Abstract
INTRODUCTION
Endometrial cancer survivors exhibit an increased incidence of subsequent neoplasms.
PRESENTATION OF CASE
We present a patient with a history of endometrial cancer who, 3 years after surgery and radiotherapy, developed synchronous neoplasms of the breast, colon and rectum. The patient underwent abdominoperineal resection, a limited right colectomy, and excision of the breast tumour and axillary lymph node dissection. 18 months after surgery, there has been no disease recurrence.
DISCUSSION
Multiple primary malignancies represent 16% of new cancer diagnoses. Research on subsequent malignancies after endometrial cancer has shown an increase in risk in colorectal, urinary bladder, lung and breast primaries.
CONCLUSION
This case report illustrates the need for physicians to be aware of and counsel patients on the risk of subsequent cancers on endometrial cancer survivors.
Keywords: Endometrial cancer, Multiple neoplasms, Synchronous
1. Introduction
The concept of multiple primary malignant neoplasms was first described by Billroth in 18891 and reviewed in detail by Warren and Gates in 1932,2 who proposed criteria for their diagnosis. The knowledge accumulated since then has not, however, been translated into clinical guidelines for their detection, with the notable exception of certain tumour syndromes. The increased risk of developing subsequent neoplasms following endometrial cancer diagnosis has been documented and its aetiology is being actively investigated.3–7 We present a case of 3 synchronous neoplasms (2 adenocarcinomas and 1 adenoma) in an endometrial cancer survivor and comment on the recent evidence.
2. Presentation of case
A 67-year-old female patient presented to the surgical outpatient clinic complaining of post-defecation hemorrhage and proctalgia over the preceding 4 months. The patient reported no weight loss, abdominal discomfort or change in bowel habits and was in good general condition. A rectal exam revealed a rigid mass close to the dentate line. The rest of the physical examination was unremarkable. The patient had undergone a total abdominal hysterectomy and bilateral salpingo-oophorectomy for endometrial cancer 3 years before and had subsequently received adjuvant chemo- and radiotherapy. She had no evidence of recurrence at last follow-up 18 months earlier.
A colonoscopy was ordered, which revealed an ulcerated mass 5 cm from the dentate line, occupying half of the circumference of the rectal lumen, a number of small (<0.5 cm) polyps and a sessile mass of the cecum (Fig. 1). Biopsy revealed that the rectal mass was a tubular adenocarcinoma with positive CK20 and CDX-2 and negative CK7 and ER immunoassays, indicating it originated from the rectal mucosa; the cecal mass appeared to be a tubulo-villous adenoma with high-grade dysplasia.
Fig. 1.
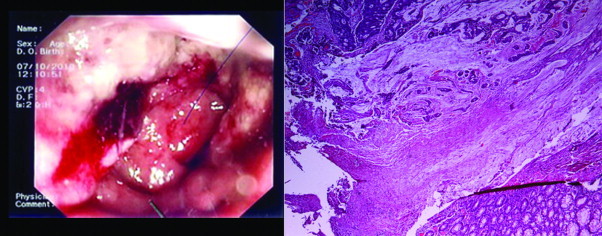
Rectal adenocarcinoma (left: endoscopic appearance; right: microscopic view).
The patient was admitted to the surgical department for further evaluation. During admission she underwent a second physical examination, which produced a new finding; there was a palpable lymph node on the left axilla. The patient had a screening mammography 2 months before, which showed some non-clustered, linear, non-branching calcifications in the left breast. Follow-up MRI mammography and core biopsy was negative for malignancy. A CT scan of the chest and abdomen failed to show any abnormal findings, while magnetic resonance imaging of the pelvis staged the tumour as T4, with invasion of the rectal sphincter. The patient's lab results, including tumour markers, were normal.
An abdominoperineal resection and a limited right colectomy, with anastomosis of the terminal ileum to the ascending colon, were performed. The palpable mass of the axilla was also excised and sent for biopsy. The patient's recovery was uneventful.
The histological examination identified the rectal mass as a mucinous adenocarcinoma measuring 5.5 cm × 1.4 cm × 1.5 cm in size, infiltrating the whole thickness of the rectal wall and extending to the dentate line (Fig. 1). There were no affected lymph nodes. The rest of the rectosigmoid showed signs of radiation injury, with irregular, flattened mucosa and numerous small polyps. The cecal mass was confirmed to be a tubulo-villous adenoma with high-grade dysplasia, without signs of infiltration. Biopsy results from the axillary mass, whose location on the tail of the breast made it clinically similar to an enlarged lymph node, showed it was a small (1.2 cm × 1 cm × 0.5 cm) ductal adenocarcinoma of the breast (Fig. 2(A)), which extended superficially up to the dermis of the skin and was excised with positive margins. Immunohistochemistry was positive for CK7, CAM 5-2, CEA, E-Cadherin, ER (80%), PR (40%), CERB 2 (3+) and negative for CK20 (Fig. 2(C)–(E)).
Fig. 2.
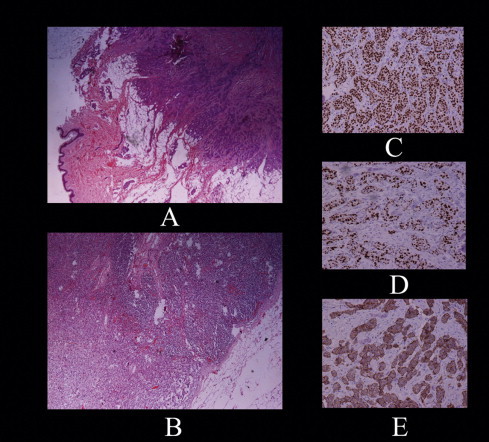
(A) Ductal adenocarcinoma of the breast. (B) Infiltrated lymph node of the left axilla. (C) Immunohistochemical staining: estrogen receptor. (D) Immunohistochemical staining: progesterone receptor. (E) Immunohistochemical staining: human epidermal growth factor receptor 2.
After receiving these results the patient was operated on again and a left upper quadrantectomy at the biopsy site and axillary lymph node dissection were performed. The pathologist could not find any residual tumor but 4 out of 15 retrieved lymph nodes were infiltrated (Fig. 2(B)).
The patient was discharged from the hospital in good condition. Her case was discussed in the multidisciplinary oncology meeting of our institution. It was decided to proceed with systemic chemotherapy and hormonotherapy for the cancer of the breast in combination with breast irradiation. During the meeting, it was decided that no radiotherapy would be administered for the treatment of the rectal cancer because of the risk of increased morbidity due to previous pelvic irradiation and due to the fact that the extent of the sphincter invasion precluded any sphincter-saving strategy. Chemotherapy consisted of docetaxel, adriamycin and cyclophosphamide in combination with transtuzumab for one year and letrozole for 5 years.
The treatment was well tolerated by the patient, who remains well, with no signs of recurrence at last follow-up, 18 months after surgery.
3. Discussion
Multiple primary malignancies represent 16% of new cancer diagnoses.8 A population-based study from the Netherlands reported a prevalence of 7%.9 Most patients (6.5%) had two cancer diagnoses, but there were also patients with three (0.5%) and four or more (0.05%) cancer diagnoses. Indeed, there have been reports of up to five malignancies in a single patient in the literature.10
In a recent commentary, the available evidence on second primary cancers according to etiology, i.e. genetic, cancer treatment and due to lifestyle/environmental influences was reviewed,8 and recommendations for future research priorities were made.
Numerous studies report associations between the specific neoplasms encountered in our patient. Research on subsequent malignancies after endometrial cancer has shown an increase in risk in colorectal, urinary bladder, lung and breast primaries.3,5,6,11
Almost one third of endometrial cancer patients receive adjuvant radiotherapy and this subgroup shows an increase in rectal and colon cancer. Interestingly, this increase persists even 10 years after endometrial cancer diagnosis and this led the authors to recommend a lengthy surveillance period.6 In another study also examining the effect of adjuvant therapy, the authors concluded that this increase could be only partially attributed to adjuvant radiation of the pelvis and, noting the bidirectional association they observed between colon and endometrial cancer, suggested the possibility of genetic associations (including hereditary nonpolyposis colorectal cancer syndrome) and environmental factors also playing a role.3 A large case series has shown a statistically significant increase in risk of breast cancer as a second primary, while also showing a trend for colorectal cancer.4 This has been corroborated by a population-based study.5 The high rates of positive family history (nearly 50%) in this group of patients again pointed towards a genetic and/or environmental interaction.
4. Conclusion
Despite the available evidence, there are at present no validated and generally accepted strategies for screening of subsequent primary tumours in patients with endometrial cancer.12 Our report constitutes a paradigm of a patient successfully treated and followed-up for endometrial carcinoma but not adequately screened for subsequent neoplasms, which remain undiagnosed until they have reached an advanced stage. The mounting evidence that endometrial cancer survivors face a considerable risk of developing other types of cancer has led to physician awareness and, in some cases, to an increase in screening compliance.7 Until future studies incorporate the increased risk for specific types of neoplasms into appropriate guidelines, physicians should ensure that endometrial cancer survivors are counseled on this risk, while not only being investigated for recurrence of the primary tumor, but also screened for other cancer types using the recommendations for the general population.
Conflict of interest
None declared.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors’ contribution
All authors have equally contributed in drafting the manuscript. Marinis A. and Rizos S critically revised it and gave final approval for submission.
Acknowledgement
The authors would like to thank Ms Myrto Kogevina for the editing of the manuscript.
References
- 1.Billroth T. Die allgemeine chirurgische pathologie and therapie. In: Reimer G., editor. 51 Vorlesungen-Ein Handbuch fur Studierende and Artze, 14. Auflage; Berlin: 1889. [Google Scholar]
- 2.Warren S., Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. American Journal of Cancer. 1932;16:1358–1414. [Google Scholar]
- 3.Brown A.P., Neeley E.S., Werner T. A population-based study of subsequent primary malignancies after endometrial cancer: genetic, environmental, and treatment-related associations. International Journal of Radiation Oncology Biology Physics. 2010;78(1):127–135. doi: 10.1016/j.ijrobp.2009.07.1692. [DOI] [PubMed] [Google Scholar]
- 4.Re A., Taylor T.H., DiSaia P.J. Risk for breast and colorectal cancers subsequent to cancer of the endometrium in a population-based case series. Gynecology Oncology. 1997;66(2):255–257. doi: 10.1006/gyno.1997.4766. [DOI] [PubMed] [Google Scholar]
- 5.Hemminki K., Aaltonen L., Li X. Subsequent primary malignancies after endometrial carcinoma and ovarian carcinoma. Cancer. 2003;97(10):2432–2439. doi: 10.1002/cncr.11372. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S., Shah J.P., Bryant C.S. Second neoplasms in survivors of endometrial cancer: impact of radiation therapy. Gynecology Oncology. 2009;113(2):233–239. doi: 10.1016/j.ygyno.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Kwon J.S., Elit L., Saskin R. Secondary cancer prevention during follow-up for endometrial cancer. Obstetrics and Gynecology. 2009;113(4):790–795. doi: 10.1097/AOG.0b013e31819c842d. [DOI] [PubMed] [Google Scholar]
- 8.Travis L.B., Rabkin C.S., Brown L.M. Cancer survivorship – genetic susceptibility and second primary cancers: research strategies and recommendations. Journal of the National Cancer Institute. 2006;98(1):15–25. doi: 10.1093/jnci/djj001. [DOI] [PubMed] [Google Scholar]
- 9.Liu L., de Vries E., Louwman M. Prevalence of multiple malignancies in the Netherlands in 2007. International Journal of Cancer. 2011;128(7):1659–1667. doi: 10.1002/ijc.25480. [DOI] [PubMed] [Google Scholar]
- 10.Cercato M.C., Colella E., Ferraresi V. Report of two cases of quintuple primary malignancies and review of the literature. Anticancer Research. 2008;28(5B):2953–2958. [PubMed] [Google Scholar]
- 11.Curtis R.E., Freedman D.M., Ron E. National Cancer Institute. NIH Publ. No. 05-5302; Bethesda, MD: 2006. New malignancies among cancer survivors: seer cancer registries, 1973–2000. [Google Scholar]
- 12.Vogel V.G. Identifying and screening patients at risk of second cancers. Cancer Epidemiol Biomarkers Prevention. 2006;15(11):2027–2032. doi: 10.1158/1055-9965.EPI-06-0416. [DOI] [PubMed] [Google Scholar]


