the results of each study identified due to heterogeneity in the study design, methods used (different measures of BMD) and due to the different way in which data was presented (raw BMD data, Z-scores and T-scores). Instead we synthesise and appraise the available evidence.
RESULTS
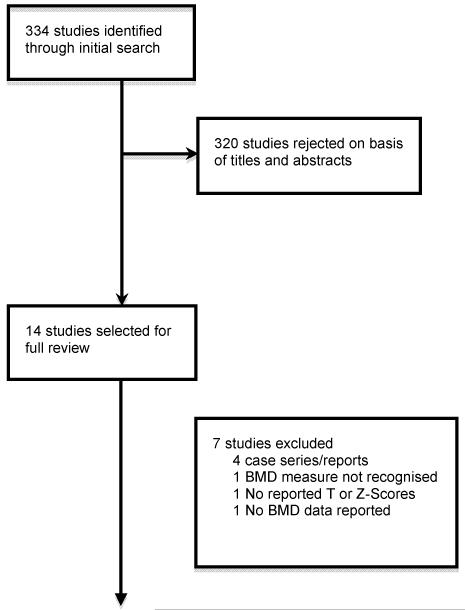
Thirty-two studies were identified by the searches. Eighteen were subsequently excluded following a review of the abstracts because they did not meet inclusion criteria and 4 were case reports/series. Of the remaining studies, 1 study did not use DEXA scans to measure BMD, 1 study whilst reporting BMD data using DEXA scans, made comparisons with their own healthy controls rather than Z or T-score reference data and 1 study did not report BMD data. After elimination, this left 7 studies for inclusion in the review (see Figure. 1).
Figure 1.

Flow diagram (selection strategy) of included studies
Demographics
Demographics and methodologies of the 7 included studies are summarised in Table 1.
Overall, the 7 studies reported data on a total of 372 patients (193 male/179 female). Of the females studied, 73 were recorded as being as premenopausal and 40 postmenopausal. This information was not specified for the remaining 66 females. Diagnoses varied; 226 were recorded as having had a diagnosis of schizophrenia, 7 schizoaffective disorder, 21 depressive disorder, 2 bipolar disorder and 5 adjustment disorder. For the remaining 111 patients the diagnosis was not specified.
Study design and bone mineral density measurement
All of the 7 studies were cross-sectional studies, report BMD data as standard deviations (SD) from reference data (Z-scores or T-scores), and all used DEXA scanning as a measure of BMD at 1 or more of the anatomical sites studied. All studies included DEXA measurements of BMD taken at the lumbar spine and hip, other than in 1 study where this was measured at the distal third of the radius only (Kishimoto et al., 2008). In addition to DEXA scans, computed tomography (pQTC) was used to measure radial BMD in 1 study (Akande et al., 2002) and ultrasound measures of bone speed of sound (QUS) at the radius, proximal phalanx of the third finger and tibia were used in another study (Becker et al., 2003). Although T-scores and Z-scores were reported for these different techniques, the implications of these findings were questionable due to a lack of data on the clinical significance of these measures.
Bone mineral density findings
All 7 included studies report BMD changes in the form of Z-scores. Five only report Z-scores (Halbreich et al., 1995; Becker et al., 2003; Meaney et al., 2004; Howes et al., 2005; O’Keane and Meaney, 2005) whilst 2 report both Z-scores and T-scores (Akande et al., 2002; Kishimoto et al., 2008).
The main findings reported in these studies and statistically significant changes in Z-scores and T-scores are summarised in Table 2.
Table 2. Summary in chronological order of the main reported findings of identified studies investigating the relationship between antipsychotic use and BMD.
| Study | Reported main findings | Key BMD findings reported as Z-scores | Key BMD findings reported as T-scores |
|---|---|---|---|
| Halbreich et al., 1995 | Statistically significant reductions in BMD in both sexes. Reduction particularly marked in males |
Males: L2-L4: 72% >1sd below mean, 28% >2sd below mean |
N/A |
| L2: 79% >1sd below mean, 35% >2sd below mean Femoral neck: 90% >1sd below mean, 52% >2sd below mean |
|||
| Females: L2-L4: 38% >1sd below mean, 6% >2sd below mean L2: 41% >1sd below mean, 12% >2sd below mean Femoral neck: 9% >1sd below mean |
|||
| Akande et al., 2002 | Statistically significant reduction in BMD in trabecular bone using pQCT but not at any sites using DEXA |
Trabecular bone: Mean −0.73, CI −1.14; −0.32, p<0.003 |
Trabecular bone: Mean −0.80, CI −1.19; −0.40, p<0.002 |
| Becker et al., 2003 | Similar BMD scores in risperidone and olanzapine groups using DEXA |
L2-L4: risperidone & olanzapine groups: Means −0.12 & −0.13 Femoral neck: 0.28 & 0.38 |
N/A |
| Statistically significant reduction in bone speed of sound (at radius, phalanx but not tibia) in risperidone group compared with olanzapine group |
Radius: risperidone & olanzapine groups: Means −0.31 & 0.58, p<0.05 Phalanx: −1.41 & 0.04, p<0.05 |
||
| Meaney et al., 2004 | High rates of osteopenia and osteoporosis (Z score >1sd below mean) in both sexes |
Males: 57% >1sd below mean (on at least one bone measure) Females: 32% >1sd below mean (on at least one bone measure) |
N/A |
| Subsequent analysis showed statistically significantly higher rates of severely reduced bMd (>2sd below mean) in ‘high- dose’ compared with ‘low-dose’ antipsychotic group |
‘High-dose’ group: 35% >2sd below mean ‘Low-dose’ group: 11.5% >2sd below mean Difference statistically significant (p<0.03) |
||
| Howes et al., 2005 | No statistically significant reductions in BMD in either sex, other than spine BMD in black males |
Males: 53.7% >−1.0 (on at least one bone measure) Females: 29.2% >−1.0 (on at least one bone measure) Black males: Mean −0.88, p<0.00001 |
N/A |
| Subsequent analysis showed no difference between patients prescribed prolactin-raising or prolactin sparing antipsychotics |
|||
| O’Keane and Meaney, 2005 | Statistically significantly higher rates of osteoporosis and osteopenia (Z score >1sd below mean) in prolactin-raising group compared to olanzapine group |
Prolactin-raising group: 65% >1sd below mean Olanzapine group: 17% >1sd below mean Difference statistically significant (p<0.005) |
N/A |
| Kishimoto et al., 2008 | Statistically significant reductions in BMD in some but not all of the age ranges defined |
Significant reductions (p<0.05) in all but 3 of the 10 age groups specified (30-34, 35-39 & 50-54). Specific Z scores not supplied |
Overall: T-score −1 to − 2.5 (osteopenia) in 37.8% of subjects. T- score >−2.5 |
| Subsequent analysis showed no difference between subjects with hyperprolactinaemia compared with those normal prolactin |
(osteoporosis) in 27.0% |
Footnotes: BMD=bone mineral density, pQCT=computed tomography, DEXA=Dual-energy X-ray absorptiometry, sd=standard deviations, L2-L4/L1-L4=lumbar spine, L2=lumbar vertebrae L2 alone, CI=confidence interval, p=level of significance
DISCUSSION
Antipsychotics and reduced bone mineral density
All of the studies that compared BMD in patients taking antipsychotics with control data identified one or more statistically significant reduction in BMD (Table 2.). This supports the hypothesis that there is an association between taking an antipsychotic and reduced BMD, and suggests that patients prescribed antipsychotics are at increased risk of developing osteoporosis. However, there are a number of caveats to the available evidence. Firstly, most of the studies reported BMD data for multiple sites, which, as none adjusted for multiple comparisons, increases the risk of false positive findings. Secondly, the strength of the relationship varies considerably between studies. This may be explained by differences between the patient populations studied: decreased BMD was particularly marked across all anatomical sites in the study by Halbreich et al. (1995), which was of in-patients, whilst in the studies of out-patients this relationship was only seen at certain sites where BMD was measured. There are a number of possible reasons why in-patients may be more affected: they are likely to have a more severe illness, and so be on higher doses of medication for longer, but also their exposure to sunlight and dietary sources of vitamin D (vitamin D is an important determinant of BMD) may be lower.
Possible mechanisms behind bone mineral density reductions - Prolactin-raising versus prolactin-sparing antipsychotics
Hyperprolactinaemia is common in patients taking antipsychotic medications (Howes et al., 2007). As previously discussed, this has led to the suggestion that reductions in BMD maybe mediated through hyperprolactinaemia caused by certain antipsychotic medications. Some of the more recent studies included in the review have attempted to investigate this by specifically grouping patients at the outset into those prescribed antipsychotic medications considered as prolactin-raising or prolactin-sparing, in order to examine BMD differences between these groups (Becker et al., 2003; O’Keane and Meaney, 2005; Howes et al., 2005; Kishimoto et al., 2008).
In 1 of these 4 studies (O’Keane and Meaney, 2005) the authors reported using Z-scores, that 65% of their prolactin-raising group had a mean BMD greater than 1 SD below the mean, compared with only 17% in the olanzapine group (p <0.005), olanzapine being considered as relatively prolactin-sparing. Interestingly, based on the assumption that BMD is normally distributed in the general population (Bonjour and Rizzoli, 1996), this figure of 17% in the olanzapine group, is essentially the same as the 16% of people in a normal population who would be expected to have a BMD greater than 1 SD below the mean, indicating that this group had essentially normal BMD.
In the other 3 studies, 2 found no difference between the high and normal prolactin groups (Howes et al., 2005; Kishimoto et al., 2008). The authors of the remaining study (Becker et al., 2003) also found no difference in BMD between the 2 groups when BMD was measured using DEXA, although they did report a statistically significant difference in BMD reduction between their risperidone (prolactin-raising) and olanzapine (prolactin-sparing) group at the phalanx (p<0.05). This difference was, however, found using bone speed of sound (QUS) as a measure of BMD, rather than the DEXA scan. The clinical implications of this finding are currently limited by the lack of data on the clinical significance of BMD measured using QUS.
Lifestyle factors
A number of lifestyle risk factors for low BMD are also seen in patients with schizophrenia and other psychotic disorders. This suggests other potential mechanisms as to how patients prescribed antipsychotic medications may develop reduced BMD. These risk factors include smoking and alcohol use (McCreadie, 2002), drug use (Regier et al., 1990), reduced exercise (Gothelf et al., 2002) and poor nutrition (Peet, 2004). The extent to which the included studies either excluded these various lifestyle factors in the study design or controlled for them in their subsequent analysis was variable.
Five of the identified studies investigated the relationship between BMD and these lifestyle risk factors (Halbreich et al., 1995; Akande et al., 2002; Meaney et al., 2004; Howes et al., 2005; Kishimoto et al., 2008). The only significant correlations seen were between BMD and BMI (spine: r=0.24, P=0.02; hip: r=0.34, P=0.001; femoral neck: r=0.28, P=0.005) (Howes et al., 2005) and between BMD at L4 and smoking (r=−0.27, P=0.04) (Meaney et al., 2004).
In the remaining 2 studies, the authors simply recorded the presence of these variables in the different groups being compared in each study, commenting that the variables were similar in each group (Becker et al., 2003; O’Keane and Meaney, 2005).
The small sample sizes in the studies make elimination of these confounding lifestyle factors difficult and only with large sample sizes can more robust conclusions be drawn on the contributing effect of these factors. A recent study with a large sample size found that schizophrenia was associated with reduced BMD, after controlling for medications and other risk factors for osteoporosis (Partti et al., 2010), suggesting that illness itself may be an independent risk factor. However, this study used ultrasound, rather than DEXA, to measure BMD.
Clinical significance of BMD measurements
In order to appraise the clinical significance of the reported statistically significant results, the identified studies were interpreted according to whether the BMD data provided was described as a T-score or as a Z-score.
The WHO criteria for interpreting BMD using DEXA T-scores define normal BMD as a T-score of −1.0 or above, osteopenia as a T-score between −1.0 and −2.5 and osteoporosis as a T-score of −2.5 or below (WHO, 1994). However, these reference ranges are based on data in postmenopausal white females, and the validity of their use in other groups such as premenopausal women and males is not established and can be misleading (Carey et al., 2009). On this basis, the International Society for Clinical Densitometry (ISCD), recommend that DEXA T-scores should only be used in postmenopausal women as per WHO definitions and DEXA Z-scores, not T-scores, should be used to inform diagnosis in premenopausal women and men aged 20-49 years (Lewiecki et al., 2004). In postmenopausal women, the use of T-scores means that these are not adjusted for age, therefore, it is not possible to infer that rates of osteopenia or osteoporosis are greater than expected in age-matched populations. However, ISCD criteria recommend the of use T-scores in postmenopausal women, as this is most closely linked to fracture risk. The ISCD recommendations do not specify cut-off ranges for normal bone density, osteopenia and osteoporosis, instead suggesting that a Z-score less than -2 indicates reduced bone mineral density. We have followed these ISCD 2005 recommendations in the following discussion of the clinical importance of the study findings.
Halbreich el al. (1995) report several statistically significantly reduced BMD Z-score values for both males and females, measured at different anatomical sites. Furthermore, these reductions are clinically significant at these sites in a large number of patients. This is particularly evident at the hip, where 52% of males are reported as having a BMD greater than 2 SD below the mean. According to ISCD criteria, this indicates that more than half of the males in the study had a BMD at the hip below the range considered as normal. Given that the distribution of BMD in healthy adults is approximately normal, in the normal population this figure would be expected to be around 2.2%. The percentages of male patients with BMD Z-scores over 2 SD from the mean at L2-L4 (28%) and L2 alone (35%) are also much greater than would be expected in the normal population. In females, this is not so evident, yet the scores over 2 SD below the mean still remain greater than would be expected in the normal population.
Meaney et al. (2004) applied WHO defined T-score cut-offs to their data and reported rates of osteopenia/osteoporosis (BMD >1 SD below the mean) of 57% in males and 32% in females. However, as discussed above, the clinical relevance of T-scores is difficult to determine in males, meaning it is not possible to interpret the male results in terms of the ISCD criteria. The females in the study were exclusively postmenopausal. As such the ISCD criteria can be applied to indicate that 32% had osteopenia/osteoporosis, but as this is not adjusted for age, it is not possible to say if the rate is higher than would be seen in age-matched postmenopausal females not taking antipsychotic medication.
The reported results by O’Keane and Meaney (2005), indicate that 65% of the prolactin-raising group had Z-scores greater than 1 SD below the mean. Although based on ISCD criteria, the use of Z-scores is appropriate, given that all females were premenopausal, the percentage with Z-scores below 2 SD below the mean is not given, so clinical interpretation in terms of ISCD criteria cannot be made.
In 3 of the studies, the findings, whilst statistically significant, are unlikely to warrant clinical investigation or intervention based on the ISCD criteria (Akande et al., 2002; Becker et al., 2003; Howes et al., 2005). Furthermore, although the studies by Becker et al. (2003) and Akande et al. (2002) reported results obtained by DEXA scanning, the significant results reported were obtained using measures of BMD other than DEXA. The clinical relevance of these measures has yet to be established (Hans et al., 1996; Petit et al., 2005).
In the remaining and most recent of the included studies, Kishimoto et al. (2008) reported both T-scores and Z-scores in a population of exclusively male patients prescribed antipsychotic medications. Whist their study reports high rates of T-scores greater than 1 SD below the mean (37.8%) and 2.5 SD below the mean (27%), the use of T-scores in males limits the clinical interpretation of these results, and the percentage of patients with Z-scores below 2 SD below the mean is not given.
In summary, clinically significant decreases in BMD based on ISCD criteria are reported in 2 out of the 7 studies (Halbreich et al., 1995; Meaney et al., 2004), whilst the clinical significance of the findings in other studies is difficult to determine because of the way the data are presented. This is probably insufficient evidence to indicate routine monitoring of BMD in patients taking antipsychotic drugs, but does suggest that clinicians have a lower threshold for investigating BMD in such patients, especially where they have additional risk factors for low BMD. This applies particularly to patients with a low BMI, corticosteroid users, females with a history of prolonged amenorrhoea, and those with a family history of osteoporosis or previous history of fractures (WHO, 1994). Where low BMD is detected in patients taking antipsychotics, treatment options include the addition of alendronic acid (Howes and Smith, 2004), or oestrogen augmentation, which may also benefit negative symptoms (Kulkarni et al., 2008).
Future directions for research
A clear recommendation for future studies is that they report data on BMD in a way that facilitates clinical interpretation; in particular, it would be useful to report the numbers of patients with BMD at levels that warrant clinical intervention according to recognised guidelines such as those from the ICSD. All the studies identified in our review use normative data for comparison. This can be seen as an advantage in these studies, given that BMD data can be compared to well established population data. The role of lifestyle risk factors in reduced BMD warrants further investigation, particularly as many of these are readily amenable to intervention. Studies comparing rates of BMD between patients taking antipsychotic medications and unmedicated patients would help shed light on whether reduced BMD is exclusively related to treatment, or may additionally be due to the disorder being treated.
CONCLUSION
The available evidence indicates that BMD is reduced in people taking antipsychotics, and that rates of clinically significant reductions are higher than expected. However, further studies that report BMD in a way that enables clinical interpretation are needed before clinical recommendations can be made.
REFERENCES
- Akande B, Wieck A, Haddad PM. Bone mineral density in premenopausal women with antipsychotic-induced hyperprolactinaemia. Eur Neuropsychopharmacol. 2002;12:311–312. [Google Scholar]
- Becker D, Liver O, Mester R, Micha R, Weizman A, Weiss M. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J Clin Psychiatry. 2003;64(7):761–766. doi: 10.4088/jcp.v64n0704. [DOI] [PubMed] [Google Scholar]
- Bonjour JP, Rizzoli R. Bone acquisition in adolescence. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. Academic Press; San Diego, CA: 1996. pp. 465–476. [Google Scholar]
- Burge RT. The cost of osteoporotic fractures in the UK: Projections for 2000-2020. J Med Econ. 2001;4:51–62. [Google Scholar]
- Carey JJ, Delaney MF, Love TE, Cromer BA, Miller PD, Richmond BJ, Manilla-McIntosh M, Lewis SA, Thomas CL, Licata AA. Dual-energy X-ray absorptiometry diagnostic discordance between Z-scores and T-scores in young adults. J Clin Densitom. 2009;12(1):11–16. doi: 10.1016/j.jocd.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Colao A, Loche S, Cappabianca P, de Divitiis E, Lombardi G. Pituitary adenomas in children and adolescents. Clinical presentation, diagnosis and therapeutical strategies. The Endocrinologist. 2000;10:314–320. [Google Scholar]
- Dennison E, Cole Z, Cooper C. Diagnosis and epidemiology of osteoporosis. Curr Opin Rheumatol. 2005;17:456–461. doi: 10.1097/01.bor.0000166384.80777.0d. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Genant HK, Cooper C, Poor G. Interim report and recommendations of the World Health Organisation task-force for osteoporosis. Osteoporosis Int. 1999;10:259–264. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]
- Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol. 2002;64:47–67. doi: 10.1146/annurev.physiol.64.081501.131049. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Falk B, Singer P, Kairi M, Phillip M, Zigel L, Poraz I, Frishman S, Constantini N, Zalsman G, Weizman A, Apter A. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry. 2002;159:1055–1057. doi: 10.1176/appi.ajp.159.6.1055. [DOI] [PubMed] [Google Scholar]
- Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64:2291–2314. doi: 10.2165/00003495-200464200-00003. [DOI] [PubMed] [Google Scholar]
- Halbriech U, Rojansky N, Palter S, Hreshchyshyn M, Kreeger J, Bakhai Y, Rosan MD. Decreased bone mineral density in medicated psychiatric patients. Psychosom Med. 1995;57:485–491. doi: 10.1097/00006842-199509000-00011. [DOI] [PubMed] [Google Scholar]
- Hans D, Dargent-Molina P, Schott AM, Sebert JL, Cormier C, Kotzki PO. Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet. 1996;348:511–514. doi: 10.1016/s0140-6736(95)11456-4. [DOI] [PubMed] [Google Scholar]
- Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psych. 2007;190:129–134. doi: 10.1192/bjp.bp.106.023671. [DOI] [PubMed] [Google Scholar]
- Howes O, Smith S. Hyperprolactinaemia caused by antipsychotic drugs. Endocrine antipsychotic side effects must be systemically assessed. BMJ. 2002;324:1278. [PMC free article] [PubMed] [Google Scholar]
- Howes O, Smith S. Alendronic acid for antipsychotic-related osteopenia. Am J Psychiatry. 2004;161:756. doi: 10.1176/appi.ajp.161.4.756. [DOI] [PubMed] [Google Scholar]
- Howes OD, Wheeler M, Meaney AM, O’Keane V, Fogelman I, Blake G, Murray RM, Smith S. Bone mineral density and its relationship to prolactin levels in patients taking antipsychotic treatment. J Clin Psychopharmacol. 2005;25(3):259–261. doi: 10.1097/01.jcp.0000162798.87249.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Wheeler MJ, Pilowsky LS, Landau S, Murray RM, Smith S. Sexual function and gonadal hormones in patients taking antipsychotic treatment for schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2007;68(3):361–367. doi: 10.4088/jcp.v68n0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JM. Role of sex steroids in the regulation of bone metabolism in the adult skeleton. Ann Endocrinol (Paris) 2006;67(2):119–122. doi: 10.1016/s0003-4266(06)72565-3. [DOI] [PubMed] [Google Scholar]
- Kayath MJ, Lengyel AM, Vieira JG. Prevalence and magnitude of osteopenia in patients with prolactinemia. Braz J Med Biol Res. 1993;26:933–941. [PubMed] [Google Scholar]
- Kishimoto T, Watanabe K, Shimada N, Nakita K, Yagi G, Kashimi H. Antipsychotic-induced hyperprolactinaemia inhibits the hypothalamo-pituitary-gonadal axis and reduces bone mineral density in male patients with schizophrenia. J Clin Psychiatry. 2008;69(3):382–391. doi: 10.4088/jcp.v69n0307. [DOI] [PubMed] [Google Scholar]
- Klibanski A, Biller BMK, Rosenthal DI, Schoenfeld DA, Saxe V. Effects of prolactin and estrogen deficiency in amenorrheic bone loss. J Clin Endocrinol Metab. 1988;67:124–130. doi: 10.1210/jcem-67-1-124. [DOI] [PubMed] [Google Scholar]
- Kulkarni J de CA, Fitzgerald PB, Gurvich CT, Bailey M, Bartholomeusz C, Burger H. Estrogen in severe mental illness: a potential new treatment approach. Arch Gen Psychiatry. 2008;65:955–960. doi: 10.1001/archpsyc.65.8.955. [DOI] [PubMed] [Google Scholar]
- Kunimatsu T, Kimura J, Funabashi H, Inoue T, Seki T. The antipsychotics haloperidol and chlorpromazine increase bone metabolism and induce osteopenia in female rats. Regul Toxicol Pharmacol. 2010;58(3):360–368. doi: 10.1016/j.yrtph.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Lewiecki EM, Watts NB, McClung MR, Petak SM, Bachrach LK, Shepherd JA, Downs RW. The International Society for Clinical Densitometry. Position Statement. Official Positions of the International Society for Clinical Densitometry. J Clin Endocrinol Metab. 2004;89(8):3651–3655. doi: 10.1210/jc.2004-0124. [DOI] [PubMed] [Google Scholar]
- McCreadie R. Use of drugs, alcohol and tobacco by people with schizophrenia: case-control study. Br J Psychiatry. 2002;181:321–325. doi: 10.1192/bjp.181.4.321. [DOI] [PubMed] [Google Scholar]
- Meaney AM, Smith S, Howes OD, O’Brien M, Murray RM, O’Keane V. Effects of long-term prolactin raising antipsychotic medication on bone mineral density in patients with schizophrenia. Br J Psychiatry. 2004;184:503–508. doi: 10.1192/bjp.184.6.503. [DOI] [PubMed] [Google Scholar]
- O’Keane V, Meaney AM. Antipsychotic drugs. A new risk factor for osteoporosis in young women with schizophrenia. J Clin Psychopharmacol. 2005;25(1):26–30. doi: 10.1097/01.jcp.0000150223.31007.e0. [DOI] [PubMed] [Google Scholar]
- Partti K, Heliövaara M, Impivaara O, Perälä J, Saarni SI, Lönnqvist J, Suvisaari JM. Skeletal status in psychotic disorders: a population-based study. Psychosom Med. 2010;72(9):933–40. doi: 10.1097/PSY.0b013e3181f7abd3. [DOI] [PubMed] [Google Scholar]
- Peet M. Diet, diabetes and schizophrenia. Br J Psychiatry. 2004;184:s102–S105. doi: 10.1192/bjp.184.47.s102. [DOI] [PubMed] [Google Scholar]
- Petit MA, Beck TJ, Kontulainen SA. Examining the developing bone: What do we measure and how do we do it? J Musculoskelet Neuronal Interact. 2005;5(3):213–224. [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse: results from the epidemiological catchment area (ECA) study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Sackett G, Unis A, Crouthamel B. Some effects of risperidone and quetiapine on growth parameters and hormone levels in young pigtail macaques. J Child Adolesc Psychopharmacol. 2010;20(6):489–493. doi: 10.1089/cap.2010.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlechte JA, Sherman B, Martin R. Bone Density in Amenorrheic Women with and without Hyperprolactinemia. J Clin Endocrinol Metab. 1983;56(6):1120–1123. doi: 10.1210/jcem-56-6-1120. [DOI] [PubMed] [Google Scholar]
- Seeman E. Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr Osteoporosis Rep. 2004;2(3):90–96. doi: 10.1007/s11914-004-0016-0. [DOI] [PubMed] [Google Scholar]
- Smith S, Wheeler M, Murray R, O’Keane V. The effects of anti-psychotic induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol. 2002;22:109–114. doi: 10.1097/00004714-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517–522. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- Vartej P, Poianac C, Vartej I. Effects of hyperprolactinaemia on osteoporotic fracture risk in premenopausal women. Gynecol Endocrinol. 2001;15(1):43–47. [PubMed] [Google Scholar]
- Wang PS, Walker AM, Tsuang MT, Orav EJ, Glynn RJ, Levin R, Avorn J. Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry. 2002;59(12):1147–1154. doi: 10.1001/archpsyc.59.12.1147. [DOI] [PubMed] [Google Scholar]
- WHO . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organisation; Geneva: 1994. (WHO Technical Report Series, No. 843). [PubMed] [Google Scholar]
- WHO . World Health Organisation Scientific Group on the assessment of osteoporosis at the primary healthcare level. World Health Organisation; Geneva: 2007. Summary Meeting Report. [Google Scholar]