Abstract
Objectives
To assess the impact of atopy and allergy on the risk of clinical malaria.
Design
A clinical and immunological allergy cross-sectional survey in a birth cohort of 175 children from 1 month to 14 years of age followed for up to 15 years in a longitudinal open cohort study of malaria in Senegal. Malaria incidence data were available for 143 of these children (aged 4 months to 14 years of age) for up to 15 years. Mixed-model regression analysis was used to determine the impact of allergy status on malaria incidence, adjusting for age, gender, sickle-cell trait and force of infection.
Main outcome measures
Asthma, allergic rhinoconjunctivitis and atopic dermatitis status, the number of clinical Plasmodium falciparum malaria episodes since birth and associated parasite density.
Results
12% of the children were classified as asthmatic and 10% as having atopic dermatitis. These groups had respectively a twofold (OR 2.12 95%; CI 1.46 to 3.08; p=8×10−5) and threefold (OR 3.15; 1.56 to 6.33; p=1.3×10−3) increase in the risk of clinical P falciparum malaria once older than the age of peak incidence of clinical malaria (3–4 years of age). They also presented with higher P falciparum parasite densities (asthma: mean 105.3 parasites/μL±SE 41.0 vs 51.3±9.7; p=6.2×10−3. Atopic dermatitis: 135.4±70.7 vs 52.3±11.0; p=0.014). There was no effect of allergy on the number of non-malaria clinical presentations. Individuals with allergic rhinoconjunctivitis did not have an increased risk of clinical malaria nor any difference in parasite densities.
Conclusions
These results demonstrate that asthma and atopic dermatitis delay the development of clinical immunity to P falciparum. Despite the encouraging decrease in malaria incidence rates in Africa, a significant concern is the extent to which the increase in allergy will exacerbate the burden of malaria. Given the demonstrated antiparasitic effect of antihistamines, administration to atopic children will likely reduce the burden of clinical malaria in these children, increase the efficacy of first-line treatment antimalarials and alleviate the non-infectious consequences of atopy.
Article summary.
Article focus
Genetic studies suggest a link between susceptibility to allergy and malaria in Africa.
We hypothesise that atopy increases susceptibility to malaria.
Key messages
Results demonstrate an association between asthma, atopic dermatitis and susceptibility to clinical Plasmodium falciparum episodes.
Genetic predisposition to asthma or atopic dermatitis impairs the acquisition of clinical immunity to malaria.
Administration of antihistamines to atopic children will likely reduce the burden of clinical malaria in these children, increase the efficacy of first-line treatment antimalarials and alleviate the non-infectious consequences of atopy.
Strengths and limitations of this study
The major strength of this study is the complete knowledge of the number of clinical P falciparum malaria episodes each individual has had since birth and the exposure level per trimester over the 15 years covering the birth cohort. No other study has such detailed information for such a length of time. The major weakness of the study is the relatively small sample size, which would have reduced power to detect an association.
Introduction
The World Allergy Organisation estimates that 40% of the world's population is concerned by allergic diseases.1 In developing countries where Plasmodium falciparum malaria is endemic, prevalence of allergy is significantly lower, but is on the increase.2 T helper type 2 (Th2) cells, their related cytokines, IgE, eosinophils and mast cells (MCs) play a major role in allergic inflammation. Orientation of the immune response towards a Th1 profile is crucial for immunity to intracellular pathogens,3 whereas orientation towards a Th2 profile drives immunity to extracellular pathogens and antigens resulting in class switching giving rise to IgE-producing B cells.4 A role of the Th1/Th2 balance in the development of clinical malaria following infection by P falciparum has been suggested by numerous studies.5–7 While it is recognised that acquired antiparasite immunity is IgG dependent,8 parasite-specific IgE also impact upon the clinical outcome of infection. For example, higher IgE but not IgG levels have been observed in patients with cerebral malaria than those with uncomplicated P falciparum infection.9 The role of IgE, however, remains unclear.10
The interplay between infectious agents and allergy is ambiguous. On the one hand, for example, severe respiratory syncytial virus infection in infants increased the risk of allergic rhinoconjunctivitis and allergic asthma.11 12 On the other hand, measles,13 hepatitis A14 and tuberculosis15 seemingly reduce atopy. Although, an atopic condition can increase incidence of disease, such as the case for the skin commensal Staphylococcus aureus in patients with atopic dermatitis,16 an atopic tendency per se does not generally lead to increased illness from infectious agents.
Genome-wide studies have identified chromosomal regions linked to clinical malaria, all of which overlap with those previously identified to be involved in atopic dermatitis, asthma, atopy and IgE levels,17–19 suggesting that common mechanisms may be involved in both pathologies.20 Chromosomal region 5q31 that has been repeatedly shown to be associated with control of parasite density and contains a cluster of cytokines, among which IL12B has been previously associated with psoriasis.21 The other regions, 13q13–q22, 5p15–p13 and 12q21–q23, contain genes involved in innate immunity, notably the interleukin 7 receptor, and several involved in tumour necrosis factor synthesis (C1q and tumour necrosis factor-related protein 3 (C1QTNF3)) and a gene involved in the complement system (C9).20
Several additional lines of evidence support the concept that susceptibility to malaria and atopy may be related to similar immunological defects. In Ethiopia, a history of malaria was associated with atopy.22 A mouse model for human atopic disease was found to be very susceptible to murine malaria and a major locus for atopic disease mapped close to the region controlling parasite density.23 This region contains several candidate genes that have effects on T cell function.23
Moreover, a direct effect of histamine in the malaria pathogenesis has been found using genetic and pharmacological approaches24 and increased levels of histamine are associated with the severity of disease in humans infected with P falciparum and in animal malaria models.25 26
To test the hypothesis that allergy impacts upon clinical P falciparum malaria, we performed a clinical allergy cross-sectional study in the family-based longitudinal cohort from Senegal previously used for the genome linkage study20 and analysed the impact of asthma, atopic dermatitis, allergic rhinoconjunctivitis on the incidence of clinical P falciparum episodes and the maximum parasite density during each episode.
Methods
Population and outcome data
The malaria research programme conducted in Dielmo village in Senegal has been ongoing since 1990 as described elsewhere.27 In brief, between 1990 and 2008, a longitudinal study involving the inhabitants of the village of Dielmo, Senegal, was carried out to identify all episodes of fever. The study design included daily medical surveillance with systematic blood testing of individuals with fever and examination of 200 oil-immersion fields on a thick blood film for malaria parasites (about 0.5 μL of blood). Each individual was given a unique identification code and details of family ties, occupation and precise place of residence were recorded on detailed maps of each household with the location of each bedroom. All households were visited daily, absenteeism recorded and the presence of fever or other symptoms assessed. We systematically recorded body temperature at home three times a week (every second day) in children younger than 5 years, and in older children and adults in cases of suspected fever or fever-related symptoms. In cases of fever or other symptoms, blood testing was carried out at the dispensary by finger prick, and we provided detailed medical examination and specific treatment. Parasitologically confirmed clinical malaria episodes were treated according to national guidelines. From 1990 to 2008, four different drug regimens were implemented: quinine from 1990 to 1994, chloroquine from 1995 to 2003, fansidar (sulfadoxine-pyrimethamine) from 2004 to mid-2006 and artemisinin-based combination therapy (Amodiaquine-sulfadoxine-pyrimethamine; ACT) from mid-2006 to 2008.
Parasite positivity was established as follows. Thick blood films were prepared and stained by 3% Giemsa stain. Blood films were examined under an oil immersion objective at ×1000 magnification by the trained laboratory technicians and 200 thick film fields were examined to count the number of asexual and gametocyte parasite stages. Asexual parasite densities (per µL) were calculated by establishing the ratio of parasites to white blood cells and then multiplying the parasite count by 8000, the average white cell count per µL of blood.
Malaria transmission in Dielmo is intense and perennial. We conducted a cross-sectional survey to estimate the prevalence of symptoms related to allergic diseases among 175 children aged from 1 month to 14 years old who were born during the malaria research programme.
Both the longitudinal and cross-sectional surveys were approved by the Ministry of Health of Senegal. Informed consent of the volunteers is renewed every year. More specifically for the cross-sectional survey, after informing about the procedures and the purpose of the study, written informed consent was obtained from parents or guardians of children either by signature or by thumbprint on a voluntary consent form written in both French and Wolof, the main local language. Consent was obtained in the presence of the school director, an independent witness.
The family structure (pedigree) was available after a demographic census performed for every volunteer at his adhesion in the project. A verbal interview of mothers or key representatives of the household was used to obtain information on genetic relationships between studied individuals, their children, their parents and to identify genetic links among the population. The total pedigree comprised 828 individuals, including absent or dead relatives, composed of 10 independent families that can be subdivided into 206 nuclear families (father–mother couples with at least one child) with an average of 3.6 children each. Genetically related nuclear families occur because of multiple marriages and marriages among related individuals. Previous typing with microsatellites has enabled the construction of a pedigree based on Identity-by-Descent using MERLIN.20 28 The mean coefficient of inbreeding is 0.0008. Newborns since this original genetic analysis were added to the family of the parents in question. The 143 children, with allergy and malaria data, belonged to 61 nuclear families and comprised 30 singletons, 102 siblings and 11 half-sibs (yielding 55 half-sib pairs). The mean genetic relatedness (by pedigree) of the 143 children is 0.0114 (range: 0.0013–0.022).
P falciparum clinical episodes
P falciparum malaria clinical episode phenotypes analysed were: (1) clinical P falciparum infections treated with antimalarial therapy and (2) the highest parasite density during the P falciparum clinical episode. A clinical P falciparum episode was defined as a clinical presentation with fever (axillary temperature ≥37.5°C) and/or other clinical signs suggestive of malaria associated with a thick blood smear positive for P falciparum and that was treated with antimalarial therapy. Repeated clinical malaria presentations within 15 consecutive days were not considered to be independent and were excluded from the analyses, unless there was a negative thick blood smear between two clinical presentations. We also excluded observations in any trimester for which the individual was not present for at least one-third of the time.
We calculated the quarterly incidence rate of clinical P falciparum episodes in children below the age of 15 years as the ratio of the total number of clinical P falciparum episodes during the trimester divided by the total number of person-trimesters surveyed. Incidence rate is expressed as cases per 100 person-trimesters (see online supplementary figure S1). This rate was used in the analysis to approximate the force of infection (exposure level) within the targeted population at the time of a given clinical P falciparum episode.
The total number of clinical presentations per trimester that were not attributable to P falciparum was tabulated. Repeated non-malaria presentations within seven consecutive days were not considered to be independent and were excluded.
Allergic diseases and atopic status
The International Study of Asthma and Allergies in Childhood (ISAAC) diagnostic criteria have been shown to be reproducible, adequate and able to discriminate children with allergic diseases in different areas of the world.2 The standardised ISAAC questionnaire originally written in English was translated into French in compliance with ISAAC guidelines29 adapting it to the usual local customs following advice from local clinicians and paediatric allergologists (acknowledgements and see online supplementary technical appendix). The adequacy and reliability of the translated questionnaire had been previously confirmed by a pilot study on 30 randomly selected children in the same community. The questionnaire was completed by specially trained health workers during an oral interview conducted in Wolof with children and their mothers or guardians.
To assess the prevalence of allergic diseases in children, we used the positive and negative predictive values of the ISAAC questionnaire diagnosis criteria developed for subtropical countries.30 Each question was scored according to the medical diagnosis of paediatricians and paediatric allergologists. Positive or negative answers were thus graded on the basis of symptom sensitivity, specificity, frequency, location or early onset. For each allergic disease, three categories of symptom severity, severe, moderate and none, were defined as follows:
Asthma—severe symptoms if the child had ‘wheezing or whistling in the chest before the age of two years’ and ‘more than three times’ or severe enough to ‘limit his/her speech’; moderate symptoms if the child had ‘wheezing or whistling in the chest before the age of two years’ and ‘in the past 12 months’; and none otherwise.
Allergic rhinoconjunctivitis—severe symptoms if the child had ‘sneezing, runny or stuffy nose in the past 12 months’ and ‘more than five times a year’ and ‘itchy, watery eyes or tropical endemic limboconjunctivitis (TELC) in the past 12 months’; moderate symptoms if the child had ‘sneezing, runny or stuffy nose in the past 12 months’, and ‘itchy, watery eyes or TELC in the past 12 months’ and none otherwise.
Atopic dermatitis—severe symptoms if the child had ‘scaly or exudating, crusted and pruritic patches in the past 12 months’ and ‘affecting any of the following characteristic areas: face, around the ears or eyes, folds of armpits or elbows or groin, behind the knees, under the buttocks’ and ‘onset of symptoms before the age of 2 years’; moderate symptoms if the child had ‘scaly or exudating, crusted and pruritic patches in the past 12 months’ and ‘affecting any of characteristic areas (see above) ’, and ‘onset of symptoms before the age of 4 years’ and none otherwise.
The inter-relationships between variables reflecting the severity of symptoms of the three allergic diseases were used to identify children at high risk of atopy. The high probability group was defined by the prevalence of at least one of any severe symptoms or two of any moderate symptoms. The probable group was defined as those with moderate symptoms from one of the three allergic diseases and remaining children were classified in the unlikely group.
Helminths
Helminthic infections are common in this region and are known to modify the clinical course and outcome of both allergic diseases and malaria.31 32 We therefore carried out a helminth survey for 91 individuals present during the cross-sectional survey. Diagnosis was performed by stool examination by microscope and by the Kato technique to search for the presence of Ascaris lumbricoides, hookworms (Ancylostoma duodenale and Necator americanus), whipworm (Trichuris trichiuria), Schistosoma mansoni and Strongyloides stercoralis. Examination for pinworms (Enterobius vermicularis) was performed by the anal scotch-test. An antihelminthic treatment was proposed for all infested individuals.
IgE titres
Specific IgE titres were measured by ELISA as previously described.33 A panel of allergens of potential pertinence to the three classes of allergy was used: (1) salivary gland extracts (SGE) of two mosquito species present in the study cohorts, Aedes aegypti and Anopheles gambiae sensu stricto and (2) P falciparum parasite extract were prepared as previously described31; (3) House dust mite spp. Dermatophagoides farinae and Dermatophagoides pteronyssinus; (4) a mix of pollen allergens from five ubiquitous graminae spp. (Cock's-foot (Dactylis glomerata), Timothy grass (Phleum pratense), Sweet Vernal grass (Anthoxanthum odoratum), Perennial ryegrass (Lolium perenne), Kentucky Bluegrass (Poa pratensis)) (all from Stallergenes, France).
Statistical analysis
Statistical analyses were performed using R V.2.12.0 (The R Foundation for Statistical Computing, Vienna, Austria). To address the effect of allergic status on the risk of clinical P falciparum episodes, we performed Generalised Linear Mixed Models (GLMM) extended to pedigree data using the pedigreemm package for R to account for the non-independence of individuals because of family relationships, shared house and for repeated measures from the same individual (see online supplementary technical appendix). Correlated individual effects due to familial relationships were taken into account by using the pedigree-based genetic relatedness matrix that contains the genetic covariance among all pairs of individuals in the study cohort and is calculated using the pedigree information.34 Shared house and repeated measures from the same individual were modelled as random effects. All random effects were assumed to be normally distributed, and conditional on these random effects, the dependent variable had: (1) a Binomial distribution when the studied phenotype was the occurrence of a clinical P falciparum episode treated with antimalarial therapy during a trimester, (2) a Gaussian distribution when the studied phenotype was the logarithm of the maximum parasite density during a given clinical P falciparum episode and (3) a Poisson distribution when the studied phenotype was the number of non-malaria episodes per trimester. The effects of allergy disease classes on these dependent variables were modelled as fixed effects. Allergy classes were reduced to two levels, severe or moderate vs none for analyses of asthma, atopic dermatitis and allergic rhinoconjunctivitis and high probability vs probable and unlikely for atopic tendency. Covariables included sickle cell trait33 gender, number of days present on site during the trimester, trimestrial incidence of P falciparum and age. Age was initially analysed as a continuous covariate. To assess the age-specific effect of allergy, age was categorised into two levels (<3.5 years of age and ≥3.5 years of age, based on the age of peak clinical incidence) and allergy class was nested within age class. The age threshold was varied from 1.5 to 5.5 years of age and the data reanalysed to assess at which age there was the strongest effect. The association of allergy classes with IgE levels was analysed by Box-Cox transforming the data and fitting a GLMM with a normal distribution.
Results
Of the 205 eligible children aged under 15 years involved in the family-based longitudinal study, 175 (85.4%) participated in the cross-sectional survey to assess the prevalence of related symptoms of allergic diseases. All eligible children present at the time of the survey were included; no explicit refusal to participate was recorded. The study cohort was aged from 1 month to 14 years 11 months. The sex-ratio (male/female) was 0.94.
From 1994 until 2008, 143 of the children participating in the cross-sectional survey were present for at least 31 days in any trimester during the study period generating a total of 3093 person-trimesters of presence (see online supplementary table S1). There were 2065 treated P falciparum clinical episodes (per individual: median 11, range 0–47; see online supplementary table S2). The age peak of incidence of P falciparum episodes occurred at 3–4 years of age (figure 1). There were 1868 non-malaria episodes (median 12, range 0–37) (see online supplementary table S2). These non-malaria clinical presentations were associated with headache (38%), chills (32%), cough (13%), vomiting (11%) and diarrhoea (6%).
Figure 1.
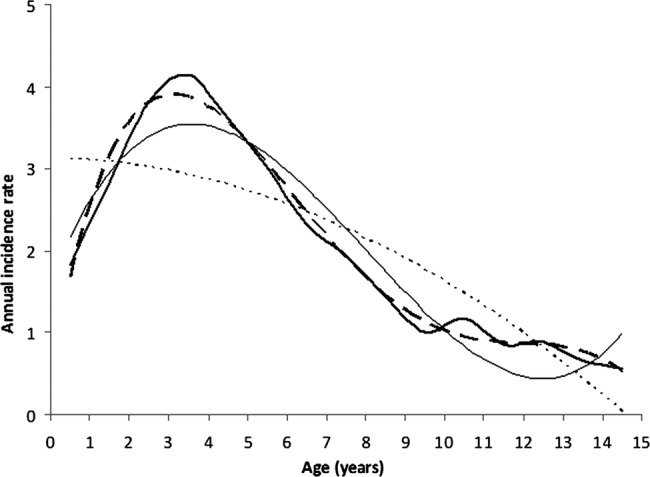
Annual incidence rate of clinical Plasmodium falciparum episodes per 100 children (bold line). In order to overcome the fluctuations of the annual incidence rate, we fit second (dotted line), third (dashed line) and fourth (solid line) degree polynomial trend lines to the data (bold line). The corresponding R2 values are 0.70, 0.91 and 0.99, respectively, indicating an accurate fit for third and fourth order polynomials. The inflexion on these two trend lines indicates the onset of acquisition of clinical immunity at approximately 3–4 years of age.
The prevalence of moderate or severe asthma symptoms was respectively 2.3% and 10.3% (table 1). The prevalence of moderate or severe allergic rhinoconjunctivitis symptoms was respectively 6.3% and 10.3%. The prevalence of moderate or severe atopic dermatitis symptoms was respectively 6.3% and 2.9%. On the basis of symptom severity, an atopic tendency was estimated to be unlikely for 68%, probable for 9.1% and highly probable for 22.9% of the 175 children. The frequency of each allergy class in children for whom malaria data were available is shown in online supplementary table S1.
Table 1.
Classification of asthma, allergic rhinoconjunctivitis, atopic dermatitis and overall Atopic status according to International Study of Asthma and Allergies in Childhood questionnaire in children aged 0–14 from a malaria birth cohort
| N (F/M) | Per cent | n-Malaria (F/M) | |
|---|---|---|---|
| Asthma symptoms | |||
| None | 153 (73/80) | 87.43 | 125 (59/66) |
| Moderate | 4 (1/3) | 2.29 | 4 (1/3) |
| Severe | 18 (6/12) | 10.29 | 14 (4/10) |
| Rhinoconjunctivitis symptoms | |||
| None | 146 (64/82) | 83.43 | 120 (52/68) |
| Moderate | 11 (8/3) | 6.29 | 9 (6/3) |
| Severe | 18 (6/12) | 10.29 | 14 (6/8) |
| Atopic dermatitis symptoms | |||
| None | 159 (75/84) | 90.86 | 128 (60/68) |
| Moderate | 11 (1/10) | 6.29 | 11 (1/10) |
| Severe | 5 (4/1) | 2.86 | 4 (3/1) |
| Atopic tendency | |||
| Unlikely | 119 (56/63) | 68.00 | 97 (46/51) |
| Probable | 16 (8/8) | 9.14 | 14 (6/8) |
| Highly probable | 40 (16/24) | 22.86 | 32 (12/20) |
N is total number of children examined and n-malaria represents those for whom malaria data were recorded. F is the number of females and M the number of males.
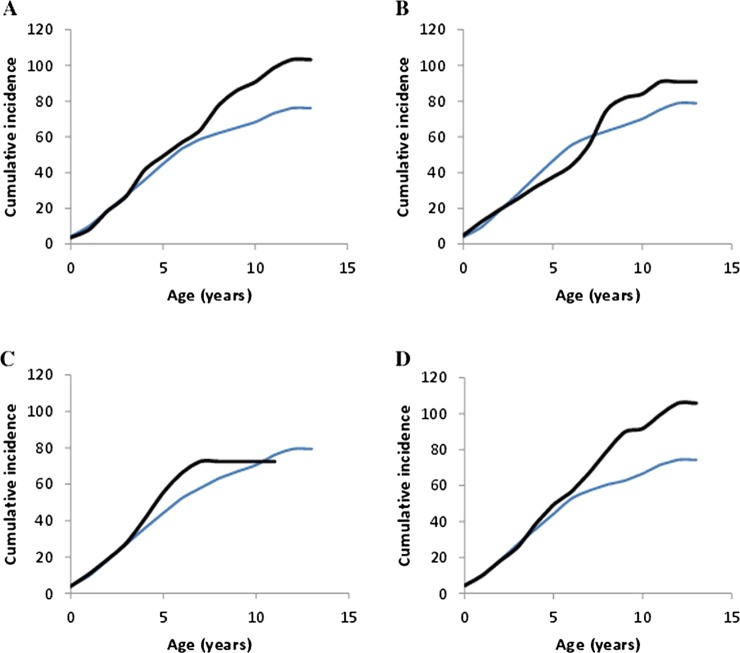
The risk of treated clinical P falciparum infections was higher for children with high probability of atopy (OR 1.65; 95% CIs 1.20 to 2.26; p=0.002; table 2), after adjusting for age, sickle-cell trait and the exposure level. Gender was not found to be significant. Analysing the impact of atopy in children younger and older than the peak age of clinical incidence (3–4 years old) revealed that atopy increased the risk of P falciparum episodes in children at an age greater than 3.5 years (OR 2.02, 1.39–2.93; p=2×10–4), but not in children of age prior to the peak clinical incidence (OR 1.38, 0.92 to 2.08; p=0.124; table 2). This increased risk resulted in an ever increasing cumulative number of P falciparum episodes with age beyond that of peak clinical incidence (figure 2; see online supplementary figure S2 for model predictions for comparison).
Table 2.
Impact of allergy status on risk of Plasmodium falciparum clinical episodes
| Age groups (3.5 years) | aOR | 95% CI |
p Value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Atopy | Both | 1.65 | 1.20 | 2.26 | 2.0×10−3 |
| <3.5 | 1.38 | 0.92 | 2.08 | 0.124 | |
| ≥3.5 | 2.02 | 1.39 | 2.93 | 2.1×10−4 | |
| Asthma | Both | 2.12 | 1.46 | 3.08 | 8.0×10−5 |
| <3.5 | 1.50 | 0.90 | 2.50 | 0.122 | |
| ≥3.5 | 2.33 | 1.50 | 3.61 | 1.5×10−4 | |
| Atopic dermatitis | Both | 1.05 | 0.65 | 1.70 | 0.842 |
| <3.5 | 0.84 | 0.49 | 1.46 | 0.539 | |
| ≥3.5 | 3.15 | 1.56 | 6.33 | 1.3×10−3 | |
| Rhinoconjunctivitis | Both | 0.96 | 0.65 | 1.41 | 0.818 |
| <3.5 | 1.05 | 0.64 | 1.72 | 0.853 | |
| ≥3.5 | 0.95 | 0.60 | 1.52 | 0.834 | |
| Age ≥3.5 | 0.48 | 0.40 | 0.57 | 2.7×10−15 | |
| Trimestrial incidence | 1.01 | 1.00 | 1.01 | 1.8×10−6 | |
| HbAS | 0.24 | 0.12 | 0.47 | 3.7×10−5 | |
Shown are the p values and adjusted ORs with 95% CIs calculated from the mixed-model analyses. Values for the covariables age (≥3.5 years of age compared with <3.5 years of age), trimestrial incidence of P falciparum clinical episodes and HbAS (β-globin sickle-cell trait; AS compared with AA) are those from the Asthma model analysis. For clarity significant covariables are shown in bold.
Figure 2.
Mean cumulative number of Plasmodium falciparum clinical episodes with age for the (A) asthma, (B) rhinoconjunctivitis and (C) atopic dermatitis classes and overall atopy class (D) (bold lines) compared with individuals without symptoms of each respective allergy type (thin lines). In all cases moderate and severe classes are combined and compared with individuals without allergy symptoms. Note there are no children older than 11 years of age with atopic dermatitis.
Analysis by allergy category revealed that asthma (severe or moderate) increases the risk of P falciparum episodes (OR 2.12; 1.46 to 3.08; p=8×10−5) and this again only in children of age greater than 3.5 years old (OR 2.33; 1.50 to 3.61; p=1.5×10−4). Atopic dermatitis increased the risk of clinical malaria in children older (OR 3.15; 1.56 to 6.33; p=1.3×10−3) but not younger than 3.5 years of age (table 2). Allergic rhinoconjunctivitis was not associated with increased risk of clinical malaria at any age (table 2). The impact of atopy, asthma and atopic dermatitis can be clearly seen in the ever-increasing number of cumulative P falciparum episodes beyond the age of the onset of clinical immunity in the population, 3.5 years of age (figure 2). There is no difference in the number of clinical malaria episodes prior to this age in individuals with or without an allergic condition. Analysis using different age thresholds (from 1.5 to 5.5 years of age) revealed similar OR for thresholds of 2.5, 3.5 and 4.5 years of age. The maximum OR for increased malaria occurred in children older than 4.5 years of age and with atopy or atopic dermatitis, whereas for the asthma group it occurred in children after 3.5 years of age (see online supplementary table S3).
There was no impact of any allergic disease on the number of non-malaria episodes by trimester (see online supplementary table S4).
The impact of atopy, asthma and atopic dermatitis on the maximum P falciparum parasite density during a given clinical malaria episode mirrored that of the risk of P falciparum episodes. Parasite density was significantly higher for children with allergic disease older than 3.5 years of age (table 3 and see online supplementary figure S3 for residuals of the fitted model). As the log-transformed data were left skewed, we additionally analysed using Box-Cox transformation and probit normalisation of the data. The results were qualitatively the same (see online supplementary text and figures S4–S8). Allergic rhinoconjunctivitis had no impact on the parasite density (table 3). Analysis using different age thresholds yielded similar qualitative conclusions as seen with the number of clinical episodes (see online supplementary table S3).
Table 3.
Impact of allergy status on the maximum Plasmodium falciparum parasite density during a clinical malaria episode
| Allergic condition | Age groups | Allergic status (no/yes) | Mean parasite density | SEM | p Value |
|---|---|---|---|---|---|
| Atopy | Both | N | 76.3 | 13.8 | |
| Y | 131.0 | 36.4 | 0.0158 | ||
| <3.5 | N | 114.3 | 23.7 | ||
| Y | 171.1 | 56.0 | 0.148 | ||
| ≥3.5 | N | 48.4 | 9.8 | ||
| Y | 114.8 | 37.1 | 9.5×10−4 | ||
| Asthma | Both | N | 78.1 | 14.4 | |
| Y | 148.5 | 44.3 | 3.8×10−3 | ||
| <3.5 | N | 114.8 | 24.3 | ||
| Y | 171.9 | 74.5 | 0.167 | ||
| ≥3.5 | N | 51.3 | 9.7 | ||
| Y | 105.3 | 41.0 | 6.2×10−3 | ||
| Atopic dermatitis | Both | N | 82.6 | 15.0 | |
| Y | 93.9 | 38.9 | 0.605 | ||
| <3.5 | N | 122.6 | 25.5 | ||
| Y | 133.9 | 63.5 | 0.425 | ||
| ≥3.5 | N | 52.3 | 11.0 | ||
| Y | 135.4 | 70.7 | 0.014 | ||
| Rhinoconjunctivitis | Both | N | 81.5 | 14.8 | |
| Y | 111.4 | 39.0 | 0.570 | ||
| <3.5 | N | 118.8 | 25.1 | ||
| Y | 166.3 | 69.9 | 0.537 | ||
| ≥3.5 | N | 54.6 | 11.3 | ||
| Y | 80.9 | 33.7 | 0.327 |
Shown are the back-transformed mean parasite densities per microlitre and SE measurements (SEM) estimated from the generalised linear mixed model analyses after taking into account the other covariables. Significantly different effects are shown in bold for clarity.
Individuals with moderate or severe symptoms of atopic dermatitis had significantly higher specific IgE titres against A aegypti (p=0.004) and A gambiae SGE (p<0.001). There were no detectable specific anti-P falciparum IgE. Individuals with moderate or severe symptoms of allergic rhinoconjunctivitis did not have significantly higher IgE titres against the tested graminae (p=0.28), although titres decreased with age (p=0.035). There was also no effect of asthma on IgE titres against the house dust mite spp. tested (D farinae p=0.60 and D pteronyssinus p=0.27).
Only five individuals were infested with helminths (two Ancylostoma, one Strongyloides, one Trichuris and one Enterobius).
Discussion
Principal findings
Establishing the allergic status of children up to the age of 15 years followed for malaria since birth, revealed an association of asthma and atopic dermatitis with susceptibility to clinical P falciparum episodes. Importantly the increase in risk of malaria associated with these allergic conditions occurred after the peak clinical incidence of disease in the population, suggesting that they delay the development of clinical immunity to malaria.
Strengths and weaknesses of the study
The major strength of this study is the complete knowledge of the number of clinical P falciparum malaria episodes each individual has had since birth and the exposure level per trimester over the 15 years covering the birth cohort. No other study has such detailed information for such a length of time. The major weakness of the study is the relatively small sample size, which would have reduced power to detect an association. In addition, although allergy diagnosis for children under 2 years of age is not considered reliable, there were only 15 individuals under 2 at the time of the allergy study of the 143 for whom malaria and allergy data were available.
Meaning of the study
Under intense malaria transmission, after repeated exposure to the parasite, children develop a clinical immunity35 whereby they tolerate elevated parasite densities without showing clinical symptoms. In this cohort, the population mean onset of clinical immunity occurred at 3–4 years of age. Although clinical immunity is accompanied by a reduction in parasite density, effective antiparasite immunity develops much more slowly36 with individuals achieving a state of premunition, whereby they maintain low-grade parasite densities in an asymptomatic state.37 We show here that children with clinically defined asthma or atopic dermatitis have an increased risk of presenting with P falciparum malaria episodes requiring treatment once passing the age of peak clinical incidence. They also had higher parasite density during clinical episodes, suggesting a reduced ability to control parasite replication. The observed increase in clinical incidence of malaria in patients with asthma or atopic dermatitis is not likely to be the result of increased frailty of such individuals; these individuals did not come more frequently to the clinic with non-malaria symptoms. Our previous genome linkage study identifying chromosomal regions20 associated with malaria that overlap with those previously shown to be linked to asthma/atopy suggests that there may be a shared genetic basis to these pathologies rather than any causative effect of one on the other. This is consistent with the increased susceptibility to malaria of mouse atopic models.23
Comparison with other studies
A previous study in Ethiopia (East Africa) found that a history of malaria (yes/no) increased risk of atopic dermatitis in 306 cases compared with 426 controls as characterised using the ISAAC questionnaire.22 The only other epidemiological study that has previously examined the link between malaria and atopy38 also interpreted the result from the perspective of the impact of malaria on atopy. They examined the reinfection rate with P falciparum over a 5-year period in 91 children who were subsequently classified as atopic or not using skin prick tests (SPT) with house dust mite antigen. Their conclusion was that, as with measles13 and tuberculosis15 malaria infection reduces atopy. However, the study lacked previous infection data since birth of the participating individuals and focused on atopy as determined by SPT against a single allergen. The case-control study of atopic dermatitis risk factors cited above found no overall association between allergen skin sensitisation and atopic dermatitis. We also found no evidence of increased IgE titres against house dust mites in the asthmatic or atopic dermatitis groups or against grass pollen in individuals with allergic rhinoconjunctivitis. Such differences likely reflect the different IgE reactivity profiles due to differences in allergen exposure in Africa.39 There was no evidence of antiparasite IgE in this cohort of children. We previously showed that circulating antiparasite IgE titres were strongly positively correlated with antimosquito saliva IgE, but became undetectable following malaria exposure, potentially being bound to effector cells.33 Only mosquito saliva, a known major local allergen, induced a specific IgE response at significantly higher titres in individuals with atopic dermatitis.
Although the immune effectors of clinical immunity are still poorly defined, there is strong evidence that acquired antiparasite immunity is IgG-dependent8 and cytophilic immunoglobulins (IgG1 and IgG3), which are capable of eliminating the parasites by opsonisation and/or by antibody dependent cellular immunity play an important role in premunition.37 The higher parasite density during symptomatic episodes observed in the asthma group suggests impaired development of acquired immunity. Impaired acquisition of immunity to malaria in children with asthma or atopic dermatitis may stem from their imbalanced Th1/Th2 response. Indeed, an atopic state may generate a tendency to develop a Th2 type immune response to P falciparum. Dendritic cells that are oriented to a Th2 phenotype are more susceptible to orient the acquired immune response towards a Th2 profile.40 Orientation of the immune response towards a Th2 profile by asthma or atopic dermatitis would result in a poor Th1 response (and hence development of protective IgG), considered to be the dominant arm of the immune response enabling resistance to infectious disease in children.41
Many studies have revealed an important role of histamine, a key downstream effector molecule in allergic reaction, in the outcome of a malaria parasite infection.24–26 42–45 Moreover, reports indicate that components of the innate immune system, including eosinophils, basophils and MCs, could play important roles in the pathogenesis of malaria.42 Increased levels of histamine in plasma and tissue, derived from basophils and MCs, notably following stimulation by IgE through the high affinity receptor FcɛR1, are associated with the severity of disease in humans infected with P falciparum and in animal malaria models.25 26 Chlorpheniramine, a HR1 agonist reversed resistance to chloroquine and amodiaquine both in vivo and in vitro.43 Moreover, astemizole, another HR1 agonist, was identified as an antimalarial agent in a clinical drug library screen.44 Finally, P falciparum produces translationally controlled tumour protein, which is a homologue of the mammalian histamine-releasing factor that causes histamine release from human basophils.45
Further research
Our results provide the first birth cohort study addressing the link between malaria and allergic diseases. They contribute to a growing body of evidence that the pathologies are related. ISAAC has revealed a steady but significant increase in prevalence rates of asthma and allergic diseases in Africa. While the majority of studies have focused on large cities, there is increasing urbanisation throughout Africa, as well as improved access to primary healthcare in many areas. A key concern for ISAAC is the extent to which such societal evolution will result in an increase in allergic diseases. Increased urbanisation in sub-Saharan Africa is changing the epidemiology of malaria and although resulting in a decrease in risk, will result in more severe clinical malaria in older individuals.46 47 Moreover, a large consumption of antimalarial drugs in the urban areas provides substantial drug pressure fostering the selection of drug-resistant parasites. Despite the encouraging recent decrease in malaria incidence rates, even in rural areas, an additional significant concern is the extent to which such an increase in allergy will exacerbate the burden of malaria. Given the demonstrated antiparasitic effect of antihistamines,48 administration of antihistamines to atopic children will likely reduce the burden of clinical malaria in these children, increase the efficacy of first-line treatment antimalarials49 and alleviate the non-infectious consequences of atopy. Clinical intervention studies should be envisaged.
Supplementary Material
Footnotes
Contributors: LB, SM and RP made substantial contributions to the concept and design of the study. MH, HB, BG, SB, FDS and AT were involved in acquisition of the data. CL, AF, OM-P, AS and RP contributed to the analysis and interpretation of the data. MH, CL, HB, BG, SB, FDS, AF, AT, LB, OM-P, SM, AS and RP critically reviewed the report and approved its final version for submission. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. MH and RP are guarantors.
Funding: Institut Pasteur de Dakar, Institut Pasteur and Agence Nationale de la Recherche, France (ANR 11-BSV1-027-01).
Competing interests: None.
Ethics approval: The allergy study was approved by the Senegalese National Ethics committee (2009/No. 46). Renewed approval of the longitudinal malaria study was obtained from the same committee (2006/No. 969).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The allergy database will be made available on-line. The longitudinal malaria data set will be made available following discussion with the coordinators of the three Institutes that govern the dataset through contact with the corresponding author.
References
- 1.WAO World Allergy Organization, 2010. http://www.worldallergy.org/index.php
- 2.Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006;368:733–43 [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol 1989;7:145–73 [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008;112:1557–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elghazali G, Perlmann H, Rutta AS, et al. Elevated plasma levels of IgE in Plasmodium falciparum-primed individuals reflect an increased ratio of IL-4 to interferon-gamma (IFN-gamma)-producing cells. Clin Exp Immunol 1997;109:84–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perlmann P, Perlmann H, ElGhazali G, et al. IgE and tumor necrosis factor in malaria infection. Immunol Lett 1999;65:29–33 [DOI] [PubMed] [Google Scholar]
- 7.Tangteerawatana P, Perlmann H, Hayano M, et al. IL4 gene polymorphism and previous malaria experiences manipulate anti-Plasmodium falciparum antibody isotype profiles in complicated and uncomplicated malaria. Malar J 2009;8:286–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature 1961;192:733–7 [DOI] [PubMed] [Google Scholar]
- 9.Perlmann H, Helmby H, Hagstedt M, et al. IgE elevation and IgE anti-malarial antibodies in Plasmodium falciparum malaria:association of high IgE levels with cerebral malaria. Clin Exp Immunol 1994;97:284–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duarte J, Deshpande P, Guiyedi V, et al. Total and functional parasite specific IgE responses in Plasmodium falciparum-infected patients exhibiting different clinical status. Malar J 2007;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–5 [DOI] [PubMed] [Google Scholar]
- 12.Sigurs N, Gustafsson PM, Bjarnason RS, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Resp Crit Care Med 2005;171:137–41 [DOI] [PubMed] [Google Scholar]
- 13.Shaheen SO, Aaby P, Hall AJ, et al. Measles and atopy Guinea-Bissau. Lancet 1996;347:1792–96 [DOI] [PubMed] [Google Scholar]
- 14.McIntire JJ, Umetsu SE, Macaubas C, et al. Immunology: hepatitis A virus link to atopic disease. Nature 2003;425:576. [DOI] [PubMed] [Google Scholar]
- 15.Shirakawa T, Enomoto T, Shimazu S, et al. The inverse association between tuberculin responses and atopic disorder. Science 1997;275:77–9 [DOI] [PubMed] [Google Scholar]
- 16.Gould HJ, Takhar P, Harries HE, et al. The allergic march from Staphylococcus aureus superantigens to immunoglobulin E. Chem Immunol Allergy 2007;93:106–36 [DOI] [PubMed] [Google Scholar]
- 17.Jang N, Stewart G, Jones G. Polymorphisms within the PHF11 gene at chromosome 13q14 are associated with childhood atopic dermatitis. Genes Immun 2005;6:262–4 [DOI] [PubMed] [Google Scholar]
- 18.Kurz T, Hoffjan S, Hayes MG, et al. Fine mapping and positional candidate studies on chromosome 5p13 identify multiple asthma susceptibility loci. J Allergy Clin Immunol 2006;118:396–402 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Leaves NI, Anderson GG, et al. Positional cloning of a quantitative trait locus on chromosome 13q14 that influences immunoglobulin E levels and asthma. Nat Genet 2003;34:181–6 [DOI] [PubMed] [Google Scholar]
- 20.Sakuntabhai A, Ndiaye R, Casademont I, et al. Genetic determination and linkage mapping of Plasmodium falciparum malaria related traits in Senegal. PLoS ONE 2008;3:e2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 2007;80:273–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haileamlak A, Dagoye D, Williams H, et al. Early life risk factors for atopic dermatitis in Ethiopian children. J Allergy Clin Immunol 2005;115:370–6 [DOI] [PubMed] [Google Scholar]
- 23.Kohara Y, Tanabe K, Matsuoka K, et al. A major determinant quantitative-trait locus responsible for atopic dermatitis-like skin lesions in NC/Nga mice is located on Chromosome 9. Immunogenetics 2001;53:15–21 [DOI] [PubMed] [Google Scholar]
- 24.Beghdadi W, Porcherie A, Schneider BS, et al. Inhibition of histamine-mediated signaling confers significant protection against severe malaria in mouse models of disease. J Exp Med 2008;205:395–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srichaikul T, Archararit N, Siriasawakul T, et al. Histamine changes in Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 1976;70:36–8 [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya U, Roy S, Kar PK, et al. Histamine & kinin system in experimental malaria. Indian J Med Res 1988;88:558–63 [PubMed] [Google Scholar]
- 27.Trape JF, Tall A, Diagne N, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis 2011;11:925–32 [DOI] [PubMed] [Google Scholar]
- 28.Abecasis GR, Cherny SS, Cookson WO, et al. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 2001;30:97–101 [DOI] [PubMed] [Google Scholar]
- 29.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995;8:483–91 [DOI] [PubMed] [Google Scholar]
- 30.Yamada E, Vanna AT, Naspitz CK, et al. International Study of Asthma and Allergies in Childhood (ISAAC): validation of the written questionnaire (eczema component) and prevalence of atopic eczema among Brazilian children. J Investig Allergol Clin Immunol 2002;12:34–41 [PubMed] [Google Scholar]
- 31.Nacher M. Malaria vaccine trials in a wormy world. Trends Parasitol 2001;17:563–5 [DOI] [PubMed] [Google Scholar]
- 32.Yazdanbakhsh M, Kremsner PG, Van Ree R. Allergy, parasites, and the hygiene hypothesis. Science 2002;296:490–4 [DOI] [PubMed] [Google Scholar]
- 33.Lawaly R, Konate L, Marrama L, et al. Impact of mosquito bites on asexual parasite density and gametocyte prevalence in asymptomatic chronic Plasmodium falciparum infections and correlation with IgE and IgG titres. Infect Immun 2012;80:2240–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado A, Loucoubar C, Grange L, et al. Human genetic contribution to the outcome of infection with malaria parasites. In: Okwa O. ed. Malaria parasites, Rijeka: INTECH, 2012:267–292 [Google Scholar]
- 35.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 2009;22:13–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh K, Snow RW. Host-parasite interaction and morbidity in malaria endemic areas. Philos Trans R Soc London B 1997;352:1385–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perignon JL, Druilhe P. Immune mechanisms underlying the premonition against Plasmodium falciparum malaria. Mem Inst Oswaldo Cruz 1994;89(Suppl. 2):51–3 [DOI] [PubMed] [Google Scholar]
- 38.Lell B, Borrmann S, Yazdanbakhsh M, et al. Atopy and malaria. Wien Klin Wochenschr 2001;113:927–9 [PubMed] [Google Scholar]
- 39.Westritschnig K, Sibanda E, Thomas W, et al. Analysis of the sensitization profile towards allergens in central Africa. Clin Exp Allergy 2003;33:22–7 [DOI] [PubMed] [Google Scholar]
- 40.De Jong EC, Vieira PL, Kalinski P, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol 2002;168:1704–9 [DOI] [PubMed] [Google Scholar]
- 41.Baird JK. Age-dependent characteristics of protection versus susceptibility to Plasmodium falciparum. Ann Trop Med Parasitol 1998;92:367–90 [DOI] [PubMed] [Google Scholar]
- 42.Mecheri S. Contribution of allergic inflammatory response to the pathogenesis of malaria disease. Biochim Biophys Acta 2011;1822:49–56 [DOI] [PubMed] [Google Scholar]
- 43.Sowunmi A, Gbotosho GO, Happi CT, et al. Enhancement of the antimalarial efficacy of amodiaquine by chlorpheniramine in vivo. Mem Inst Oswaldo Cruz 2007;102:417–19 [DOI] [PubMed] [Google Scholar]
- 44.Chong CR, Chen X, Shi L, et al. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat Chem Biol 2006;2:415–16 [DOI] [PubMed] [Google Scholar]
- 45.MacDonald SM, Bhisutthibhan J, Shapiro TA, et al. Immune mimicry in malaria: Plasmodium falciparum secretes a functional histamine-releasing factor homolog in vitro and in vivo. Proc Natl Acad Sci USA 2001;98:10829–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Silva PM, Marshall JM. Factors contributing to urban malaria transmission in sub-Saharan Africa: a systematic review. J Trop Med 2012. 819563.10.1155/2012/819563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bob NS, Diop BM, Renaud F, et al. Parasite polymorphism and severe malaria in Dakar (Senegal): a West African urban area. PLoS ONE 2010;5:e9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musonda CC, Whitlock GA, Witty MJ, et al. Chloroquine-astemizole hybrids with potent in vitro and in vivo antiplasmodial activity. Bioorg Med Chem Lett 2009;19:481–4 [DOI] [PubMed] [Google Scholar]
- 49.Egan TJ, Kaschula CH. Strategies to reverse drug resistance in malaria. Curr Opin Infect Dis 2007;20:598–604 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.