Abstract
Adipose-derived stem cells (ADSCs) are an attractive cell source for tissue engineering, and recently a modified aggregate culture of human ADSCs (hADSCs) was established based on preparation of three-dimensional (3D) cell aggregates in growth factor–enriched low serum medium using the hanging droplet method. Growth and differentiation factor 5 (GDF5) plays a critical role in chondrogenesis and cartilage development. In the present study, we examine (1) whether the modified aggregate culture is feasible for chondrogenic induction of hADSCs, (2) whether overexpressed GDF5 can promote chondrogenesis, and (3) the gene expression profile during chondrogenesis in this aggregate culture. hADSCs were infected with an adenovirus carrying the GDF5 gene (Ad-GDF5). Cells were cultured with chondrogenic media either in a modified aggregate culture or in an attached micromass culture that served as a control. The chondrogenic phenotype was assessed by morphology (n=8), biochemistry (n=3), and histology (n=2). Expression of 12 genes was determined by quantitative real-time polymerase chain reaction (n=3). We found that ADSCs cultured in the modified aggregates exhibited denser pellets and higher content of sulfated glycosaminoglycan (sGAG) compared with those cultured in the micromass. Infection of cells with Ad-GDF5 increased the aggregate size and sGAG content. It also up-regulated expression of GDF5, aggrecan, and leptin and down-regulated expression of COL I, while expression of COL II and COL 10 remained unchanged. We concluded that the modified aggregate culture is feasible for chondrogenic induction of human ADSCs. Infection with Ad-GDF5 appears to promote the chondrogenesis. These findings suggest that genetic modification of ADSCs with GDF5 in the modified aggregate culture could be useful for treating diseases with cartilage defects.
Key words: aggregate culture, cartilage regeneration, chondrogenesis, GDF5, genetic modification, human adipose-derived stem cell
Introduction
Degeneration of articular cartilage is commonly accompanied by trauma, malposition, and aging. It may cause severe clinical problems, such as substantial joint pain and degenerative arthritis due to the poor intrinsic capacity of injured cartilage for healing.1 Autologous chondrocyte implantation and matrix-associated chondrocyte implantation have proven to be effective surgical techniques for management of articular cartilage defects.2,3 However, both involve a minimum of two operations; the first is to harvest healthy cartilage biopsy for chondrocyte isolation and expansion, which imply donor site morbidity and chondrocyte dedifferentiation, respectively.1,2,4,5 Therefore, various groups have sought alternative cell sources such as mesenchymal stem cells (MSCs).6 Among the various sources, adipose tissue has been suggested to provide a superior stem cell source (adipose-derived stem cells [ADSCs]) due to their higher yield and the minimally invasive procedure to collect them.1,4,7
Chondrogenic induction of stem cells has been carried out in the presence of scaffolds4 or in their absence.8–11 In the case of scaffold-free chondrogenesis, cells are commonly cultured in either pellets8,9 or micromass10,11 to maintain a high density state. In an initial attempt to explore an optimal delivery method of human ADSCs (hADSCs) for therapeutic purpose, we established a technique for preparing three-dimensional (3D) cell aggregates in growth factor–enriched low serum medium with the hanging droplet method.12
Multiple growth factors have been explored to stimulate MSC chondrogenesis, such as transforming growth factor β,13 insulin-like growth factor 1,13 fibroblast growth factor 2,13 and growth and differentiation factor 5 (GDF5).8,9,14,15 Among these growth factors, GDF5, also called bone morphogenetic protein 1416 or cartilage-derived morphogenetic protein 1,8,15,17–19 regulates a variety of musculoskeletal processes, including chondrogenesis,8,9,14,15 joint formation,15,20 maintenance or repair of intervertebral disc,20–23 and tendon and ligament maintenance.24–27 Specifically, we demonstrated that overexpression of GDF5 via infection with an adenovirus carrying a human GDF5 gene fragment (Ad-GDF5) significantly augmented chondrogenesis of rat ADSCs.9
Based on our previous findings,9,12 we determined the following in the present study: (1) whether the reported 3D aggregates formulated as modified aggregates were able to induce chondrogenesis, (2) whether overexpressed GDF5 could enhance chondrogenesis of hADSCs with the modified aggregate culture, and (3) if changes in gene expression occurred with regard to this chondrogenesis.
Materials and Methods
Study design
Our study design with four experimental groups is depicted in Table 1. Variables included the culture method and cell type. Basic medium (BM) was Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 50 μg/mL ascorbic acid, while chondrogenic medium (CM) was DMEM with 1% FBS, 1% penicillin/streptomycin, 50 μg/mL ascorbic acid, 10 nmol/L dexamethasone, and 1% ITS-Premix.13 Experimental groups C and D were designed for the first purpose, and the cells were infected with Ad-GDF5 to prepare modified aggregates12 or micromass cultures.10,11 Micromass cultures were employed as control due to their simple preparation. Modified aggregates made from cells with or without Ad-GDF5 infection were employed in groups A–C sequentially denoted as non–Ad-GDF5/BM, non–Ad-GDF5/CM, and Ad-GDF5/CM for the other two purposes. Three replicates were performed for either proteoglycan or gene expression analysis, and each sample contained three aggregates or micromasses. An additional two aggregates or micromasses were applied to cryosectioning for histological and immunostaining analysis.
Table 1.
Experimental Groups in This Study
| Group | Culture method | Cell/Ad-GDF5 | Medium |
|---|---|---|---|
| A | Aggregate | No | BM |
| B | Aggregate | No | CM |
| C | Aggregate | Yes | CM |
| D | Micromass | Yes | CM |
BM, basic medium; CM, chondrogenic medium.
Cell isolation and treatment
We obtained human adipose tissue from surgical waste of a nondiabetic patient (29-year-old woman with body mass index of 45.4 Kg/m2) who underwent liposuction at University of Virginia Medical Center. The surgical waste usage protocol was reviewed and approved by the internal research board of University of Virginia.
ADSCs were isolated using previously described methods.28 Briefly, tissue was washed with complete Hanks buffer and then isolated with enzyme digestion and filtration. Pelleted stromal cells were filtered twice with 250-μm and 105-μm mesh, and the erythrocytes were removed with osmotic buffer. Cells were cultured in DMEM:Nutrient Mixture F-12 (DMEM/F-12) with 10% FBS and 1% antibiotic-antimycotic at 37°C in a humidified incubator (Forma Scientific Inc., Marietta, OH). Nonadherent cells were removed 24–48 h after plating. Culture medium was changed every 2 days.
We generated an adenovirus carrying the GDF5 gene (Ad-GDF5) using the AdEasy™ adenoviral vector system (Stratagene, La Jolla, CA) as described previously.9 We infected the human ADSCs at passage 3 with Ad-GDF5 at a multiplicity of infection of 150 for 24 h.
For the modified cell aggregate culture, cells were suspended at a concentration of 125,000 cells/mL in growth factor-enriched low-serum medium (DMEM/F-12, 0.1 mmol/L L-glutamine, 10−8 mol/L dexamethasone, 100 μmol/L ascorbic acid 2-phosphate, 0.50% ITS+3, 0.05% fatty acid supplement, 1% nonessential amino acids, 10−8 mol/L estradiol, 10−8 mol/L progesterone, 500 ng/mL hydrocortisone, 10 ng/mL epidermal growth factor, 1 ng/mL platelet-derived growth factor, 1 ng/mL stem cell growth factor β, 1 ng/mL tumor necrosis factor α, 1 ng/mL interleukin 1β, 1% antibiotic/antimycotic) with 1% human serum.11 Forty microliters of cell suspension was dropped on a culture dish lid, and the lid was inverted to induce aggregate formation using a “hanging droplets” method.29,30 After 24 h of culture, cells in the resulting hanging droplets formed 3D aggregates comprised of ∼5000 cells, and the aggregates were transferred into ultralow-attachment culture plates (Corning, Lowell, MA) for different treatments.
For the micromass culture, ADSCs were suspended at a concentration of 250,000 cells/mL in DMEM/F-12 with 10% FBS and 1% antibiotic-antimycotic. Twenty-microliter droplets were placed in the bottom of an adhesive 96-well culture plate and seated for 2 h before addition of extra medium. The aggregates were treated with either BM or CM.9 All cultures were maintained up to 3 weeks with medium changed every 2 days. Aggregates were harvested at 1, 2, or 3 weeks for morphology assessment. Microphotographs of aggregates were taken under an inverted light microscope, and the sizes of eight aggregates in each group were analyzed with the software ImageJ (National Institutes of Health, Bethesda, MD), expressed as fold of change and normalized to non-GDF5/BM group at 1 week.
Quantitative real-time polymerase chain reaction, biochemical, histological, and immunostaining analyses
For real-time reverse-transcription polymerase chain reaction (PCR), we isolated total RNA using the RNeasy® Mini kit (Qiagen, Valencia, CA) and synthesized cDNA with the Reverse Transcription System kit (Promega, Madison, WI), following the manufacturers' instructions. Real-time PCR was performed with the QuantiTect® SYBR Green PCR master mix (Qiagen). Gene expression was calculated using the delta delta Ct method31 and normalized to 18S, and expressed as fold of change. Expression of all genes except for leptin was expressed as fold of change over the non–Ad-GDF5/BM group. For leptin, since the expression in this group was not detectable, gene expression was expressed as fold of change over the non–Ad-GDF5/CM group. The primer sequences are listed in Table 2.
Table 2.
Primer Sequences Used for Real-Time Polymerase Chain Reaction
| Molecule | Primer sequence | Size of product (bp) |
|---|---|---|
| Growth and differentiation factor 5 | 5′-ATG AGA CTC CCC AAA CTC CTC AC-3′ (sense) | 237 |
| 5′-GCC TCC CTT TGC CCT GGC ATT-3′ (antisense) | ||
| Aggrecan | 5′-CGC TAC TCG CTG ACC TTT-3′ (sense) | 106 |
| 5′-GCT CAT AGC CTG CTT CGT-3′ (antisense) | ||
| Collagen Ia1 | 5′-GCC ATC AAA GTC TTC TGC-3′ (sense) | 145 |
| 5′-ATC CAT CGG TCA TGC TCT-3′ (antisense) | ||
| Collagen IIa1 | 5′-TCC CAG AAC ATC ACC TAC C-3′ (sense) | 131 |
| 5′-AAC CTG CTA TTG CCC TCT-3′ (antisense) | ||
| Collagen 10a1 | 5′-TGC TAG TAT CCT TGA ACT TGG TTC AT-3′ (sense) | 98 |
| 5′-CTG TGT CTT GGT GTT GGG TAG TG-3′ (antisense) | ||
| Collagen 15a1 | 5′-GTT GTC CAC CTA CCG AGC AT-3′ (sense) | 197 |
| 5′-TGT CTC GAC CAT CAA AGG AG-3′ (antisense) | ||
| Glypican 3 | 5′-AAT GAA GGG CCC TGA GC-3′ (sense) | 228 |
| 5′-GCC AGT TCT GCA AGG AAG C-3′ (antisense) | ||
| Keratin 19 | 5′-GCA CTA CAG CCA CTA CTA CAC GA-3′ (sense) | 159 |
| 5′-CTC ATG CGC AGA GCC TGT T-3′ (antisense) | ||
| Matrix metalloproteinase 3 | 5′-TGA AGA GTC TTC CAA TCC TAC TGT TG-3′ (sense) | 113 |
| 5′-CTA GAT ATT TCT GAA CAA GGT TCA TGC-3′ (antisense) | ||
| CD105 | 5′-GCCAGCATT GTCTCACTTCATG-3′ (sense) | 176 |
| 5′-GCAACAAGCTCTTTCT TTAGTACCA-3′ (antisense) | ||
| Hypoxia inducible factor 1α | 5′-GTC GCT TCG GCC AGT GTG-3′ (sense) | 152 |
| 5′-GGA AAG GCA AGT CCA GAG GTG-3′ (antisense) | ||
| Leptin | 5′-GTG CGG ATT CTT GTG GCT TT-3′ (sense) | 174 |
| 5′-GGA ATG AAG TCC AAA CCG GTG-3′ (antisense) | ||
| 18S | 5′-GTG ACC AGT TCA CTC TTG GT-3′ (antisense) | 99 |
| 5′-GAA TCG AAC CCT GAT TCC CCG TC-3′ (antisense) |
For biochemical analyses, cell aggregates or micromasses were digested in 200 μL of papain digestion buffer (United States Biological, Swampscott, MA; prepared 125 mg/mL in sterile phosphate-buffered saline, pH 6.0, with 5 mmol/L cysteine hydrochloride) for 18 h at 60°C. Sulfated glycosaminoglycan (sGAG) levels were measured with a spectrophotometer after incubation with 1,9-dimethylmethylene blue-chloride (Polysciences, Inc., Warrington, PA) dye and normalized to total DNA. DNA concentration was measured with the bisbenzimide Hoechst 33258 DNA quantification kit (Bio-Rad Laboratories, Inc., Hercules, CA) using calf thymus DNA (Sigma-Aldrich, St. Louis, MO) as standard.5
For histology and immunostaining, cell aggregates were fixed with 4% paraformaldehyde at room temperature for 3 h and embedded with Tissue-Tek® O.C.T Compound (Sakura Finetek USA, Inc., Torrance, CA). Cryo-microsections (6 μm) were prepared and stained with Safranin O. Immunochemical staining of collagen II was also performed with a mouse antihuman collagen II antibody together with a mouse immunoCruz™ system according to the manufacturer's protocol (Santa Cruz Biotechnology, Inc., Dallas, TX). Briefly, sections were treated with 2% bovine testicular hyaluronidase for 30 min at 25°C, followed by incubation with a mouse monoclonal antibody (COL2A1 [003-02]; Santa Cruz Biotechnology, Inc.) at a dilution of 1:250 for 1 h at room temperature. After color development with the mouse immunoCruz system, cells were counterstained for 10 min at room temperature with a fluorescent DNA dye YOYO-1 (Life Technologies, Grand Island, NY). Microphotographs were taken under an Olympus BX51 microscope equipped with an Olympus DP70 digital camera (Olympus America Inc., Center Valley, PA).
Data analysis
Data from aggregate/micromass size, biochemical assay, and gene expression analysis were expressed as mean±SD. Statistical evaluation was performed with two-tailed Student's t-test using Microsoft Excel® (Microsoft Corporation, Redmond, WA) to determine the difference between two groups.
Results
Modified aggregate culture is superior to micromass culture for chondrogenesis
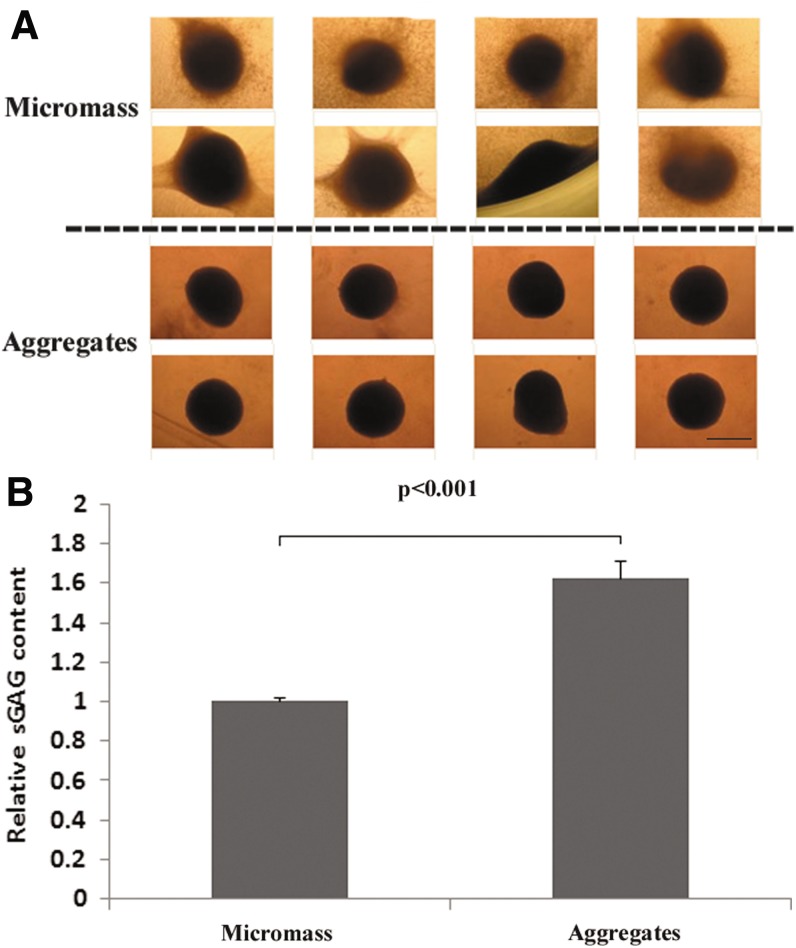
After 3 weeks' culture, the aggregates made from the hanging droplet method were dense and spherical with few cells growing outward, whereas micromass cultures were irregular or sphere-like with cells growing outward. In addition, micromass cultures occasionally had aggregates attached to the side of the plate (Fig. 1A). Furthermore, biochemical analysis showed aggregates produced higher (150%; p<0.001) amounts of sGAG than micromass cultures produced (Fig. 1B).
FIG. 1.
Chondrogenesis of human adipose-derived stem cells (ADSCs) were improved by a modified aggregate culture. ADSCs were cultured in aggregates or in micromass with CM for 3 weeks. (A) The morphology of eight individuals cultured in aggregates or in micromass. The aggregates were dense and spherical with few cells growing outward, whereas micromass cultures were irregular or sphere-like with cells growing outward. Scale bar=400 μm. (B) Glycosaminoglycan (GAG) content. The aggregates or micromass cultures were digested with papain, and the sulfated glycosaminoglycan (sGAG) and DNA content were measured as described in the Materials and Methods. Relative sGAG content was obtained by normalizing sGAG content to DNA content and further to micromass cultures. ADSCs in aggregates produced higher (150%) amounts of sGAG than those in micromass cultures.
Overexpressed GDF5 enhanced cell aggregate size and matrix production during chondrogenesis
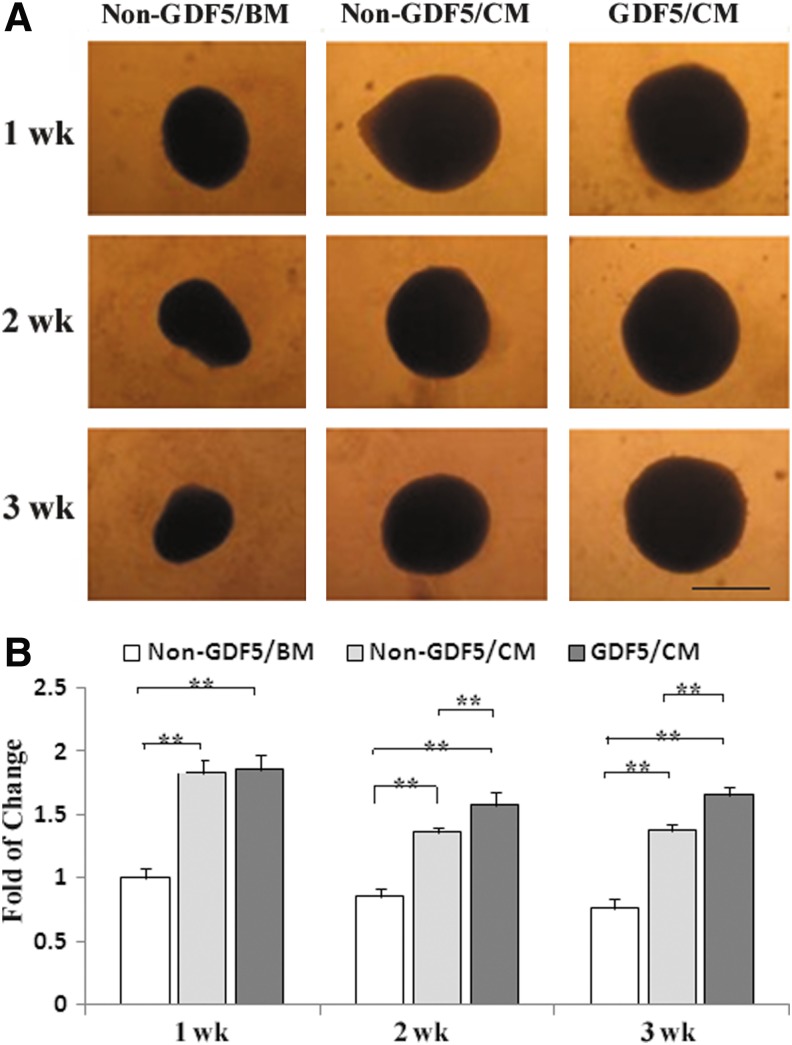
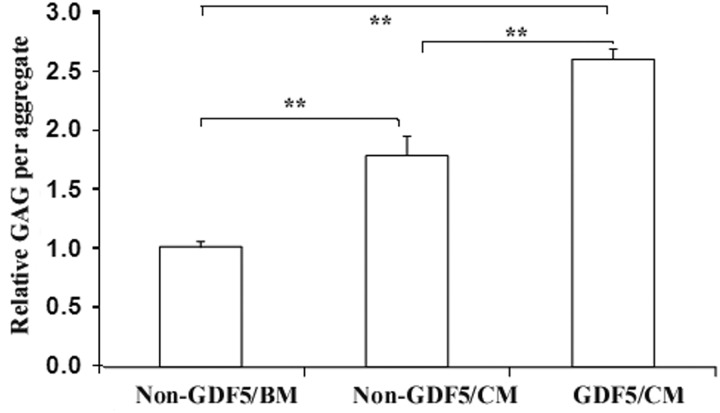
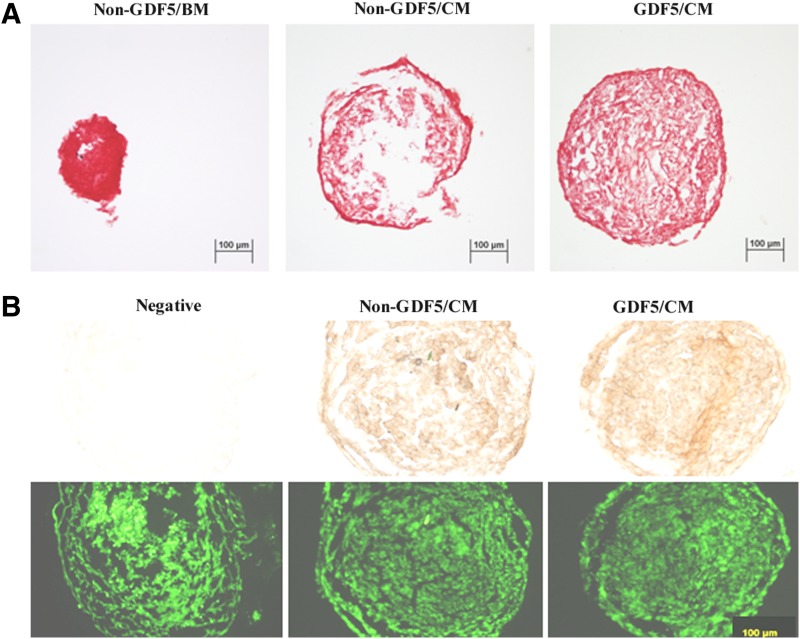
The aggregate sizes were at their maximum at week 1 for all three groups (Ad-GDF5/CM=non–Ad-GDF5/CM>non–Ad-GDF5/BM; Fig. 2). At weeks 2 and 3, aggregates in the Ad-GDF5/CM group were larger (p<0.01) than in the non–Ad-GDF5/CM group. The content of sGAG in the aggregates was significantly different among these three groups. Compared to non–Ad-GDF5/BM, non–Ad-GDF5/CM and Ad-GDF5/CM had an 80% and 160% increase, respectively (Fig. 3). Safranin O staining revealed a similar result, and strong staining was seen in every group (Fig. 4). However, immunostaining analysis showed no apparent difference in COL II between non–Ad-GDF5/CM and Ad-GDF5/CM (Fig. 4).
FIG. 2.
Growth and differentiation factor 5 (GDF5) promotes chondrogenesis of human ADSCs. ADSCs with GDF5 overexpression formed a larger aggregate. ADSCs with (GDF5) or without Ad-GDF5 infection (non-GDF5) were cultured in basic or chondrogenic medium (BM or CM, respectively) for up to 3 weeks. (A) The morphology was photographed at 1, 2, and 3 weeks. Bar=300 μm. (B) The aggregate size was measured and analyzed. The bar graph shows quantification of the aggregate size. **p<0.001.
FIG. 3.
GDF5 enhances the contents of sGAG in human ADSCs. ADSCs with (GDF5) or without Ad-GDF5 (non-GDF5) infection were cultured in aggregates with BM or CM for 3 weeks, and the sGAG content was measured as described in the Materials and Methods. Relative sGAG per aggregate was obtained by normalizing sGAG content per aggregate to the non-GDF5/BM group. **p<0.001.
FIG. 4.
Safranin O staining of aggregates (A) and immunostaining of type II collagen in aggregates (B, top) of human adipose stem cells with (GDF5) or without (non-GDF5) infection of ad-GDF5 and cultured in BM or CM for 3 weeks. Counterstaining was performed with a DNA fluorescent dye YOYO-1 (B, bottom). The negative control group was the non-GDF5/CM group without incubation of the primary antibody. Bar=100 μm.
Gene expression profiles during chondrogenesis
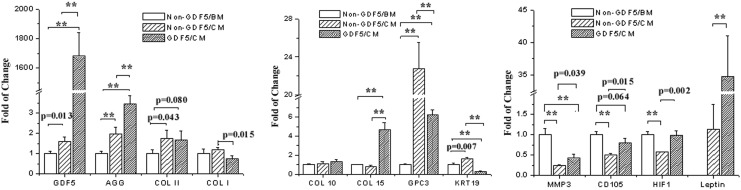
Twelve genes in total were investigated in aggregates cultured in different conditions for 3 weeks by real-time RT-PCR analysis, including a growth factor, GDF5; matrix proteins, aggrecan (AGG), collagens type I (COL I), II (COL II), 10 (COL 10), and 15 (COL 15), glypican 3 (GPC3), and keratin 19 (KRT19); a matrix degrading enzyme, matrix metalloproteinase 3 (MMP3); an MSC surface marker, endoglin (CD105); a transcript factor, hypoxia inducible factor 1α (HIF1); and an adipose-derived hormonal protein, leptin [Ob(Lep)] (Fig. 5). Compared to the non–Ad-GDF5/BM group, the mRNA level of six genes was elevated in either the non–Ad-GDF5/CM group [GDF5, AGG, COL II, GPC3, KRT19, and Ob(Lep)] or the Ad-GDF5/CM group [GDF5, AGG, COL II, COL 15, GPC3, and Ob(Lep)]. Three genes (MMP3, CD105, and HIF1) in the non–Ad-GDF5/CM group and two (KRT19 and MMP3) in the Ad-GDF5/CM group decreased, while three (COL I, COL 10, and COL 15) in the non–Ad-GDF5/CM group and four (COL I, COL 10, CD105, and HIF1) in the Ad-GDF5/CM group remained unchanged. Compared with the non–Ad-GDF5/CM group, the differential pattern in gene expression in Ad-GDF5/CM group was as follows: seven genes up-regulated, GDF5, AGG, COL 15, MMP3, CD105, HIF1, and Ob(Lep); three down-regulated, COL I, GPC3, and KRT19; and two unchanged, COL II and COL 10.
FIG. 5.
GDF5 induced gene expression in human ADSCs. Cells with (GDF5) or without (non-GDF5) infection of ad-GDF5 were cultured in BM or CM for 3 weeks. Total RNA was isolated from cell aggregates after 3 weeks of culture, measured by real-time reverse-transcription polymerase chain reaction (PCR). The mRNA level was normalized to 18S. **p<0.001. AGG: aggrecan; COL I, II, 10, and 15: collagens type I, II, 10, and 15, respectively; GPC3: glypican 3; KRT19: keratin 19; MMP3: matrix metalloproteinase 3; CD105: endoglin; HIF1: hypoxia inducible factor 1.
Discussion
In this pilot study, we investigate for the first time chondrogenesis by hADSCs in modified aggregates and with overexpressed GDF5. Our data demonstrated that the modified aggregate culture produced better outcomes in both morphology and matrix production (Fig. 1). This observation is probably partly due to the cell outgrowth and subsequent dedifferentiation in the case of micromass cultures, but the evidence is obviously lacking at this point. Another possible contributor might have been the specific medium used for the aggregate preparation, which was enriched in growth factors with low serum. Indeed, the aggregates were found to have high expression of extracellular matrix proteins such as biglycan, various types of collagens, as well as growth factors beneficial to chondrogenesis, such as insulin-like growth factor 1 and transforming growth factor β after 6 days' culture in the medium.12 In the present study, strong positive Safranin O staining was consistently seen in the non–Ad-GDF5/BM group (Fig. 4A), and only a moderate increase (less than twofold) in sGAG content was achieved in either non–Ad-GDF5/CM or Ad-GDF5/CM group compared to the non–Ad-GDF5/BM group (Fig. 3). These results imply that the modified aggregates might undergo chondrogenesis even in the absence of induction. As such, we provide herein a novel 3D culture technique for chondrogenesis, in addition to those previously described, such as pellet8,9 and hydrogel culture.4,23 Meanwhile, the modified aggregates have been implicated to have the ability of accelerating diabetic wound healing.12 In order to broaden the scope of their potential use, the impact of the specific medium on differentiating ability (chondrogenesis, osteogenesis and adipogenesis, etc.) by the modified aggregates deserves further investigation.
Improvement of chondrogenesis and cartilage development by GDF5 has been well established during the past two decades.8,9,14,15,19,23 In humans, it has been reported to enhance the chondrogenic differentiation in bone marrow MSC from the fetus8 and adult,23,32 and rheumatoid fibroblast-like synoviocytes,33 as well as the chondrogenic transdifferentiation in dermal fibroblasts.19 In the present study, influence of GDF5 on hADSC chondrogenesis was investigated by taking advantage of the modified aggregate culture technique. Measurement of GDF5 at the protein level by ELISA has previously demonstrated that Ad-GDF5 is an effective tool to mediate GDF5 overexpression in rat ADSCs.9 The Ad-GDF5 appears to work effectively in hADSCs as well, since after infection the mRNA level of GDF5 robustly increases >1000-fold, in terms of data from real-time RT-PCR analysis (Fig. 5A). Furthermore, GDF5 gene overexpression could augment the chondrogenesis by hADSCs, revealed by aggregate size (Fig. 2), proteoglycan production (Fig. 3), and gene expression of chondrogenic markers (Fig. 5A). These results are in accordance with our previous study related to rat ADSC chondrogenesis.9 Additionally, the aggregate size became smaller over time for all three groups; however, overexpressed GDF5 appears capable of slowing the decrease in aggregate size (Fig. 2). More investigation is necessary to clarify the related mechanisms.
Pilgaard et al.34 reported the transcriptional signature of hADSCs preconditioned for chondrogenesis by hypoxia. It is appealing to investigate the gene expression pattern during chondrogenesis by hADSCs in the current aggregate culture. As an initial attempt, the present experiment depicts expression of a total of 12 genes by real-time RT-PCR (Figs. 5A–C). Obviously, further experiments are required to verify the significance of gene expression pattern in this system with regard to GDF5 regulation. However, some new findings are of interest. First, we demonstrate that leptin expression is ultimately absent in human ADSC aggregates cultured in basic medium, but markedly elevated after 3-week induction, in particular by >30-fold in the presence of overexpressed GDF5 (Fig. 5C). Leptin is known mostly for its role as a key regulator of body weight and food intake.35 More importantly, its pivotal modulation has also been suggested in endochondral ossification during bone formation, partly via prevention of premature mineralization of the prehypertrophic chondrocytes at the growth plate.36 Second, our present data show that GDF5 causes no increase in type II collagen expression, as evidenced by both immunostaining (Fig. 4) and real-time PCR (Fig. 5A) analyses, which seemed inconsistent with those we previously reported.9 The inconsistency might be attributed to the different culture conditions (modified aggregate versus pellet; pretreatment with growth factor–enriched medium versus without the pretreatment) and source of cells (human versus rat). Third, overexpressed GDF5 inhibit expression of COL 1 (Fig. 5A) but do not affect COL 10 (Fig. 5C). Combined with the fact that leptin expression is robustly elevated by GDF5 overexpression, it seems that in our system (i.e., modified aggregates and GDF5 genetic modification), chondrogenic hypertrophy might be prevented. Lastly, we provide some new information on the expression pattern of genes such as CD105, COL 15, GPC3, HIF1, KRT19, and MMP3, although to speculate on the significance of these findings is a challenging task at this stage. It is worth noting that the current modified aggregate culture along with GDF5 genetic modification is quite different from other culture systems previously reported for chondrogenic induction. In addition, the exact roles of these genes during chondrogenesis are not defined yet due to insufficient experimental data. For example, CD105 is generally characterized as a specific surface marker of MSCs, and a CD105-enriched population within hADSCs has a stronger capacity for chondrogenesis and osteogenesis as well as a weaker capacity for adipogenesis.37 However, no data have been published to date on the regulation of CD105 expression itself during chondrogenesis.
Our study has several limitations. First, cells used in the experiments were obtained from only one subject, and this may cause uncertainty in the biological significance of the present findings. Second, culture media with different compositions were used for aggregate and micromass cultures in the present study. This inconsistency might prevent an effective comparison of the two methods. Additionally, adhesive micromass cultures were not optimal controls for modified aggregates, due to their adhesive properties. In spite of more complexity in preparation, nonadhesive pellets would be a better control.7–9 Third, we did not compare the ADSCs to chondrocytes. Since the ultimate purpose of the research was to find an alternative source for autologous chondrocytes, such a control would better define any limitations of the ADSCs. Lastly, the present study provides experimental data on gene expression only at the mRNA level and lacks data at the protein level. In the future, Western blot or ELISA should be conducted to confirm the findings by real-time RT-PCR, especially for those genes of extreme significance such as GDF5, leptin, COL I, and COL 10.
The merit of this study was in providing a putative cell therapy in the absence of scaffolds. Although scaffolds can help retain cells in the desired location and provide appropriate mechanical properties and/or biochemical signals in tissue engineering, the long-term safety issues of the biomaterial components, such as the retention and degradation of synthetic materials in situ and infectious risk and immunological reaction caused by biological materials, remains a big concern.38 Therefore, using a scaffold-free culture system has been proposed as an alternative to avoid those unknown risks.38 As such, further investigation deserves to be made to evaluate the in vivo efficacy of the modified aggregates of hADSCs with overexpressed GDF5 in treating cartilage defects. Furthermore, the aggregates might be engineered with different numbers of cells to form desirable-sized spheroids,12 providing flexibility of either filling defects of clinically relevant size or examining the dose-dependent effect of the cell transplantation treatment with an animal model.
Genetically modified stem cells have been proposed as a promising tool for cell therapy. They have been proven safe and effective in tissue repair or disease treatment by numerous animal tests and are now ready for human clinical trials.39 Our present data, together with those previously reported,9 support the notion that genetically engineered adipose stem cells with GDF5 might be applied for cartilage regeneration. It is worth noting that a variety of techniques for gene delivery that are based on viral40 and nonviral vectors41 have been extensively investigated from basic to translational research.
In conclusion, this is the first attempt to apply the specific aggregate culture for a chondrogenic induction, as well as to determine the impact of GDF5 on chondrogenesis by a primary human adipose stem cell culture. In spite of its preliminary nature, this study highlights the potential use of the ADSC aggregate with GDF5 genetic modification for cartilage repair.
Acknowledgments
The study was performed at the Orthopedic Research Laboratory, University of Virginia School of Medicine. This work was supported by funding from the AO Foundation (Dübendorf, Switzerland) and the Scoliosis Research Society (Milwaukee, WI).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hildner F. Albrecht C. Gabriel C. Redl H. Van Griensven M. State of the art and future perspectives of articular cartilage regeneration: a focus on adipose-derived stem cells and platelet-derived products. J Tissue Eng Regen Med. 2011;5:e36–e51. doi: 10.1002/term.386. [DOI] [PubMed] [Google Scholar]
- 2.Bedi A. Feeley BT. Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 3.Gomoll AH. Farr J. Gillogly SD. Kercher J. Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:2470–2490. [PubMed] [Google Scholar]
- 4.Awad HA. Wickham MQ. Leddy HA. Gimble JM. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Romero J. Gaillard JP. Grogan SP. Nesic D. Trub T. Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005;202:731–742. doi: 10.1002/jcp.20164. [DOI] [PubMed] [Google Scholar]
- 6.Hwang NS. Elisseeff J. Application of stem cells for articular cartilage regeneration. J Knee Surg. 2009;22:60–71. doi: 10.1055/s-0030-1247728. [DOI] [PubMed] [Google Scholar]
- 7.He F. Pei M. Extracellular matrix enhances differentiation of adipose stem cells from infrapatellar fat pad toward chondrogenesis. J Tissue Eng Regen Med. 2013;7:73–84. doi: 10.1002/term.505. [DOI] [PubMed] [Google Scholar]
- 8.Bai X. Xiao Z. Pan Y, et al. Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun. 2004;325:453–460. doi: 10.1016/j.bbrc.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Feng G. Wan Y. Balian G. Laurencin CT. Li X. Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors. 2008;26:132–142. doi: 10.1080/08977190802105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang JI. Zuk PA. Jones NF, et al. Chondrogenic potential of multipotential cells from human adipose tissue. Plast Reconstr Surg. 2004;113:585–594. doi: 10.1097/01.PRS.0000101063.27008.E1. [DOI] [PubMed] [Google Scholar]
- 11.Parker A. Shang H. Khurgel M. Katz A. Low serum and serum-free culture of multipotential human adipose stem cells. Cytotherapy. 2007;9:637–646. doi: 10.1080/14653240701508452. [DOI] [PubMed] [Google Scholar]
- 12.Amos PJ. Kapur SK. Stapor PC, et al. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A. 2010;16:1595–1606. doi: 10.1089/ten.tea.2009.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortier LA. Barker JU. Strauss EJ. McCarrel TM. Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman CM. Tuan RS. Functional role of growth/differentiation factor 5 in chondrogenesis of limb mesenchymal cells. Mech Dev. 2003;120:823–836. doi: 10.1016/s0925-4773(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 15.Tsumaki N. Tanaka K. Arikawa-Hirasawa E, et al. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol. 1999;144:161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhabra A. Zijerdi D. Zhang J. Kline A. Balian G. Hurwitz S. BMP-14 deficiency inhibits long bone fracture healing: a biochemical, histologic, and radiographic assessment. J Orthop Trauma. 2005;19:629–634. doi: 10.1097/01.bot.0000177108.38461.9c. [DOI] [PubMed] [Google Scholar]
- 17.Bobacz K. Gruber R. Soleiman A. Graninger WB. Luyten FP. Erlacher L. Cartilage-derived morphogenetic protein-1 and -2 are endogenously expressed in healthy and osteoarthritic human articular chondrocytes and stimulate matrix synthesis. Osteoarthritis Cartilage. 2002;10:394–401. doi: 10.1053/joca.2002.0522. [DOI] [PubMed] [Google Scholar]
- 18.Gruber R. Mayer C. Schulz W, et al. Stimulatory effects of cartilage-derived morphogenetic proteins 1 and 2 on osteogenic differentiation of bone marrow stromal cells. Cytokine. 2000;12:1630–1638. doi: 10.1006/cyto.2000.0760. [DOI] [PubMed] [Google Scholar]
- 19.Yin S. Cen L. Wang C, et al. Chondrogenic transdifferentiation of human dermal fibroblasts stimulated with cartilage-derived morphogenetic protein 1. Tissue Eng Part A. 2010;16:1633–1643. doi: 10.1089/ten.TEA.2009.0570. [DOI] [PubMed] [Google Scholar]
- 20.Cui M. Wan Y. Anderson DG, et al. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8:287–295. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Li X. Leo BM. Beck G. Balian G. Anderson GD. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine (Phila Pa 1976). 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 22.Liang H. Ma SY. Feng G. Shen FH. Joshua Li X. Therapeutic effects of adenovirus-mediated growth and differentiation factor-5 in a mice disc degeneration model induced by annulus needle puncture. Spine J. 2010;10:32–41. doi: 10.1016/j.spinee.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoyanov JV. Gantenbein-Ritter B. Bertolo A, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater. 2011;21:533–547. doi: 10.22203/ecm.v021a40. [DOI] [PubMed] [Google Scholar]
- 24.James R. Kumbar SG. Laurencin CT. Balian G. Chhabra AB. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011;6:025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller TC. Hogan MV. Kesturu G. James R. Balian G. Chhabra AB. Growth/differentiation factor-5 modulates the synthesis and expression of extracellular matrix and cell-adhesion-related molecules of rat Achilles tendon fibroblasts. Connect Tissue Res. 2011;52:353–364. doi: 10.3109/03008207.2010.534208. [DOI] [PubMed] [Google Scholar]
- 26.Park A. Hogan MV. Kesturu GS. James R. Balian G. Chhabra AB. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng Part A. 2010;16:2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saiga K. Furumatsu T. Yoshida A, et al. Combined use of bFGF and GDF-5 enhances the healing of medial collateral ligament injury. Biochem Biophys Res Commun. 2010;402:329–334. doi: 10.1016/j.bbrc.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Katz AJ. Tholpady A. Tholpady SS. Shang H. Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee M. Bhonde RR. Application of hanging drop technique for stem cell differentiation and cytotoxicity studies. Cytotechnology. 2006;51:1–5. doi: 10.1007/s10616-006-9001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelm JM. Timmins NE. Brown CJ. Fussenegger M. Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83:173–180. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Xu D. Gechtman Z. Hughes A, et al. Potential involvement of BMP receptor type IB activation in a synergistic effect of chondrogenic promotion between rhTGFbeta3 and rhGDF5 or rhBMP7 in human mesenchymal stem cells. Growth Factors. 2006;24:268–278. doi: 10.1080/08977190601075865. [DOI] [PubMed] [Google Scholar]
- 33.Liu FL. Lin LH. Sytwu HK. Chang DM. GDF-5 is suppressed by IL-1beta and enhances TGF-beta3-mediated chondrogenic differentiation in human rheumatoid fibroblast-like synoviocytes. Exp Mol Pathol. 2010;88:163–170. doi: 10.1016/j.yexmp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Pilgaard L. Lund P. Duroux M, et al. Transcriptional signature of human adipose tissue-derived stem cells (hASCs) preconditioned for chondrogenesis in hypoxic conditions. Exp Cell Res. 2009;315:1937–1952. doi: 10.1016/j.yexcr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Ahima RS. Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 36.Kishida Y. Hirao M. Tamai N, et al. Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone. 2005;37:607–621. doi: 10.1016/j.bone.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Jiang T. Liu W. Lv X, et al. Potent in vitro chondrogenesis of CD105 enriched human adipose-derived stem cells. Biomaterials. 2010;31:3564–3571. doi: 10.1016/j.biomaterials.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 38.Maeda S. Fujitomo T. Okabe T. Wakitani S. Takagi M. Shrinkage-free preparation of scaffold-free cartilage-like disk-shaped cell sheet using human bone marrow mesenchymal stem cells. J Biosci Bioeng. 2011;111:489–492. doi: 10.1016/j.jbiosc.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Meyerrose T. Olson S. Pontow S. Kalomoiris S. Jung Y. Annett G, et al. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010;62:1167–1174. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakuma T. Barry MA. Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443:603–618. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y. Satterlee A. Huang L. In vivo gene delivery by nonviral vectors: overcoming hurdles? Mol Ther. 2012;20:1298–1304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]