Abstract
Anorexia nervosa (AN) is a condition of severe undernutrition characterized by alterations in multiple neuroendocrine axes and peptides that signal or regulate energy intake. These alterations include a state of hypogonadotropic hypogonadism, a nutritionally acquired resistance to growth hormone (GH) with low IGF-1 levels, relative hypercortisolemia, low total T3 despite normal TSH, low levels of leptin and insulin, and elevated levels of ghrelin, peptide YY (PYY) and possibly adiponectin. Although many of these changes are adaptive to low weight, they can impact bone metabolism, body composition, reproductive function and statural growth. Low bone mass is characteristic of AN in both adolescent boys and girls. In girls, sites of trabecular bone are more likely to be affected than sites of cortical bone, whereas in boys with AN, sites of cortical bone are more commonly affected. Bone microarchitecture is also affected in adolescent girls with AN, with a decrease in trabecular thickness and bone trabecular volume, and an increase in trabecular separation. Important predictors of low bone density include nutritional factors, body composition, hypogonadism, low IGF-1, elevated cortisol and PYY levels, with possible contributions of low insulin. Weight gain is associated with a stabilization of bone density, although residual deficits persist in the short term, and in some cases, long term.
Anorexia nervosa (AN) is a primary psychiatric condition with high mortality characterized by severe self imposed nutritional deprivation and reduction in caloric intake associated with (1) weight loss, a failure to gain weight or to maintain weight leading to body weight that is less than 85% of what is considered ideal for age and for height, (2) BMI less than 17.5 kg/m2 in older adolescents, (3) an intense fear of gaining weight, (4) an impaired body image, and (5) in postmenarchal girls, amenorrhea for at least three consecutive cycles [1]. 0.2–4.0% of adolescent girls and college aged young women suffer from this eating disorder, and this has been reported to be the third common chronic illness in teenage girls [2]. In the restrictive form of AN, which is more common in adolescents, reduction in caloric intake is primarily a consequence of a marked decreases in absolute fat intake, whereas protein and carbohydrate intake do not significantly differ from normal-weight healthy adolescents [3]. This nutritional deprivation is associated with significant alterations in various endocrine axes, and these are typically adaptive responses to a state of reduced energy availability. In addition, AN is associated with significant impairment of normal bone metabolism, a consequence of both the low energy state and of adaptive changes in various endocrine axes. This review describes alterations that occur in these endocrine axes in AN and reviews the pathophysiology underlying low bone density in adolescents with AN. Electrolyte abnormalities such as hypokalemia and hypophosphatemia can occur in patients with eating disorders, as well as hematologic abnormalities, but will not be reviewed here.
Hypothalamo-Pituitary-Gonadal Axis
AN is characterized by hypogonadotropic hypogonadism, and at this time, amenorrhea remains a necessary diagnostic criterion for AN according to the DSM-IV. The condition may present as primary or secondary amenorrhea, and in one study, 28% of adolescent girls 12–18 years old with AN were premenarchal compared to only 11% of healthy normal-weight adolescents of the same age range [4]. Studies by Boyar et al. [5] in adult women with AN have demonstrated that luteinizing hormone (LH) pulsatility reverts to either a prepubertal pattern of low amplitude LH pulses or an early pubertal pattern of nighttime entrainment of LH pulses. Of note, with recovery, the pattern of gonadotropin secretion recapitulates normal pubertal patterns. Estradiol levels in adolescent girls with AN are lower than in healthy adolescent girls, even when the latter are examined in the early follicular phase of their cycles when estradiol levels are at a nadir [4], and free testosterone levels trend lower in girls with AN than in controls [6]. Similarly, boys with AN have lower testosterone and estradiol levels than healthy adolescent boys [7].
Hypogonadotropic hypogonadism is likely the consequence of a marked reduction in energy availability associated with low fat mass, and resumption of menstrual function in girls with AN is associated with significant increases in fat mass [8] (fig. 1). In one study, all girls whose fat mass reached 24% resumed menstrual function, whereas none of the girls with percent fat mass of less than 18% resumed menses [8]. Research is ongoing to identify neuroendocrine factors that signal a state of low energy availability to hypothalamic neurons in conditions of low energy availability, but possible signals include alterations in levels of hormones such as leptin, ghrelin, cortisol, insulin and IGF-1. Low leptin and high ghrelin levels have been demonstrated to predict lower levels of estradiol and gonadotropins in AN [9, 10]. Menstrual recovery is expected to occur within six months of weight gain (to 90% of ideal body weight) in girls with AN, however, this is not universal. A corresponding increase in fat mass is necessary, and persistent alterations in hormones such as ghrelin, leptin and cortisol may prevent resumption of menses in some instances even after adequate weight gain. In addition, some girls with AN who do not resume menses with weight gain have features of the lean form of polycystic ovary syndrome (PCOS), and examination for clinical and biochemical correlates of PCOS may become necessary in such instances. It is unknown whether such girls revert to their pre-morbid phenotype, or, whether weight gain in a subset of girls alters hypothalamic- pituitary-ovarian sensitivity.
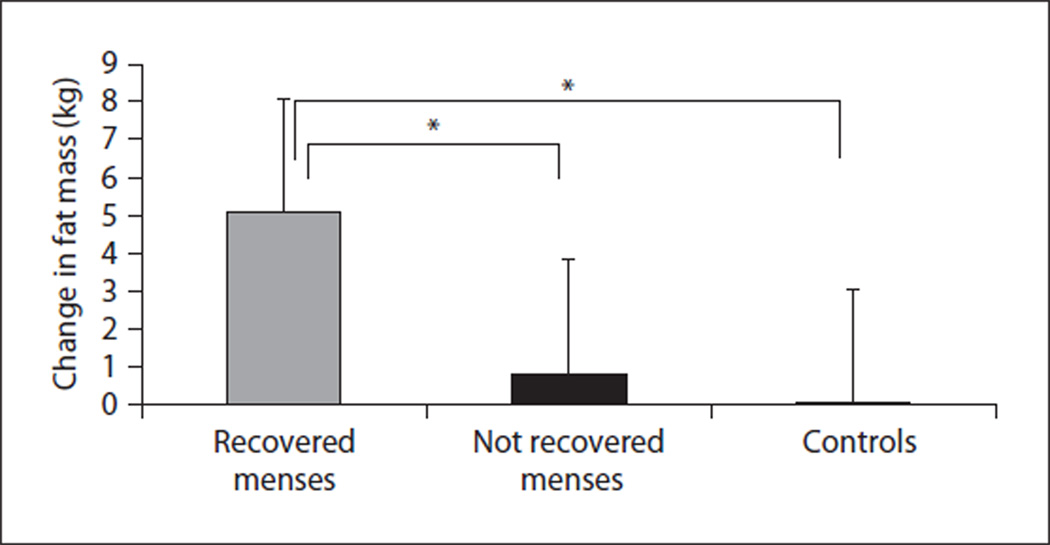
Fig. 1.
Changes in fat mass in AN girls who recovered menses (n = 19) (gray bar), AN girls who did not recover menses (n = 14) (black bar) and controls (n = 33) (white). ANOVA demonstrated a significant difference between the groups (p < 0.0001). AN girls who recovered menses had greater increases in fat mass than AN girls who did not resume menses and controls (p < 0.05 for both). Reprinted with permission from Misra et al. [8].
Growth Hormone-Insulin-Like Growth Factor Axis
In adolescents with AN, levels of insulin-like growth factor-1 (IGF-1) are low despite high concentrations of growth hormone (GH), indicating a state of hepatic GH resistance that is nutritionally acquired [11, 12]. Low levels of GH-binding protein in adults with this disorder suggest that reduced expression of the GH receptor may account for resistance to GH effects [13]. IGF-1 levels are a direct marker of nutritional status and correlate inversely with GH concentrations [11]. Elevated GH concentrations appear to be a consequence of (1) reduced negative feedback from low IGF-1 levels resulting from inadequate IGF-1 liver production, (2) an increase in ghrelin, which is a GH secretatgogue [14], with the possible contribution of (3) low leptin levels [9–11, 15]. Deconvolution analyses indicate that increased GH concentrations in AN are consequent to increases in both basal and pulsatile GH secretion [11, 15], and the latter results from increased secretory burst frequency [11, 15] and burst mass [15] (fig. 2). Studies have proposed both a decrease in hypothalamic somatostatinergic tone and an increase in gonadotropin hormone-releasing hormone pulses in AN, although definitive data are lacking [16]. Increased approximate entropy scores in AN in both adults and adolescents also indicate an increased disorderliness of GH secretion [11, 15].
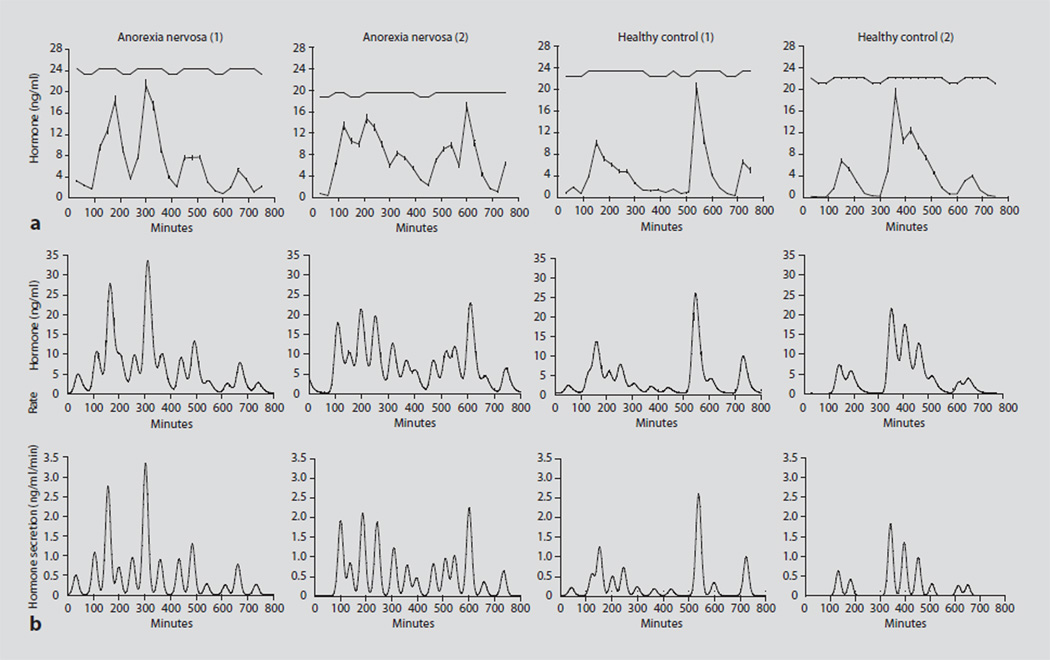
Fig. 2.
Cluster and deconvolutional analyses in AN patients and controls. a Cluster analysis in 2 girls with AN (two left panels) and 2 healthy adolescent girls (two right panels). The mean and nadir GH concentrations and the total AUC were greater in girls with AN than in controls. b Deconvolutional analysis in the 2 girls with AN and the 2 healthy controls analyzed by cluster in a. The upper panels show GH concentrations over the sampling period; the lower panels show the individual secretory bursts. Girls with AN had higher basal GH secretion and a greater number of secretory episodes than healthy adolescents of comparable chronological and bone ages. Reprinted with permission from Misra et al. [11].
Although absolute suppression of GH following an oral glucose load does not differ from controls, because baseline GH concentrations are higher in AN, nadir GH concentrations are also higher [17]. Weight gain is associated with an increase in IGF-1 levels and a lowering of GH concentrations. Because GH is a counter-regulatory hormone, an increase in GH concentrations in this state of severe undernutrition should help maintain euglycemia, and likely represents an adaptive response to an undernourished state.
IGF-binding proteins have been studied in AN, and the results are variable. IGFBP-1 and IGFBP-2 levels are typically high in AN and decrease with weight gain [12, 13]. IGFBP-3 levels, however, have been variably reported to be low [12, 13] or normal in AN [6, 18], and unlike other catabolic states, AN is not associated with increased proteolysis of IGFBP-3 [19]. IGFBP-4 and IGFBP-5 have important effects on bone and are both very low in AN [20].
Hypothalamo-Pituitary-Adrenal Axis
Both 24-hour urinary cortisol concentrations and serum cortisol measured by frequent sampling overnight are higher in adolescent girls with AN compared with controls, associated with an increased frequency of secretory bursts and longer half-life [21] (fig. 3). The frequency of secretory bursts decreases following weight gain, with or without menstrual recovery. Diurnal rhythmicity of cortisol secretion is preserved in AN. Consistent with a state of cortisol excess, cortisol levels suppress to a higher nadir in girls with AN following an oral glucose load than in healthy adolescents [21]. Similarly, in adults with AN, suppression of cortisol following administration of dexamethasone is suboptimal [22] and subsequent stimulation with corticotropin-releasing hormone (CRH) results in higher cortisol concentrations than in controls. Important predictors of high cortisol concentrations in adolescents with AN are low glucose and insulin levels and nutritional markers such as low BMI and fat mass [21]. High ghrelin and low leptin levels in AN independently predict cortisol concentration and secretory characteristics. The level of activation of the hypothalamo-pituitary-adrenal axis has not been well elucidated, but appears to involve CRH hypersecretion, and elevated cerebrospinal fluid levels of CRH have been reported.
Fig. 3.
Cluster analysis of cortisol concentration and deconvolutional analysis of cortisol secretion AN and healthy adolescents. a Cluster analysis in 2 girls with AN (two left panels) and 2 healthy adolescent girls (two right panels). Mean, nadir, valley mean, peak mass, peak amplitude of cortisol concentration, and total AUC were greater in girls with AN than in controls. b Deconvolutional analysis in the 2 girls with AN and the 2 healthy controls analyzed by cluster in a. The upper panels show cortisol concentrations over the sampling period, whereas the lower panels show the individual secretory bursts. Girls with AN had a greater number of secretory episodes than healthy adolescents of comparable CA and BA and higher pulsatile and total cortisol secretion. Reprinted with permission from Misra et al. [11].
Because cortisol stimulates gluconeogenesis, an increase in cortisol concentrations, in addition to high GH levels, may be yet another adaptive mechanism to maintain euglycemia in this condition of severe undernutrition. In addition, glucocorticoids are endogenous antagonists of leptin and insulin. Some studies suggest that high cortisol concentrations in AN may predict greater increases in fat mass with recovery [8] and particularly an increase in trunk fat [23]. High cortisol concentrations in AN are also an independent predictor of lower extremity lean mass, consistent with known effects of cortisol on muscle [24].
Hypothalamo-Pituitary-Thyroid Axis
Changes in the hypothalamo-pituitary-thyroid axis in adolescents with AN are reminiscent of the sick euthyroid syndrome. TSH levels are usually normal, total T3 levels are low, and free T4 levels are normal or low normal [21]. The low T3 levels are consequent to increased peripheral deiodination of T4 to reverse T3 rather than T3. Total T3 levels correlate inversely with measures of nutritional status in AN including BMI, fat mass, insulin, glucose, IGF-1 and leptin. A decrease in thyroid hormone levels could contribute to the low resting energy expenditure observed in AN, and preservation of consumed energy for vital functions. A blunted response of TSH to exogenously administered TRH has been previously reported in up to 50% of adults with AN [25]. Changes in thyroid hormones normalize with weight gain.
Peptides and Cytokines that Signal Energy Availability
Various peptides and cytokines are affected by the state of energy availability and may impact other endocrine axes and also bone metabolism. AN is associated with marked reductions in BMI and fat mass, and adolescents with AN have as much as a 50% reduction in fat mass and 40% reduction in percent body fat compared with normal-weight adolescents [4]. Consistent with reductions in fat mass, there is a marked reduction in the adipokine, leptin [10]. In addition, peptides regulating appetite, food intake and satiety are markedly altered in this state of nutritional deprivation, usually as adaptive phenomena [9, 10, 26]. In addition to leptin, data are available regarding levels of insulin, adiponectin, ghrelin and PYY in AN.
Insulin and Adiponectin
Low body weight and low fat mass in AN are associated with lower levels of fasting insulin and glucose compared with normal-weight adolescent girls [21, 27]. Measures of insulin resistance, such as HOMA-IR, are also markedly reduced in AN [27]. CT and MRI measures of regional body composition are limited in AN, however, reports using dual-energy X-ray absorptiometry indicate marked reductions in trunk fat, which would be consistent with increased insulin sensitivity in AN [23, 28]. Adiponectin is an adipokine that mediates insulin sensitivity and resistance through effects on PPAR-γ, and levels of adiponectin have been variably reported to be elevated, unchanged or low in AN and may reflect difficulties assessing this adipokine in extreme states of reduced fat mass. [27, 29–31]. Although adiponectin levels are typically high in conditions of increased insulin sensitivity, as observed in some studies of AN, it is also possible that high levels of adiponectin may not be evident if fat mass is markedly reduced given that adiponectin is secreted by adipocytes. In fact, in at least one study in which absolute adiponectin levels were reported to be unchanged compared with controls, levels were higher per unit fat mass in AN than in normal-weight controls [27].
Leptin
Leptin is an adipokine that correlates strongly with fat mass [10]. Adolescent girls with AN have almost 72% lower levels of leptin than normal-weight girls, associated with increases in the soluble leptin receptor, which is the binding protein for circulating leptin. The free leptin index, which is the ratio of leptin and the soluble leptin receptor is 84 % lower in AN than in controls [32]. Lower leptin levels in girls with AN are a consequence of reduced leptin burst mass and basal secretion, and are predicted strongly by fat mass [10]. Because leptin inhibits the orexigenic neuropeptide Y (NPY) and AgRP neurons and stimulates the anorexigenic POMC and CART neurons in the hypothalamus, low leptin levels in AN may represent an adaptive response to nutritional deprivation, and an attempt to suppress anorexigenic and facilitate orexigenic stimuli and thereby stimulate caloric intake. Leptin also has a potentiating effect on gonadotropin-releasing hormone neurons, and resumption of menstrual function in AN is associated with and may in part be mediated by an increase in leptin levels [8, 10]. Leptin concentrations increase with weight gain and an increase in fat mass, although this increase may not be evident with only partial weight recuperation. Interestingly, in contrast to girls with AN, boys with AN do not have lower leptin levels than normal-weight boys, despite lower fat mass [7]. Larger studies in boys are necessary to confirm these findings.
Ghrelin
Ghrelin is an orexigenic peptide that is secreted by the oxyntic cells of the stomach [33], and levels of ghrelin typically peak right before a meal and reach a nadir between 30 and 60 min following a meal. Ghrelin stimulates the NPY and AgRP neurons in the arcuate nucleus of the hypothalamus, and girls with AN have significantly higher ghrelin levels than normal-weight controls [9, 34]. Nadir ghrelin levels following an oral glucose load are higher in AN than in controls [9,17]. High ghrelin levels are a consequence of increased ghrelin secretory burst mass [9], and decrease with weight gain. Low insulin levels and lower HOMA-IR are important predictors of high ghrelin levels in AN, with other predictors being lower BMI, fat mass, IGF-1 and leptin. High ghrelin levels in AN are an indicator of low energy availability, and the increase in ghrelin is likely an adaptive response to stimulate hunger and food intake. In addition, ghrelin is a GH and ACTH secretagogue, and also has inhibitory effects on gonadotropin pulsatility as demonstrated in animal and human studies [35, 36]. In our studies, higher ghrelin levels in AN were an independent predictor of higher GH and cortisol and lower LH and estradiol levels [9]. Stimulation of ACTH and subsequently cortisol may also be an adaptive response to stimulate hunger. Importantly, in contrast to high ghrelin levels in girls with AN, levels in boys with AN are not significantly higher than in controls [7]. A recent study reports high levels of obestatin, a ghrelin gene product that inhibits appetite and gastric motility, in adults with AN [37] with positive associations with acyl- and desacyl-ghrelin, and inverse associations with BMI, glucose, insulin and leptin. Further studies are necessary to better understanding the implication of high levels of this hormone in AN.
Peptide YY
PYY is an anorexigenic peptide secreted by the L or endocrine cells of the distal gut in response to food intake. Levels rise within 15–30 min of food intake, and induce satiety through the binding of PYY to the Y2 receptor of NPY, which causes a reduction in NPY secretion. Typically, PYY levels would be expected to be low in AN as an adaptive response to a low energy state in order to reduce satiety, and would be expected to be high in obesity. However, contrary to expectations, adolescent girls with AN have high levels of PYY [26] whereas obese individuals have low levels of PYY [38]. PYY levels are predicted inversely by body weight and BMI, and the reason behind this paradox remains unclear at this time. Whether elevated PYY levels represent a more primary disease mechanism rather than an adaptation to starvation is unknown. Similar to girls with AN, PYY levels are high in boys with AN [7].
Other Hormones
There are limited data regarding other gut and hypothalamic peptides in AN. Activation of NPY neurons stimulates feeding behavior, and leptin, ghrelin and PYY all impact NPY secretion with consequent effects on appetite. It is unclear whether peripheral NPY levels in any way reflect central expression of NPY, and data regarding NPY levels in AN are conflicting. The serotoninergic system has been strongly implicated in eating disorders, and specific polymorphisms in the promoter of the 5-HT 2A receptor gene have been associated with restrictive AN and with reduced caloric intake. Similar to NPY, the importance of peripheral serotonin levels, which are gut rather than brain derived, in AN, remains unknown. Gut hormones such as GLP-1 and GLP-2, amylin, cholecystokinin, bombesin and gastrin inhibitory peptide are altered in states of energy deficit and excess; however, there are limited data regarding these hormones in AN.
Bone Metabolism
An important consequence of impaired nutrition and alterations in various neuroendocrine axes in AN is low bone density. The impact of this disorder on the development of peak bone mass can result in failure of bone mass accrual and marked reduction in bone mass at multiple skeletal sites. AN is characterized by impaired bone metabolism in both adults [39] and adolescents [4, 40] (fig. 4, 5). In adults, more than 90% of ambulatory nonhospitalized women with AN are osteopenic and almost 40% are osteoporotic by WHO criteria based on bone density assessment by dual-energy X-ray absorptiometry (DXA) [39]. In adolescents with AN, about half have Z-scores of < −1 at one or more skeletal site, and 11% have Z-scores of < −2 [4]. Both trabecular and cortical bone are affected in this condition, although trabecular bone appears to be affected more than cortical based on lower Z-scores (for adolescents) and T-scores (for adults) for the spine, than for the hip or femoral neck [4, 39]. In adolescents, low spine bone density is a consequence of decreased spine bone mineral content for bone area, with sparing of bone area for height. In contrast, low whole body bone density is a consequence of low bone area for height, with a sparing of bone mineral content for bone area [41]. Lower bone density as assessed by DXA is corroborated by reports of impaired bone microarchitecture as assessed by ultra-high resolution CT of the radius. Adults with AN have a decrease in trabecular number and bone trabecular volume and an increase in trabecular separation, as well as a decrease in cortical thickness [42]. In addition, in adults with AN, lower bone density at the spine is associated with greater marrow fat corroborating the bone-fat connection [43]. In adolescents with AN, trabecular thickness and bone trabecular volume are lower than in controls, whereas trabecular separation is increased [44].
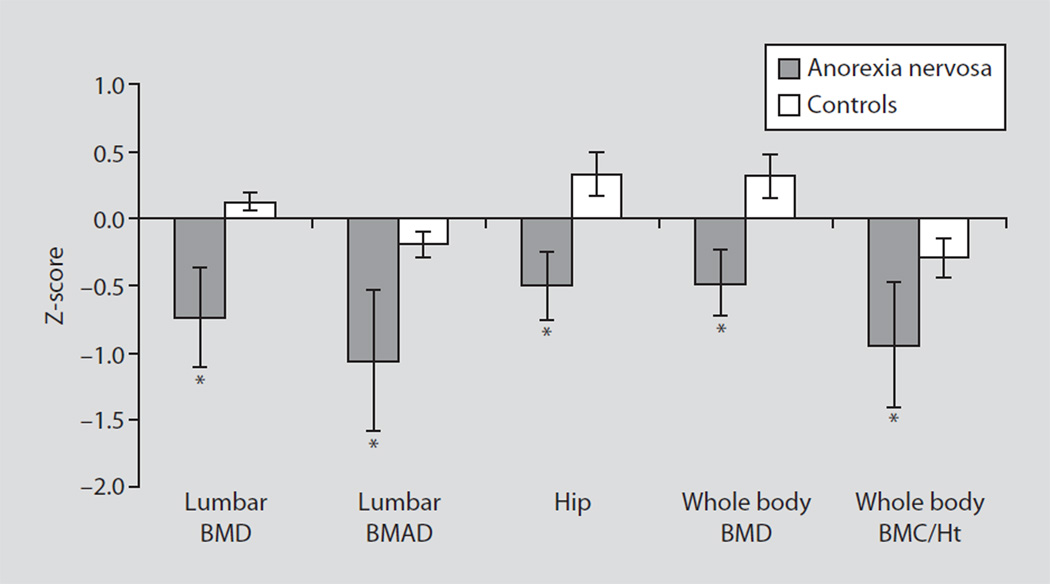
Fig. 4.
Bone density Z-scores in adolescent girls with AN and controls. Z-scores for lumbar spine bone mineral density (BMD), lumbar spine bone mineral apparent density (BMAD), hip bone density, whole body bone density and whole body bone mineral content/height (BMC/Ht) were lower in girls with AN (black bars) than in healthy controls (white bars). * p < 0.05.
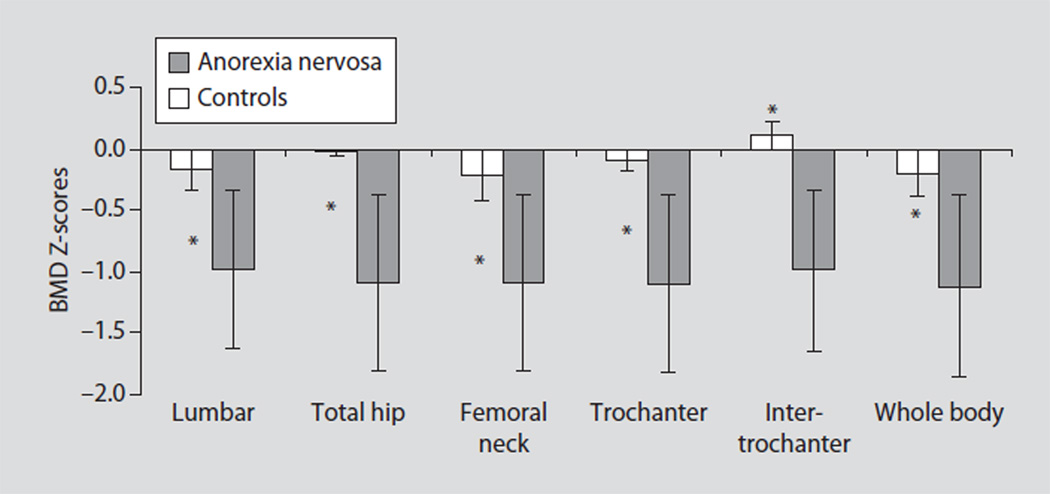
Fig. 5.
Bone density Z-scores in adolescent boys with anorexia nervosa and controls. Z-scores of the lumbar spine, total hip and its sub-regions (femoral neck, trochanter, intertrochanteric region) and the whole body were significantly lower in boys with anorexia nervosa than in controls. * p < 0.05. Reprinted with permission from Misra et al. [7].
Similar to females with AN, males with AN are also at high risk for low bone density. Adolescent boys and young men with AN have significantly lower bone density than normal-weight controls at all sites [7, 45]. However, unlike females, males with AN have greater involvement of the hip and femoral neck than of the spine [7, 45].
Bone turnover is uncoupled in adults with AN with a decrease in surrogate markers of bone formation and an increase in surrogate markers of bone resorption [46]. In male and female adolescents with AN, both bone formation and bone resorption markers are lower than in healthy adolescents, indicating a coupled decrease in bone turnover [6, 7, 47]. This is in contrast to normal adolescence, typically a high bone turnover state. With weight recovery, markers of bone formation and resorption both increase, and are predicted by the corresponding increase in lean mass and IGF-1 [48].
Nutritional markers such as BMI and lean mass are important determinants of low bone density in AN in both males and females [4, 6, 7], consistent with the known beneficial effects of weight loading and muscle pull on bone. In the absence of weight gain, girls with AN have a decrease in bone density and corresponding Z-scores over time [41, 48]. This is in contrast to healthy girls, who have the expected pubertal increase in bone mass and areal density, and maintain their Z-scores over the follow-up period. In one study, areal bone density at the spine decreased at an annual rate of 0.3% in girls not recovering weight or menses, and increased at the rate of 2.8% per year in normal-weight girls (fig. 6). Similar trends were observed with bone mineral content. Because the adolescent years are a critical time during which to optimize bone mass accrual towards attainment of peak bone mass, this decreased rate of bone mass accrual in AN is of significant concern. In fact, data indicate that individuals who develop AN during the adolescent years have lower subsequent bone density than those who develop this disorder in adult life, despite a similar duration of amenorrhea [49]. Of note, in girls with AN who gain weight and resumed menses, spine areal bone density increases at an intermediate rate of 1.4% per year, and bone mineral content for bone area at the spine, and bone area for height for the whole body also increase [41]. Although this rate of bone mass accrual falls short of complete catch-up, the trend is positive, emphasizing the importance of early and sustained weight gain and menstrual recovery in AN. Associated increases in lean mass with weight gain are an important predictor of increases in levels of bone turnover markers and bone density in girls with AN [48].
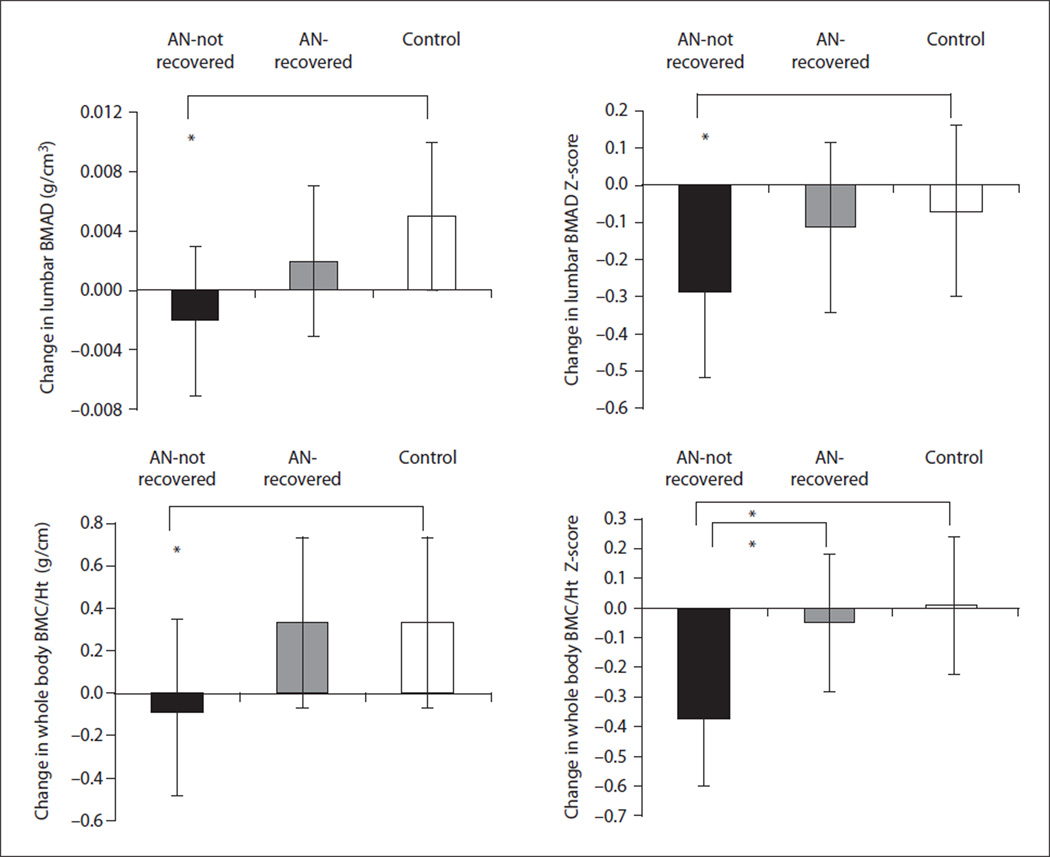
Fig. 6.
Change in lumbar BMAD and WB BMC/Ht measures in AN-not recovered (black bar), AN-recovered (gray bar), and healthy adolescents (white bar). AN-not recovered continued to lose bone mass over the 1-year follow-up period, and change in bone density measures was significantly lower in this group, compared with controls (Tukey-Kramer test for multiple comparisons). AN-recovered did not differ from controls for change in bone density parameters and differed significantly from AN-not recovered for change in whole body bone density Z-scores. * p < 0.05. Reprinted with permission from Misra et al. [41].
In adults with AN, in the absence of weight or menstrual recovery, women lose bone density at an annual rate of 2.6% at the spine and 2.4% at the hip [50]. Weight gain favors an increase in bone density at the hip, whereas menstrual recovery is associated with an increase in bone density at the spine, consistent with known effects of estrogen deficiency on trabecular bone density.
Although an optimal nutritional status is essential for optimizing bone mass accrual and calcium and vitamin D are nutrients essential for bone mineralization, multiple studies have demonstrated that adults and adolescents are not calcium or vitamin D deficient. Intake of calcium and vitamin D in this population is typically better than in controls, subsequent to increased use and patient acceptance of noncaloric supplements. However, supplementation with calcium and vitamin D is not effective in increasing bone density in AN [6, 51].
Hormonal Determinants of Low Bone Density in Anorexia Nervosa
Hypogonadism
Hypogonadism is an important contributor to low bone density in AN. The duration of amenorrhea predicts the extent of bone loss in females with AN [40], and low testosterone levels are an independent predictor of low bone density in both boys and girls with AN [7, 48]. Estrogen is primarily antiresorptive and decreases osteoclast differentiation and activity through its effect on pro-inflammatory cytokines and the RANK-RANKL-osteoprotegerin (OPG) pathway [52]. It inhibits secretion of the proinflammatory cytokines and increases OPG, with a consequent reduction in osteoclast differentiation and activation and an increase in osteoclast apoptosis. A state of hypogonadism would thus be expected to result in an increase in pro-inflammatory cytokines and a decrease in OPG. Levels of cytokines such as IL-6 are elevated in AN [27]; however, OPG levels are also high [53]. OPG levels correlate inversely with bone density measures suggesting that high OPG levels in AN may be an adaptive response to preserve bone mass. Testosterone has direct anabolic effects on bone but a major impact of its effect on bone metabolism is through its aromatization to estrogen [54].
Importantly, contrary to expectations, bone density does not increase with administration of oral estrogen in both adults and adolescent females with AN [51, 55, 56]. This has been attributed to IGF-1 and androgen suppressive effect of oral estrogens, although it is also possible that weight normalization is necessary for beneficial effects of estrogen replacement on bone. In addition, oral contraceptives may further decrease IGF-1 levels, and in adolescents, the doses of estrogen in such preparations may not be physiological. The effect of testosterone replacement on bone density in hypogonadal males with AN has not been reported. Data regarding levels of adrenal androgens in AN are conflicting with studies indicating either normal or low levels of DHEAS in adolescents and young adults with AN [48, 57]. Long-term DHEA administration does not increase bone density in AN after controlling for effects of weight gain [57].
GH Resistance and Low IGF-1
Another important cause of low bone density in AN is the state of GH resistance, with low levels of IGF-1, a hormone known to be anabolic to bone [11]. GH has direct and IGF-1 mediated effects on osteoblast and osteoclast differentiation and activity, and low IGF-1 levels in AN predict both lower bone density and lower levels of bone formation markers [6]. In addition, whereas GH concentrations are positive predictors of bone turnover markers in healthy adolescent girls, this association is absent in girls with AN, suggesting that the resistance to GH effects seen at the liver, may also occur in bone [11]. Increases in IGF-1 levels seen with weight gain are associated with increases in markers of bone formation and in bone density [6]. Short-term administration of rhIGF-1 at a dose of 30 mcg/kg twice daily selectively increases bone formation markers in adults with AN within days [58]. A higher dose of 100 µg/kg twice daily increases markers of both bone formation and bone resorption. Chronic administration of rhIGF-1 (30 µg/kg s.c. doses twice daily) with daily estrogen given as an oral contraceptive for nine months (an anabolic and an antiresorptive agent, respectively) caused a significant increase in spine bone density in adult women with AN [58]. This effect was not seen with estrogen alone, highlighting the importance of an anabolic agent in the treatment of this low formation state. This combination was associated with an increase in surrogate markers of bone formation and a decrease in surrogate markers of bone resorption, consistent with known effects of IGF-1 and estrogen on bone. It is important to emphasize that IGF-1 is locally produced by bone and the impact of circulating versus locally produced IGF-1 is unknown.
Other Hormones
Hypercortisolemia has multiple deleterious effects on bone. Cortisol excess inhibits osteoblasts and stimulates osteoclasts, impairs calcium absorption from the gut and the renal handling of calcium, and impacts negatively on the GH-IGF-1 axis. Cortisol levels are higher in both adults and adolescents with AN than in controls, and are an important and inverse predictor of bone density [21, 49]. In girls with AN, elevated cortisol concentrations independently predicted low levels of bone formation markers [21], again emphasizing the importance of weight gain and weight maintenance in AN.
Other possible determinants of low bone density include high levels of adiponectin (in relation to fat mass) [27], ghrelin [59] and PYY [26, 60] and low levels of insulin and leptin [27]. Adiponectin has been demonstrated to increase osteoclast activity through effects on RANKL and OPG, and ghrelin increases osteoblast activity in in vitro models. In addition, PYY, which acts through the Y2 receptor of NPY, may impact osteoblast activity based on data from the Y2 receptor knockout mouse, which demonstrates increased osteoblastic activity and high bone mass [61]. Thyroid hormones stimulate local IGF-1 secretion at the level of bone, and low total T3 levels may contribute to lower bone density by reducing local IGF-1 secretion. In adolescents with AN, high PYY and low levels of insulin are important predictors of lower levels of bone turnover markers [26, 27]. High PYY predicts lower bone density in adults with AN [60], and adiponectin is an inverse predictor of bone density measures in adolescents with AN [27]. Ghrelin was an independent predictor of bone density in controls, but not in an AN population, and one may postulate a resistance to ghrelin effects in AN given that effects of ghrelin on bone in controls seem to be mediated through increases in GH and cortisol by ghrelin [59].
Body Composition
Both fat and lean mass are lower in adults and adolescents with AN as compared to normal-weight controls. Regional fat distribution is also affected with a decrease in percent trunk fat and a decrease in the trunk/extremity fat ratio in adult and adolescent females with AN [23, 28]. With weight gain, percent trunk fat and trunk/extremity fat in adolescent girls with AN increase and approximate that seen in healthy adolescent girls [28]. In contrast, weight gain in adults with AN is associated with an increase in percent trunk fat during recovery such that this may exceed percent trunk fat in healthy adults [23]. Reassuringly, MRI measures of body composition indicate that although there is an acute increase in visceral fat with weight gain in adult women with AN to exceed that seen in controls, this normalizes over time with sustained weight gain. In adolescent girls with AN, high GH concentrations predict lower trunk fat, whereas high cortisol levels predict lower extremity lean mass [24]. Boys with AN have a sparing of trunk fat unlike females with AN, which appears to be related to their low testosterone levels [62].
Statural Growth
Reports have indicated lower than expected, normal and even higher than expected stature in girls with AN compared with genetic potential or controls [63–65]. Similarly, both height deficits and height within range of genetic potential have been reported in boys with AN [7, 66]. Whether or not height is affected may depend on the age of onset, severity and duration of the disease, and the pubertal stage of the specific individual. Greater severity and longer duration of the disorder, and onset of AN before puberty or in early puberty (when significant growth potential exists) may cause statural deficits. In contrast, later onset of AN (when growth is almost complete) would not be expected to cause significant statural deficits. Studies that have reported no change in height potential or height SDS in AN suggest that a delay in bone age associated with high GH concentrations (and direct GH effects on the growth plate) may cause a sparing of height deficits in adolescents with AN, particularly when the disease is not very severe [63].
Conclusion
AN is associated with adaptive changes in multiple endocrine systems leading to hypogonadotropic hypogonadism, a nutritionally acquired resistance to GH with low IGF-1 levels, relative hypercortisolemia, low levels of insulin and leptin, and high levels of ghrelin and adiponectin. In addition, PYY levels are high in AN. The adaptive changes, however, can cause significant pathology, particularly in the context of bone metabolism. Low bone density and impaired bone microarchitecture are important consequences of nutritional, body composition and hormonal changes in AN and in some patients bone deficits can persist throughout adult life.
Acknowledgments
Grant Support
This work was supported in part by NIH grant K23 RR018851.
The author has consulted for Ipsen within the past year and has previously received grant support from Tercica.
References
- 1.Diagnostic and Statistical Manual of Mental Disorders. ed 3. Washington: American Psychiatric Association; 1987. [Google Scholar]
- 2.Lucas AR, Beard CM, O’Fallon WM, Kurland LT. 50-year trends in the incidence of anorexia nervosa in Rochester, Minn.: a population-based study. Am J Psychiatry. 1991;148:917–922. doi: 10.1176/ajp.148.7.917. [DOI] [PubMed] [Google Scholar]
- 3.Misra M, Tsai P, Anderson EJ, et al. Nutrient intake in community-dwelling adolescent girls with anorexia nervosa and in healthy adolescents. Am J Clin Nutr. 2006;84:698–706. doi: 10.1093/ajcn/84.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misra M, Aggarwal A, Miller KK, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114:1574–1583. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 5.Boyar RM, Katz J, Finkelstein JW, et al. Anorexia nervosa. Immaturity of the 24-hour luteinizing hormone secretory pattern. N Engl J Med. 1974;291:861–865. doi: 10.1056/NEJM197410242911701. [DOI] [PubMed] [Google Scholar]
- 6.Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87:4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 7.Misra M, Katzman DK, Cord J, et al. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab. 2008;93:3029–3036. doi: 10.1210/jc.2008-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra M, Prabhakaran R, Miller KK, et al. Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa. Pediatr Res. 2006;59:598–603. doi: 10.1203/01.pdr.0000203097.64918.63. [DOI] [PubMed] [Google Scholar]
- 9.Misra M, Miller K, Kuo K, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E347–E356. doi: 10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- 10.Misra M, Miller KK, Kuo K, et al. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E373–E381. doi: 10.1152/ajpendo.00041.2005. [DOI] [PubMed] [Google Scholar]
- 11.Misra M, Miller K, Bjornson J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88:5615–5623. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- 12.Scacchi M, Pincelli A, Caumo A, et al. Spontaneous nocturnal growth hormone secretion in anorexia nervosa. J Clin Endocrinol Metab. 1997;82:3225–3229. doi: 10.1210/jcem.82.10.4275. [DOI] [PubMed] [Google Scholar]
- 13.Counts D, Gwirtsman H, Carlsson L, Lesem M, Cutler G. The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75:762–767. doi: 10.1210/jcem.75.3.1381372. [DOI] [PubMed] [Google Scholar]
- 14.Takaya K, Ariyasu H, Kanamoto N, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908–4911. doi: 10.1210/jcem.85.12.7167. [DOI] [PubMed] [Google Scholar]
- 15.Stoving R, Veldhuis J, Flyvbjerg A, et al. Jointly amplified basal and pulsatile growth hormone secretion and increased process irregularity in women with anorexia nervosa: indirect eveidence for disruption of feedback regulatin within the growth hormone-insulin like growth factor-I axis. J Clin Endocrinol Metab. 1999;84:2056–2063. doi: 10.1210/jcem.84.6.5734. [DOI] [PubMed] [Google Scholar]
- 16.Stoving RK, Andersen M, Flyvbjerg A, et al. Indirect evidence for decreased hypothalamic somatostatinergic tone in anorexia nervosa. Clin Endocrinol (Oxf) 2002;56:391–396. doi: 10.1046/j.1365-2265.2002.01485.x. [DOI] [PubMed] [Google Scholar]
- 17.Misra M, Miller KK, Herzog DB, et al. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. J Clin Endocrinol Metab. 2004;89:1605–1612. doi: 10.1210/jc.2003-031861. [DOI] [PubMed] [Google Scholar]
- 18.Grinspoon S, Miller K, Herzog D, Clemmons D, Klibanski A. Effects of recombinant human insulin-like growth factor (IGF)-I and estrogen administration on IGF-I, IGF binding protein (IGFBP)-2, and IGFBP-3 in anorexia nervosa: a randomized-controlled study. J Clin Endocrinol Metab. 2003;88:1142–1149. doi: 10.1210/jc.2002-021402. [DOI] [PubMed] [Google Scholar]
- 19.Stoving RK, Flyvbjerg A, Frystyk J, et al. Low serum levels of free and total insulin-like growth factor I (IGF-I) in patients with anorexia nervosa are not associated with increased IGF-binding protein-3 proteolysis. J Clin Endocrinol Metab. 1999;84:1346–1350. doi: 10.1210/jcem.84.4.5622. [DOI] [PubMed] [Google Scholar]
- 20.Munoz MT, Argente J. Anorexia nervosa in female adolescents: endocrine and bone mineral density disturbances. Eur J Endocrinol. 2002;147:275–286. doi: 10.1530/eje.0.1470275. [DOI] [PubMed] [Google Scholar]
- 21.Misra M, Miller KK, Almazan C, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89:4972–4980. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 22.Lawson EA, Misra M, Meenaghan E, et al. Adrenal glucocorticoid and androgen precursor dissociation in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:1367–1371. doi: 10.1210/jc.2008-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinspoon S, Thomas L, Miller K, et al. Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. Am J Clin Nutr. 2001;73:865–869. doi: 10.1093/ajcn/73.5.865. [DOI] [PubMed] [Google Scholar]
- 24.Misra M, Miller KK, Almazan C, et al. Hormonal determinants of regional body composition in adolescent girls with anorexia nervosa and controls. J Clin Endocrinol Metab. 2005;90:2580–2587. doi: 10.1210/jc.2004-2041. [DOI] [PubMed] [Google Scholar]
- 25.Leslie RD, Isaacs AJ, Gomez J, Raggatt PR, Bayliss R. Hypothalamo-pituitary-thyroid function in anorexia nervosa: influence of weight gain. Br Med J. 1978;ii:526–528. doi: 10.1136/bmj.2.6136.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027–1033. doi: 10.1210/jc.2005-1878. [DOI] [PubMed] [Google Scholar]
- 27.Misra M, Miller KK, Cord J, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92:2046–2052. doi: 10.1210/jc.2006-2855. [DOI] [PubMed] [Google Scholar]
- 28.Misra M, Soyka L, Miller K, et al. Regional body composition in adolescents with anorexia nervosa and changes with weight recovery. Am J Clin Nutr. 2003;77:1361–1367. doi: 10.1093/ajcn/77.6.1361. [DOI] [PubMed] [Google Scholar]
- 29.Housova J, Anderlova K, Krizova J, et al. Serum adiponectin and resistin concentrations in patients with restrictive and binge/purge form of anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2005;90:1366–1370. doi: 10.1210/jc.2004-1364. [DOI] [PubMed] [Google Scholar]
- 30.Pannacciulli N, Bunt JC, Ortega E, et al. Lower total fasting plasma adiponectin concentrations are associated with higher metabolic rates. J Clin Endocrinol Metab. 2006;91:1600–1603. doi: 10.1210/jc.2005-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tagami T, Satoh N, Usui T, et al. Adiponectin in anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2004;89:1833–1837. doi: 10.1210/jc.2003-031260. [DOI] [PubMed] [Google Scholar]
- 32.Misra M, Miller KK, Almazan C, et al. Hormonal and body composition predictors of soluble leptin receptor, leptin, and free leptin index in adolescent girls with anorexia nervosa and controls and relation to insulin sensitivity. J Clin Endocrinol Metab. 2004;89:3486–3495. doi: 10.1210/jc.2003-032251. [DOI] [PubMed] [Google Scholar]
- 33.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 34.Soriano-Guillen L, Barrios V, Campos-Barros A, Argente J. Ghrelin levels in obesity and anorexia nervosa: effect of weight reduction or recuperation. J Pediatr. 2004;144:36–42. doi: 10.1016/j.jpeds.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92:3202–3205. doi: 10.1210/jc.2007-0593. [DOI] [PubMed] [Google Scholar]
- 36.Vulliemoz NR, Xiao E, Xia-Zhang L, et al. Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab. 2004;89:5718–5723. doi: 10.1210/jc.2004-1244. [DOI] [PubMed] [Google Scholar]
- 37.Nakahara T, Harada T, Yasuhara D, et al. Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry. 2008;64:252–255. doi: 10.1016/j.biopsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 39.Grinspoon S, Thomas E, Pitts S, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133:790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84:4489–4496. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 41.Misra M, Prabhakaran R, Miller KK, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93:1231–1237. doi: 10.1210/jc.2007-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milos G, Spindler A, Ruegsegger P, et al. Cortical and trabecular bone density and structure in anorexia nervosa. Osteoporos Int. 2005;16:783–790. doi: 10.1007/s00198-004-1759-2. [DOI] [PubMed] [Google Scholar]
- 43.Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bredella MA, Misra M, Miller KK, et al. Distal radius in adolescent girls with anorexia nervosa: trabecular structure analysis with high-resolution flat-panel volume CT. Radiology. 2008;249:938–946. doi: 10.1148/radiol.2492080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro J, Toro J, Lazaro L, Pons F, Halperin I. Bone mineral density in male adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2002;41:613–618. doi: 10.1097/00004583-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Grinspoon S, Baum H, Lee K, et al. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3864–3870. doi: 10.1210/jcem.81.11.8923830. [DOI] [PubMed] [Google Scholar]
- 47.Misra M, Prabhakaran R, Miller KK, et al. Prognostic indicators of changes in bone density measures in adolescent girls with anorexia nervosa-II. J Clin Endocrinol Metab. 2008;93:1292–1297. doi: 10.1210/jc.2007-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soyka L, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anroexia nervosa. J Clin Endocrinol Metab. 2002;87:4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 49.Biller B, Saxe V, Herzog D, et al. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68:548–554. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- 50.Miller KK, Lee EE, Lawson EA, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91:2931–2937. doi: 10.1210/jc.2005-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klibanski A, Biller B, Schoenfeld D, Herzog D, Saxe V. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898–904. doi: 10.1210/jcem.80.3.7883849. [DOI] [PubMed] [Google Scholar]
- 52.Riggs B. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106:1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Misra M, Soyka LA, Miller KK, et al. Serum osteoprotegerin in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2003;88:3816–3822. doi: 10.1210/jc.2003-030088. [DOI] [PubMed] [Google Scholar]
- 54.Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 55.Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135–143. doi: 10.1016/s1083-3188(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 56.Strokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: a double-blind, placebo-controlled study. J Adolesc Health. 2006;39:819–827. doi: 10.1016/j.jadohealth.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935–4941. doi: 10.1210/jc.2002-020545. [DOI] [PubMed] [Google Scholar]
- 58.Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87:2883–2891. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- 59.Misra M, Miller KK, Stewart V, et al. Ghrelin and bone metabolism in adolescent girls with anorexia nervosa and healthy adolescents. J Clin Endocrinol Metab. 2005;90:5082–5087. doi: 10.1210/jc.2005-0512. [DOI] [PubMed] [Google Scholar]
- 60.Utz AL, Lawson EA, Misra M, et al. Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone. 2008;43:135–139. doi: 10.1016/j.bone.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baldock PA, Sainsbury A, Couzens M, et al. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Misra M, Katzman DK, Cord J, et al. Percentage extremity fat, but not percentage trunk fat, is lower in adolescent boys with anorexia nervosa than in healthy adolescents. Am J Clin Nutr. 2008;88:1478–1484. doi: 10.3945/ajcn.2008.26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prabhakaran R, Misra M, Miller KK, et al. Determinants of height in adolescent girls with anorexia nervosa. Pediatrics. 2008;121:e1517–e1523. doi: 10.1542/peds.2007-2820. [DOI] [PubMed] [Google Scholar]
- 64.Roze C, Doyen C, Le Heuzey MF, et al. Predictors of late menarche and adult height in children with anorexia nervosa. Clin Endocrinol (Oxf) 2007;67:462–467. doi: 10.1111/j.1365-2265.2007.02912.x. [DOI] [PubMed] [Google Scholar]
- 65.Nussbaum M, Baird D, Sonnenblick M, Cowan K, Shenker IR. Short stature in anorexia nervosa patients. J Adolesc Health Care. 1985;6:453–455. doi: 10.1016/s0197-0070(85)80052-8. [DOI] [PubMed] [Google Scholar]
- 66.Modan-Moses D, Yaroslavsky A, Novikov I, et al. Stunting of growth as a major feature of anorexia nervosa in male adolescents. Pediatrics. 2003;111:270–276. doi: 10.1542/peds.111.2.270. [DOI] [PubMed] [Google Scholar]