Abstract
To achieve an easily established, safe, and reproducible animal model for the study of heterotopic bone formation around vessels, a small animal series using New Zealand White rabbits was performed. Three different dosages of recombinant human bone morphogenic protein (rhBMP-2) carried by fibrin matrix were tested. A guided tissue regeneration (GTR) membrane sheet was formed into a tube and allowed to harden; it served both to maintain the space around the vessel bundle and to separate the fibrin matrix with rhBMP-2 from skeletal muscle. Wrapped around the femoral vessel bundle and fixed in place, the tube was filled with the fibrin matrix containing rhBMP-2. The surgical site was closed in layers, and the postoperative healing was uneventful. All animals resumed their full preoperative daily activities 3–4 days after the operation. No adverse events such as wound dehiscence or infection occurred, and all animals could be sacrified at the scheduled date. Micro–computed tomography and histological investigations showed heterotopic bone formation around the vessel bundle in the medium- and high-dosage rhBMP-2 groups. An easy, safe, and reproducible animal model that allows the study of heterotopic bone formation around vessels was successfully established.
Key words: biomaterials, growth factor, tissue engineering
Introduction
Reconstruction of large-size bone defects has been a critical challenge for maxillofacial and orthopedic clinicians, even though surgical techniques have been refined and bone engineering research has undergone remarkable improvement. Tissue engineering using in vitro bioreactors functions successfully only within the dimension of critical-size bone defects.1 Warnke et al.2 have demonstrated the possibility of creating large amounts of bone tissue in vivo in a region with appropriate bone inductive conditions.2 However, osteoinductive conditions in their study only provided engineered bone with an unpredictable perfusion pattern. As life expectancy rises due to progress in the medical field, the number of elderly, medically compromised patients increases. Prolonged operation time with two operation sites, as needed in microvascular flap procedures, submits these patients to increased comorbidities such as infection, reduced postoperative mobilization, and flap failure due to peripheral vascular atheromatous changes. Creating heterotopic bone around previously dissected facial vessels laid in a load-bearing custom-made scaffold of resorbable material might represent an alternative technique in reconstructive procedures of jaw bones, providing a predictable perfusion pattern, a shorter operation time, and less comorbidity for such patients.
Bone morphogenetic proteins (BMPs) play a key role in osteogenesis and chondrogenesis.3 BMPs have been approved by the U.S. Food and Drug Administration for clinical use as osteoinductive mediators.4 Combination with other growth factors such as vascular endothelial growth factor (VEGF) in an appropriate delivery system may improve their osteoinductive effect.5 Since BMP-2 may be quickly flushed away after its administration,6 suitable delivery systems are needed to provide retention and optimal release for continuous stimulation of bone formation.5 Recent studies have demonstrated that fibrin matrices provide such prolonged retention and release of embedded recombinant human BMP-2 (rhBMP-2) to the surrounding tissue.6–9
The aim of the present study was to establish an animal model in New Zealand white rabbits in which (1) heterotopic bone generation around easily accessible vessels could be studied without compromising the animals' daily activities, and (2) an easy, reproducible, and safe surgical procedure could be performed without intra- and postoperative complications.
Materials and Methods
Preparation of rhBMP-2 and fibrin gel
RhBMP-2 was prepared according to the protocol described in our previous studies.10,11 Briefly, the rhBMP-2 was diluted in Tris-HCl (1 mM) and supplemented with thrombin solution (Tisseel Lyo®, Baxter, Vienna, Austria). Fibrinogen, the other component of Tissel Lyo, was diluted with Tris-buffered saline (TBS). Fibrin gels were formed by 1:1 mixing of fibrinogen and thrombin using ready-to-use syringes (Duploject system; Baxter) during the surgery. Mixing fibrinogen and thrombin solution immediately led to formation of the fibrin gel. After adding it to the fibrin solution, rhBMP-2 was precipitated in the fibrin maxtrix and then slowly solublized.
Animal model
The research protocol of the present study was approved by the Committee on the Use of Live Animals for Teaching and Research (CULATR No.1637-08), the University of Hong Kong. The surgical technique was developed and refined in three New Zealand white rabbits from other trials after sacrifice (unpublished data of authors), before carrying out the current study with rhBMP-2.
Six adult (6–9 months old) New Zealand white rabbits (3.0–4.2 kg) were used in this pilot trial. These rabbits were randomly assigned to three groups, each with two rabbits, with rhBMP-2 at high (250 μg, group H), medium (125 μg, group M), and low dosages (62.5 μg, group L).
Surgical procedure
A standardized surgical procedure was carried out in all animals on the inside of the right upper thigh (Fig. 1) by the same surgeons (R.A.Z., W.-X.C.).
FIG. 1.
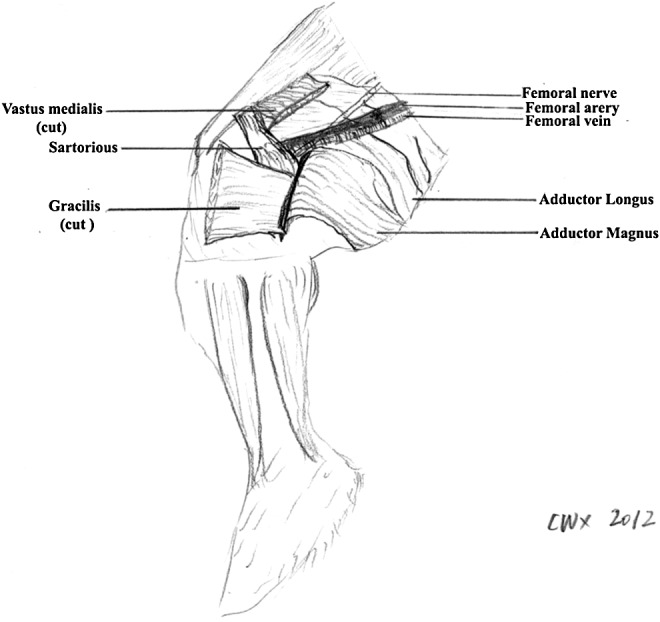
Regional anatomy of a rabbit's thigh. The femoral artery and vein, originating from the external iliac vessels, were dissected. The surgical site was closed in layers, using medial vastus and gracilis muscle tissue as a muscle pouch to achieve adequate wound healing without dehiscences.
After administering 30 mg/kg of long-acting oxytetracycline (Troy Laboratories Pty Limited, Glendenning, Australia) and 30 μg/kg Temgesic® (Reckitt Benckiser Health, Slough, United Kingdom) as preoperative antibiotic and analgesic medications, respectively, the veterinarian anesthetized the rabbits by intramuscular acepromazine (1 mg/kg; Delvet Pty. Ltd., Asquith, Australia), ketamine (45 mg/kg; Alfasan International B.V., Woerden, Holland), and xylazine (5 mg/kg; Alfasan) mixture into the gluteal muscle.
After intramuscular anesthesia, the hair on the inner side of the right posterior thigh was shaved. The rabbit was put in a supine position on the operating table and anaesthetic maintenance during the surgery was performed by 1.5–2% Forane® (Halocarbon Laboratories, River Edge, NJ) inhalation administered via face mask.
Flexing the right knee joint revealed the subcutaneous course of the saphenous vein and the artery bundle medial to it. With the skin lifted by two toothed forceps, a stab incision was performed with a scalpel. Subcutaneous dissection alternating with extension of the incision up to a length of 4 cm was performed with blunt scissors parallel to the anterior border of the right thigh (Fig. 2a, b). Following the course of the saphenous vein and artery proximally, the covering muscle fascia was gently transsected. At the site where the vessels descended between the medial vastus and gracilis muscles, the fascial incision was extended ∼5 mm to the surface of the gracilis muscle, which was gently transsected. Further dissection to the depth of the femoral artery and vein was performed bluntly with arterial forceps, transsecting muscle tissue where necessary. Before the femoral vessel bundle was bluntly dissected from the underlying adductor magnus muscle, the overlying saphenous nerve was carefully dissected and kept away from the vessels with a vessel loop or a retractor (Fig. 3a, 3b). Once the femoral vessel bundle, which presented no branching at the middle third of the thigh, had been completely dissected from the surrounding muscles, a moist gauze was put on the surgical site. On a draped side table, a biodegradable tube (Fig. 4), 1 cm long and 7 mm in diameter, was prepared out of an Inion® guided tissue regeneration (GTR) membrane sheet (Inion Oy, Tampere, Finland) according to the company's instructions.
FIG. 2.
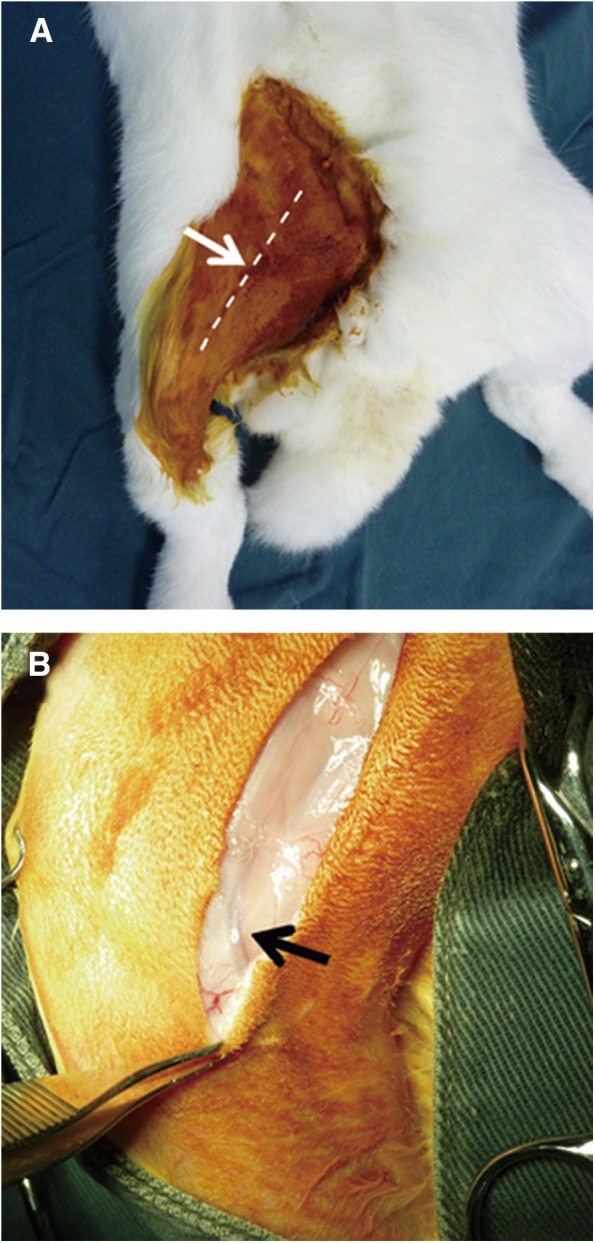
(A) Skin incision (arrow). A 4.0-cm-long straight incision (dashed line) was performed through the skin in the middle of the inner side of the right thigh. (B) The saphenous vascular bundle (arrow) was used as a guiding anatomical structure to approach the femoral vascular bundle in the depth of the femoral muscles.
FIG. 3.
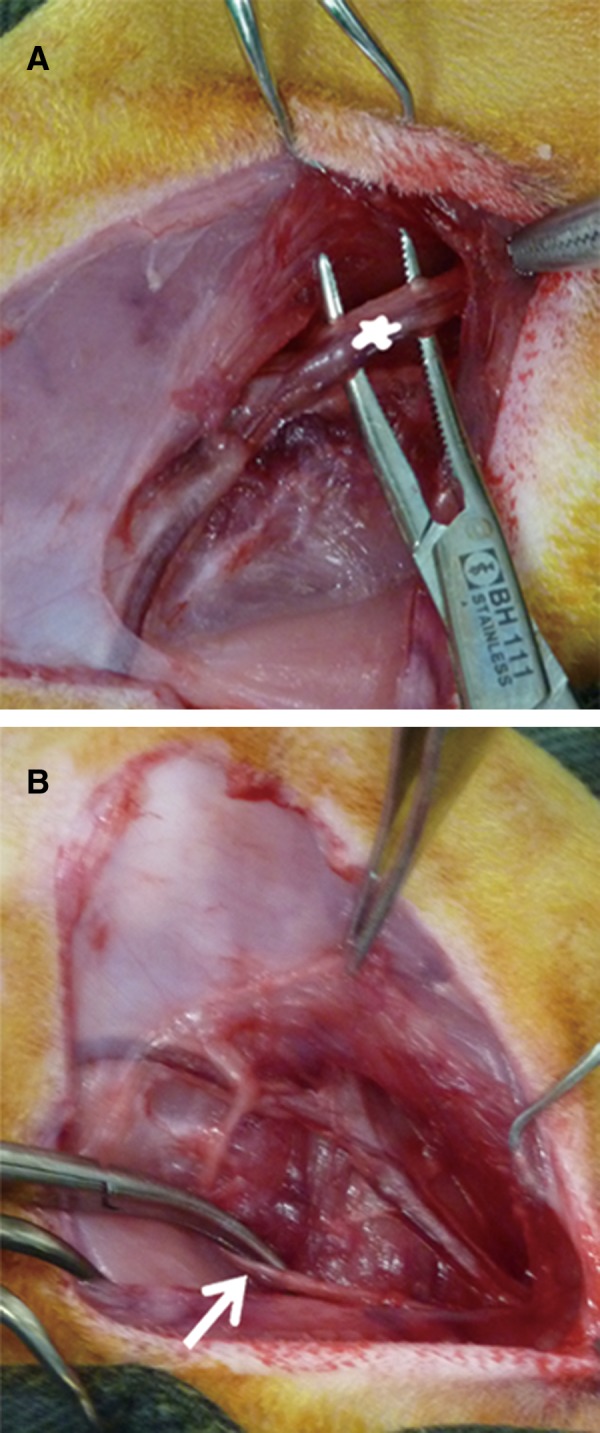
(A) The femoral vascular bundle (asterisk) was dissected from the underlying adductor magnus muscle to wrap around the Inion membrane tube. (B) The overlying saphenous nerve (arrows) was dissected carefully and kept further away from the vessels.
FIG. 4.
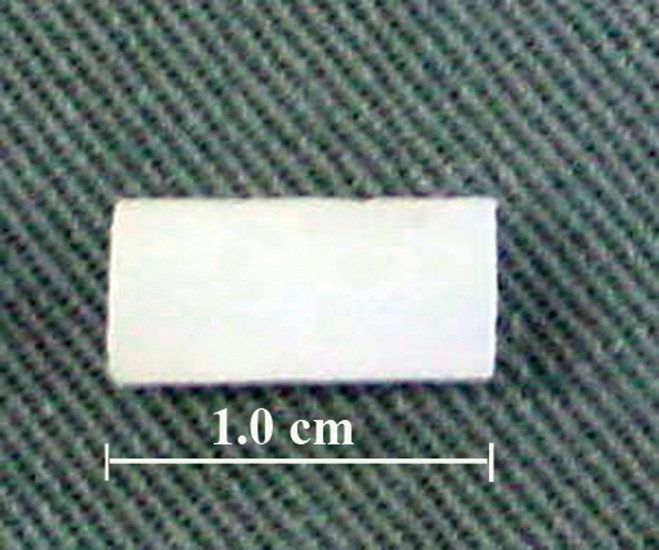
Inion membrane tube, ∼7 mm in diameter and ∼1 cm in length, after washing out with normal saline solution and before wrapping around the femoral vascular bundle.
The hardened tube was then wrapped around the femoral vessel bundle, secured with two single stitches of Vycril® 4/0 (Johnson & Johnson, Hong Kong, China) at each end, and fixed to the underlying adductor magnus with another single stitch of the same suture material (Fig. 5). The proximal end of the tube was then secured with a previously prepared piece of sterile hand glove and artery forceps to avoid proximal discharge of the fibrin gel during its injection and the hardening process in the tube. After the tube was filled with the rhBMP-2 and fibrin/thrombin mixture, the hand glove piece was removed as soon as the tisseel had gelatinized. The tube was carefully covered by the medial vastus and adductor gracilis muscles. The wound closure of the muscles and their fasciae was performed with Vycril 4/0 (Fig. 6), and Ethilon 5/0 (Johnson & Johnson, Hong Kong, China) was used to close the skin, both in single-suture technique.
FIG. 5.
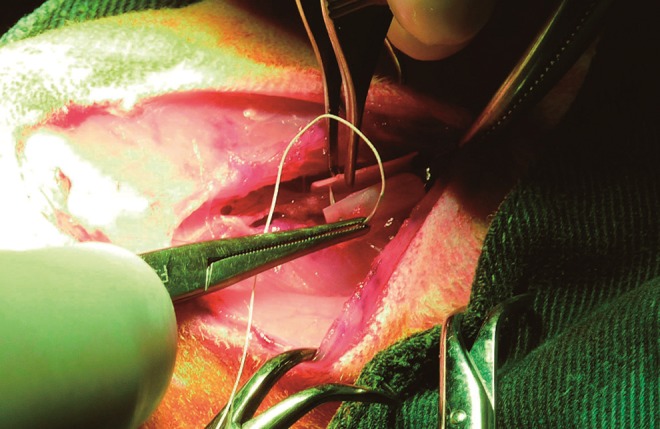
The hardened tube was wrapped around the femoral vascular bundle and secured in its tube shape with two single stitches of Vicryl 4-0 at each end. Thereafter, it was fixed to the underlying adductor magnus muscle with one single stitch (Vicryl 4-0).
FIG. 6.
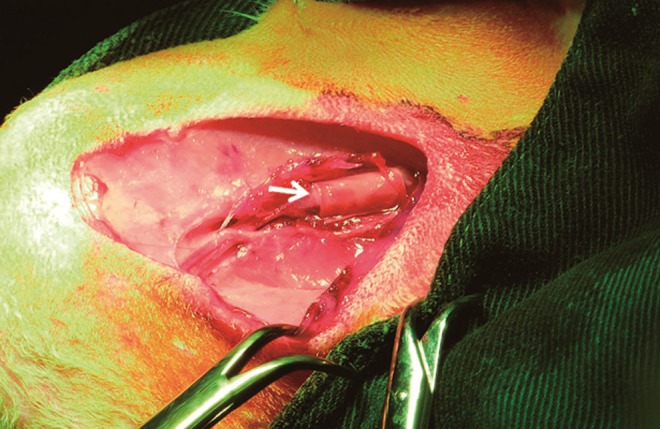
After filled up with fibrin gel carrying recombinant human bone morphogenic protein (rhBMP-2; arrow), the tube was carefully covered by tissue of the medial vastus and adductor gracilis muscles.
Postoperative care
Long-acting oxytetracycline (30 mg/kg; Troy Labs) was administered intramuscularly twice per week postoperatively for 2 weeks. Buprenorphine (0.05 mg/kg; Temgesic) was administered subcutaneously twice daily for 3 days as pain relief. Meloxicam (0.2 mg/kg; Metacam®, Ingelheim am Rhein, Germany) was administered subcutaneously once daily thereafter for 2 weeks. The rabbits were fed rabbit diet 5L25 (PicoLab® Rabbit Diet HF, Richmond, IN) and provided with water by a experienced technician. The behavior of the animals was continuously monitored and recorded by closed circuit television.
Specimen harvesting
Eight weeks postoperatively, the rabbits were sacrificed by administration of pentobarbital sodium (150 mg/kg; Alfasan). Afterward, the scar from the surgical approach was incised. Under palpation the site of the specimen was localized and an incision was made with the scalpel at a safe distance (∼1 cm), taking the vessel bundles proximally and distally as the transverse specimen-guiding structure. After careful dissection and mobilization of the specimen, the proximal and distal vascular pedicle were clipped with artery forceps to avoid bleeding and the specimen was harvested. The specimens were kept in 10% formalin solution for further management.
Assessment methods for specimens
Micro–computed tomography
Each specimen was placed into a sample holder filled with 10% formalin and oriented with the longitudinal axis of the cylinder parallel to the sample holder axis. The specimen was then examined morphologically and quantitatively by a computed tomography (CT) scan machine (SkyScan1076, Bruker, Kontich, Belgium) at 59 kV and 149 μA intensity with a 12-μm pixel resolution using an aluminum filter (0.5 mm). The reconstruction data was analyzed with CT Analyzer v. 1.9 software (Skyscan, Kontich, Belgium). The volume of the newly generated bone was evaluated with the area of bone tissue outlined as region of interest.
Histology
The specimens were demineralized at room temperature with 12.5% ethylene diamine tetraacetic acid (EDTA; Sigma, New York, NY) solution (pH 7.2) for about 2 weeks. Each specimen was cut in quarters, and the demineralized specimens were embedded in paraffin wax. Cross-sections with a thicknesses of 6 μm were performed using a microtome (Leica RM2155; Nussloch, Germany). The sections were stained with hematoxylin/eosin (H&E) and examined under a light microscope (Leica DMLB) at 2× and 10× magnifications.
Results
All rabbits showed a decrease in their daily food intake, with weight loss up to 0.42 kg in the first postoperative week. By 2 weeks postoperative, all had resumed their normal weight and diet. In line with the weight, behavioral patterns and daily activities normalized within the same time frame. Neither intra- nor postoperative adverse events occurred during the entire period of the trial. Wound healing proceeded uneventfully and the sutures could be removed 2 weeks postoperative.
Specimen
Clinical examination of the specimens revealed that the Inion membrane tube was filled with bone-like tissue, especially in groups H and M (Figs. 7 and 8), whereas such clinical observation was nonexistent in group L.
FIG. 7.
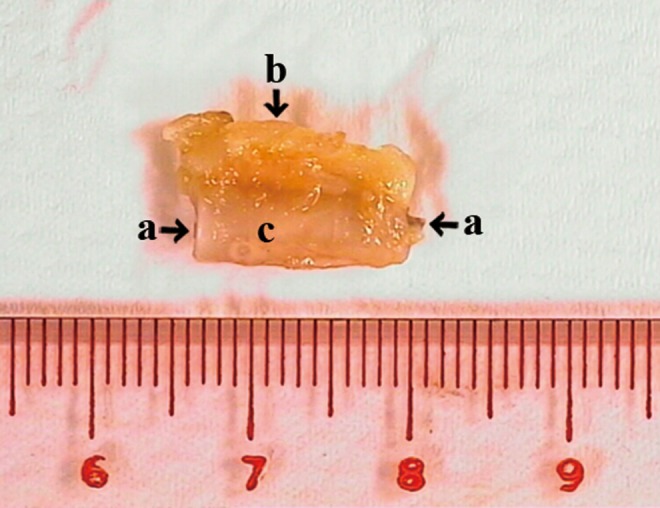
Longitudinal view of the harvested specimen. Femoral vessels (a) wrapped by the Inion membrane tube (c) together with the surrounding soft tissue (b) was harvested and subjected to clinical examination, micro-CT and histological examination. CT, computed tomography.
FIG. 8.
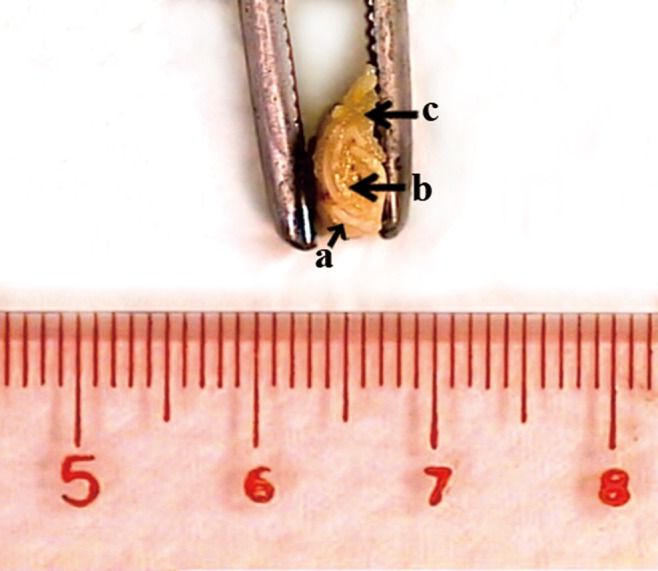
Cross-sectional view of the harvested specimen. Bone-like tissue (b) could be detected within the membrane tube (a). Surrounding soft tissue (c) was also harvested.
Micro-CT
The bone formation in the Inion cylinder was quantified by micro-CT analysis. In micro-CT images of groups H and M, newly formed bone could be detected within the Inion membrane tube (Fig. 9a, b), whereas only limited bone tissue was detected in group L. The mean calculated volumes of this newly formed bone were 11.02±0.02 mm3, 7.16±0.31 mm3, and 3.32±3.64 mm3 within groups H, M and L, respectively.
FIG. 9.
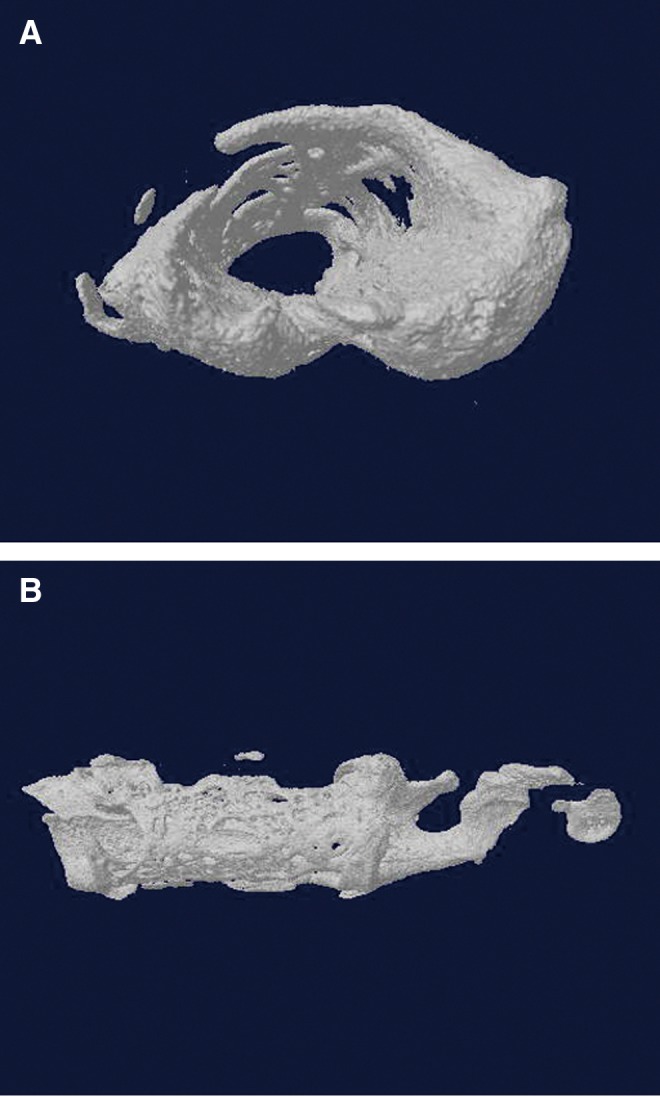
(A) Cross-sectional view of the newly formed bone within the membrane tube in micro-CT three-dimensional reconstruction (Group H). (B) Longitudinal view. The newly formed bone was detected within the membrane tube around the femoral vessel bundle (Group H).
Histological examination
Histological features of groups H and M did not show significant differences. H&E staining of the sections demonstrated the intact patency of the femoral vessel bundle (Fig. 10a, b). Bone tissue was disclosed within the Inion membrane tube. No bone tissue was histologically visible in group L.
FIG. 10.
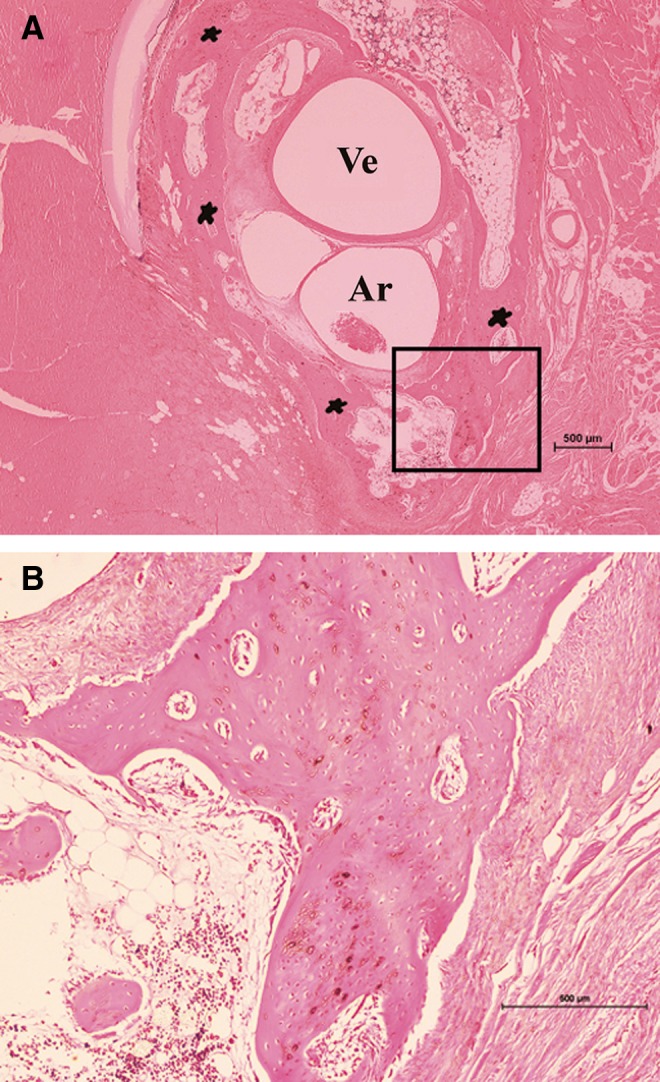
(A) Cross sections of histological images show newly formed bone around the vessels (H&E staining). (B) The framed area from (A) at higher magnification. The intact patency of the femoral artery (Ar) and femoral vein (Ve) was demonstrated. Bone (*) tissue was disclosed in cross sections of the harvested tissue from Group H. Magnification: (A) 2×, (B) 10×.
Discussion
This study generated two findings: (1) an easy, reproducible animal model that is safe to perform to study heterotopic bone generation around vessels, and (2) heterotopic bone formation around vessels by means of rhBMP-2 in a fibrin gel matrix separated from surrounding skeletal muscles with a dimensionally stable membrane tube.
Several conditions render this animal model ideal for studying heterotopic bone formation around vessels, including the easy approach through the skin, the subcutaneous superficial saphenous vascular bundle serving as a guiding anatomical structure toward the femoral vascular bundle, an exceedingly suitable muscular pouch for adequate wound closure, and a straight section of nonbranching femoral vascular bundle. The supine position with the abducted and flexed hindlimb further provides a clear surgical site with a good overview, that allows a safe and straightforward surgical procedure. The membrane tube was covered with medial vastus and adductor gracilis muscle tissue after adequate dissection and mobilization.12 This measure helped to avoid anatomical dead space with consequent bacterial infection and wound breakdown.13 Lounev and coworkers14 demonstrated that vascular smooth muscle cells did not contribute to BMP-induced heterotopic bone formation. To our knowledge this study is the first to detect heterotopic bone formation in direct contact with and around vessels. A dimensionally stable membrane tube14 prevented contact with surrounding skeletal muscle tissue. Heterotopic bone formation around vessels, other than the already widely described bone formation in a skeletal muscle pouch, may expand the indication for the technique in medically compromised patients.
Important characteristics of an ideal carrier material are (1) the provision of space, where new bone can form, (2) the ability for controlled release kinetics of growth factors, and (3) the simplicity to use as well as the biodegradability of the material.13 Recent research has already demonstrated that fibrin matrices represent ideal carriers and release systems for rhBMP-2.6–9 In this study fibrin gel was therefore used as matrix to carry and release rhBMP-2. Its injection into the Inion GTR membrane tube around the intratubular femoral vascular bundle resulted in concentric bone tissue formation. This might generate hope that large amounts of heterotopic bone might be fabricated along vessels in the future, leading eventually to heterotopic bone with an inherent perfusion pattern, even without direct contact with skeletal muscle tissue in an artificial, load-bearing matrix.
The volume of heterotopically generated bone was found to be dependent on rhBMP-2 dose. Whereas a low rhBMP-2 dose in the fibrin sealant was associated with hardly any new bone, increased doses led to heterotopic bone generation. However, no significant difference was found between high and medium dosages. This finding might therefore suggest that a dose of 125 μL rhBMP-2 would be ideal for generating heterotopic bone in this animal model, especially because toxic BMP dosages still are lacking in literature to our knowledge.
The Inion GTR membrane is biodegradable and the degradation products, alpha-hydroxy acids, can be metabolized by the body.13 It is a two-layer membrane of bioabsorbable copolymers composed of L-lactide, D-lactide, glycolic acid, and trimethylene carbonate. The N-methyl-2-pyrrolidone (NMP) solvent, which acts as a plasticizer, diffuses into the polymer, making the membrane malleable enough to adapt its form to different sites in the body. Once the desired shape is achieved, the solvent can be flushed out with a saline solution and the membrane hardens. The membrane then becomes stable in its desired form and retains the space within. Furthermore, NMP can increase the bioactivity and availability of autologous BMP by increasing the kinase activity, thus promoting bone formation in vivo.15
Some shortcomings are inherent in this experiment. First, the pilot study aimed to investigate the possibility and safety of a novel animal model to study heterotopic bone formation around vessels. The sample size was therefore not big enough for statistical evaluation. A second shortcoming was the somewhat difficult filling procedure of the membrane tube. As the gelation process after injection takes some time, a high chance for leakage at the proximal tube end exists. The sealing of the distal tube end with a piece of hand glove to avoid leakage needs to be improved in future trials because it did not represent the kind of tight closure that was intended. Thirdly, a duploject two-syringe system is needed to ensure equal volumes of the two sealant componets. The dead space volume of the joining piece of the duploject two-syringe system and the needle should be calculated for the total amount of rhBMP-2 and fibrin gel volume needed to fill the Inion GTR membrane tube. Moreover, the syringe system is not environmentally friendly because it can not be recycled due to blocking fibrin gel.
Since this new animal model proved to be safe and successful, future research in this field can be performed with a larger number of animals to provide more adequate data for statistical evaluation. It also would be of interest to investigate vessels sprouting out of the vascular bundle inside the Inion GTR membrane tube when adding VEGF in different ratios to rhBMP-2 in the fibrin matrix. VEGF has been reported not only to modulate angiogenesis but also to play an important role in bone development.16–20 The animal model presented here could thus be helpful to further study the hypothesis to create heterotopic bone with an inherent perfusion pattern and pedicle.
Acknowledgments
This study was supported by the GRF of Hong Kong (GRF funding Project No 784009). We appreciate the valuable technical support provided by the Laboratory Animal Unit and the Hard Tissue Laboratory of the Faculty of Dentistry, both of the University of Hong Kong.
Disclosure Statement
No competing financial interests exist.
References
- 1.Warnke PH. Wiltfang J. Springer I, et al. Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials. 2006;27:3163–3167. doi: 10.1016/j.biomaterials.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 2.Warnke PH. Springer ING. Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 3.Zheng LW. Cheung LK. Effect of recombinant human bone morphogenetic protein-2 on mandibular distraction at different rates in a rabbit model. Tissue Eng. 2006;12:3181–3188. doi: 10.1089/ten.2006.12.3181. [DOI] [PubMed] [Google Scholar]
- 4.Herford AS. Stoffella E. Tandon R. Reconstruction of mandibular defects using bone morphogenic protein: can growth factors replace the need for autologous bone grafts? A systematic review of the literature. Plast Surg Int. 2011;2011:165824. doi: 10.1155/2011/165824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung RE. Schmoekel HG. Zwahlen R. Kokovic V. Hammerle CH. Weber FE. Platelet-rich plasma and fibrin as delivery systems for recombinant human bone morphogenetic protein-2. Clin Oral Implants Res. 2005;16:676–682. doi: 10.1111/j.1600-0501.2005.01183.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmoekel H. Schense J. Weber F, et al. Bone healing in the rat and dog with nonglycosylated BMP-2 demonstrating low solubility in fibrin matrices. J Orthop Res. 2004;22:376–381. doi: 10.1016/S0736-0266(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 7.Schmoekel HG. Weber FE. Seiler G, et al. Treatment of nonunions with nonglycosylated recombinant human bone morphogenetic protein-2 delivered from a fibrin matrix. Vet Surg. 2004;33:112–118. doi: 10.1111/j.1532-950x.2004.04018.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmoekel HG. Weber FE. Schense JC. Gratz KW. Schawalder P. Hubbell JA. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol Bioeng. 2005;89:253–262. doi: 10.1002/bit.20168. [DOI] [PubMed] [Google Scholar]
- 9.Schmoekel H. Weber F. Hurter K, et al. Enhancement of bone healing using non-glycosylated rhBMP-2 released from a fibrin matrix in dogs and cats. J Small Anim Pract. 2005;46:17–21. doi: 10.1111/j.1748-5827.2005.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 10.Weber FE. Eyrich G. Gratz KW. Maly FE. Sailer HF. Slow and continuous application of human recombinant bone morphogenetic protein via biodegradable poly(lactide-co-glycolide) foamspheres. Int J Oral Maxillofac Surg. 2002;31:60–65. doi: 10.1054/ijom.2001.0154. [DOI] [PubMed] [Google Scholar]
- 11.Jung R. Glauser R. Schärer P. Hämmerle C. Sailer H. Weber F. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res. 2003;14:556–568. doi: 10.1034/j.1600-0501.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 12.Wingerd BD. Rabbit Dissection Manual. Johns Hopkins University Press; Baltimore, MD: 1985. [Google Scholar]
- 13.Pirhonen E. Pohjonen T. Weber F. Novel membrane for guided bone regeneration. Int J Artif Organs. 2006;29:834–840. doi: 10.1177/039139880602900904. [DOI] [PubMed] [Google Scholar]
- 14.Lounev VY. Ramachandran R. Wosczyna MN, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miguel B. Ghayor C. Ehrbar M, et al. N-methyl pyrrolidone as a potent bone morphogenetic protein enhance for bone tissue regeneration. Tissue Eng Part A. 2009;15:2955–2963. doi: 10.1089/ten.TEA.2009.0009. [DOI] [PubMed] [Google Scholar]
- 16.Maes C. Carmeliet P. Moermans K, et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73. doi: 10.1016/s0925-4773(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 17.Gerber HP. Vu TH. Ryan AM. Kowalski J. Werb Z. Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 18.Zelzer E. McLean W. Ng YS, et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–1904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- 19.Maes C. Stockmans I. Moermans K, et al. Soluble VEGF isoforms are essential for establishingepiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest. 2004;113:188–199. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng H. Usas A. Olshanski A, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20:2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]


