Summary
C. elegans is a premier model genetic system for discovering new information about the assembly and maintenance of striated muscle. The localization of a protein within a nematode muscle cell can reveal important clues to its function. In C. elegans, proteins can be localized by two different methods at the light microscopy level: GFP tagged proteins and indirect immunofluorescence. Although there are advantages and disadvantages of each method, antibodies can be used to localize proteins expressed at endogenous levels and without tags that might interfere with function. Immunolocalization requires efficient and effective methods of fixation. Here, we describe in detail two different methods for fixation of adult worms, the Nonet method and the Constant Spring method. We also discuss the advantages and disadvantages of each, and how to choose between them. These methods are also useful for localizing proteins expressed in other cell types.
Keywords: C. elegans, muscle, fixation, immunostaining, methods
1. Introduction
Sarcomeres, highly ordered assemblages of several hundred proteins, perform the work of muscle contraction. Despite ever increasing knowledge of the components and functions of sarcomeric proteins, we do not have a clear picture about how sarcomeres are assembled, and maintained in the face of muscle contraction. C. elegans is an excellent model genetic system in which to investigate these questions. With this system, most often, a muscle component is identified by a mutation and the eventual molecular identification of the encoded protein, or by RNAi screens of known genes. The localization of a protein within C. elegans adult body wall muscle is an important component of this analysis (1-9). This is true for both determining the localization of a new component of the sarcomere, as well as characterizing the phenotype of a mutant by localizing already known sarcomeric components. Localization can also validate protein-protein interactions that are determined by other methods.
Proteins may be localized by two different methods at the light microscopy level: GFP tagged proteins and indirect immunofluorescence. Generation and localization of a GFP tagged protein can usually be completed sooner than generating and localizing an antibody, and GFP tagged proteins can be visualized in live animals. A disadvantage of GFP tagging is that the usual way the required transgenic worms are created leads to overexpression of the GFP fusion, and consequently the danger that the protein may localize to places other than its endogenous location. In addition, overexpression may sometimes affect function. Antibodies can be used to localize native proteins expressed at normal levels. Many monoclonal and polyclonal antibodies are available to known C. elegans sarcomeric proteins (10) and as new antibodies are discovered they are detailed on http://www.wormbase.org/ with the information found for each individual gene or protein. Immunostaining requires efficient and effective methods of fixation. Here, we describe in detail two different methods for C. elegans fixation, the Nonet method (11) and the Constant Spring method (12, 13). While both of these fixation methods were originally developed for neuronal staining, they are also effective for the fixation of body wall muscle and many other tissues and cell types.
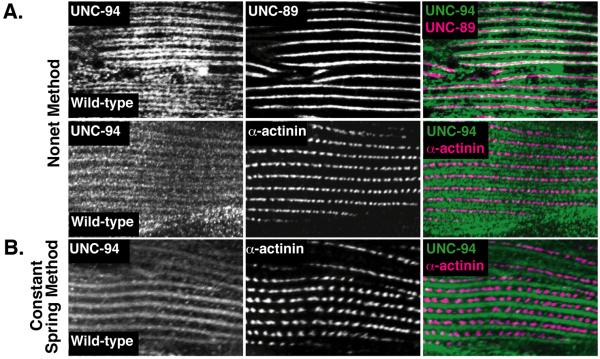
The Nonet method of fixation has many advantages including ease of use and results in usually sharper sacromeric striations upon immunostaining, possibly due to faster penetration and fixation. One disadvantage of the Nonet method, however, is that it destroys the fluorescence signal from GFP. In contrast, the Constant Spring method preserves the GFP signal, but often results in less sharp localization by immunostaining (Figure 1) (4). Table 1 compares and contrasts the Nonet and Constant Spring methods. Thus, whenever possible we use Nonet fixation but we have found that this method does not always work with monoclonal antibodies. For example when staining with 6 different monoclonal antibodies (KT3, KT6, KT9, KT10, KT11, and KT12) all worked with the Nonet method except for KT11 (14). In these situations, we perform Constant Spring Fixation. Thus, both Nonet method and Constant Spring fixation methods are important tools for evaluating C. elegans muscle and for understanding protein function.
Figure 1. Comparison of immunolocalization after Nonet or Constant Spring fixation.
Figure modified from (4). (A) Nonet method fixation of wild type worms with anti-UNC-94 (TMD-1) and either anti-UNC-89 (obscurin) or anti-α-actinin. The Nonet method shows clearly that UNC-94 (TMD-1) localizes to two closely spaced parallel lines flanking the M-line. (B) Constant-spring method fixation of wild type worms with anti-UNC-94 (TMD-1) shows broad I-band localization with no hint of the lines revealed by the Nonet method. The localization obtained with the Nonet method is consistent with likely localization to the pointed ends of the thin filaments and the known biochemical activity of other tropomodulins.
Table 1.
A comparison and contrast of the two methods for C. elegans fixation.
| Nonet Method of C. elegans Fixation |
Constant Spring Method of C. elegans Fixation |
|
|---|---|---|
| Freezing worms during fixation | Yes | Yes |
| Time for experienced user | ~ 5 hours | ~ 7 hours |
| Preserves GFP signal | No | Yes |
| Sharpest Striations | Yes | No |
| Store worms for at least a month after fixation |
Yes | Yes |
2. Materials
Prepare all solutions using deionized water and analytical grade reagents. Prepare and store all reagents at 4°C (unless otherwise indicated). Follow all local waste disposal regulations when disposing of waste materials.
2.1 Solutions
1 M MgSO4: Weigh out 120.4 g MgSO4 and dissolve in water, bringing the final volume to 1 L. Sterile filter and store at room temperature.
M9: Weigh out 6 g Na2PO4, 3 g KH2PO4, and 5 g NaCl, and dissolve in water to a final volume of 1 L. Autoclave, let solution cool, and then add 1 mL sterile 1 M MgSO4, and store at room temperature.
Bouin’s fixative: Mix together 75 mL saturated picric acid (see Note 1), 25 mL formalin, and 5 mL glacial acetic acid.
20% Trition X-100: Weigh out 50 g trition X-100 liquid into a 250 mL bottle (see Note 2). Using a graduated cylinder add 200 mL water. Using a stir rod allow Triton X-100 to dissolve. This may take as much as overnight, so if that is the case tighten the lid to prevent evaporation.
50X Borate Buffer (BO3): 1 M H3BO3 and 0.5 M NaOH with the pH being not less than 9.5 (see Note 3). Add approximately 70 mL water to a small plastic beaker. Weigh out 3.1 g Boric Acid (H3BO3) and add to the beaker, using a stir rod to dissolve. Add 5 mL 5 M NaOH and then using a plastic 100 mL cylinder bring solution to 100 mL with water.
BT: 1X Borate buffer and 0.5% trition X-100. Add 10 mL 50X Borate Buffer and 12.5 mL 20% Triton X-100 and using a graduate cylinder bring volume to 500 mL with water.
BTB: 1X BT + 2% β-mercaptoethanol
Phosphate Buffered Saline (PBS): For 1 L of 10X PBS add 2 g KCl, 2 g KH2PO4, 11.5 g Na2HPO4.7H2O, 80 g NaCl. Check that the pH is 7.2. Sterile filter; do not autoclave.
Antibody Buffer A (AbA): 1X PBS, 1% bovine serum albumin (BSA), 0.5% Triton X-100, 1 mM NaN3, and 1 mM EDTA (see Note 4).
4X MRWB: 320 mM KCl, 80 mM NaCl, 40 mM EGTA pH 7.4, 20 mM spermidine, 60 mM Pipes pH 7.4. Add 5.3 mL 3 M KCl, 800 uL 5 M NaCl, 20 mL 0.1 M EGTA, 1 mL 1 M spermidine, 6 mL 0.5 M PIPES pH 7.4 bring to 50 mL with water.
TTB (Tris-Triton Buffer): 100 mM Tris-HCl pH 7.4, 1% trition X-100, 1 mM EDTA. Into a graduated cylinder add 50 mL 1 M Tris pH 7.4, 25 mL 20% triton X-100, and 1 mL 0.5 M EDTA. Bring solution to 500 mL, filter sterilize, and store at room temperature.
Antibody Buffer B (AbB): 1X PBS, 0.1 % BSA, 0.5% trition X-100, 1 mM NaN3, and 1 mM EDTA (see Note 4). Add approximately 150 mL water to a beaker. Add 25 M 10X PBS, 6.25 mL 20% triton X-100, 1.25 mL 200 mM NaN3, 500 μL 0.5 M EDTA, and 0.25 g BSA. Add BSA last by sprinkling BSA powder on top of the liquid and allow to dissolve slowly before stirring (otherwise lumps will form). Using a graduate cylinder bring volume to 250 mL.
0.1 M Dithioerythritol (DTT): Shortly before it is needed measure 15.4 mg DTT and dissolve in 1 mL water.
DABCO: 20 mM Tris-HCl pH 8.0, 0.2 M 1,4-diazabicyclo-2,2,2-octane (DABCO) and 90% glycerol.
2.2 Additional Materials
Methanol
Liquid nitrogen
Glass centrifuge tube with screw cap closure: cat. #99502-10 (Corning, Acton, MA).
15 mL polypropylene conical
Saturated picric acid
Formalin (formaldehyde solution, 36.5%, cat. # F8775, Sigma-Aldrich, St. Louis, MO).
Paraformaldehyde, 16% solution, 10 mL ampoules, cat. #15700 (Electron Microscopy Sciences, Ft. Washington, PA, USA). We aliquot ~300 μL into 1.5 mL Eppendorf tubes and store at −20° C. For each fixation, thaw 1 aliquot. If crystals appear, simply heat at 60° C for several minutes and vortex until dissolved.
30% H2O2 solution
Bovine serum albumin (BSA)
Secondary antibodies: Donkey anti-Rabbit Alexa 488 cat. #A21206 and Goat anti-Mouse Alexa 594 cat. #A-11005 (Invitrogen, Carlsbad, California).
Microscopy slides (3“ × 1” × 1 mm) and coverslips (22 × 22 mm)
3. Methods
Carry out all procedures at room temperature unless otherwise indicated.
3.1 Collect worms
Collect un-starved worms from four 100 mm plates using M9 buffer (see Note 5). Use 3-5 mL of M9 per plate and tilt back and forth to resuspend worms transfer to glass tube using glass pipette (see Note 6).
Spin down worms 1 minute at 282xg in a table top centrifuge.
Wash 2 more times by adding 10-14 mL M9 buffer, wait for 15 min for worms to settle by gravity (see Note 7), and then aspirate M9 buffer off. After the second time leave about 50 μL of M9 left.
Proceed to either Nonet method fixation or Constant Spring fixation (see introduction about which method to choice).
3.2 Nonet fixation method
Put worms on ice for 2 min (see Note 8). During this time prepare fixative solution in a separate tube. (10 μL β-mercaptoethanol, 400 μL Bouin’s fixative, and 400 μL methanol).
Remove excess M9 buffer and add fixative to worms. Transfer worms from glass tube into a 1.5 mL Eppendorf-type tube (see Note 9).
Mix by rotation on nutator for 30 minutes. Obtain liquid nitrogen during this time.
Freeze worms quickly by dipping tube into liquid nitrogen (see Note 10). Let it stay frozen for at least 5 min, but this step can be used as a stopping point by placing the tube in a −80°C freezer overnight.
Thaw tube in warm tap water until fully thawed (see Note 11).
Mix by rotation on nutator for 30 minutes.
Centrifuge in microfuge for ~12 sec (enough time for centrifuge to reach full speed) and pipette supernatant into waste container.
Wash at least 3 times by adding 1.4 mL BTB solution, centrifuging ~12 sec, and pipetting supernatant into waste container (see Note 12). The BTB solution needs to be made fresh every time.
After washing, resuspend worms in 1 mL BTB and mix by rotation on nutator for 1 hour (see Note 13).
Centrifuge ~12 sec and pipette supernatant into waste container. Add 1 mL BTB and rock by nutator for 2-3 hours.
Centrifuge ~12 sec and pipette supernatant into waste tube. Wash by adding 1 mL BT (not BTB), inverting 3-4 times, centrifuging, and pipetting supernatant into waste container.
Wash twice by adding 1 mL AbA buffer, inverting 3-4 times, centrifuging ~12 sec, and removing supernatant. Be very careful when aspirating off supernatant since the small worm pellet may become loose after washing with the PBS-based AbA.
After washing, resuspend worms in 1 mL AbA buffer and rotate on nutator for at least 30 minutes.
Centrifuge ~12 sec, and pipette off supernatant. Leave worms in ~100 μL (at least a 1:1 ratio) with AbA buffer. Fixed worms can be kept for up to a month at 4°C.
3.3 Constant Spring fixation
Put worms on ice for 2 min (see Note 8). During this time prepare fixative solution in a separate tube. (900 μL 4X MRWB buffer, 2850 μL methanol, and 250 μL 16% paraformaldehyde).
Remove excess M9 buffer and add fixative to worms. Transfer worms from glass tube into a 15 mL polypropylene conical (see Note 14). Mix by inversion.
Freeze worms in liquid nitrogen and let stay frozen for at least 3 minutes (see Note 10).
Thaw worms by hand (or in tap water) until reaching glacial stage instead of completely liquid stage (see Note 15).
Repeat the freeze/thaw cycle 4 more times (only need to freeze until frozen; no need to wait 3 minutes in between).
After final thaw, incubate worms on ice for 1 hour. Inverting 3-4 times ~ every 10 minutes.
Spin at 282xg for 1 minute and aspirate off supernatant.
Wash twice by re-suspending in 5 mL 1X TTB, inverting 3 times, and spinning at 282xg for 1 min, and aspirating off supernatant.
Re-suspend in 5 mL 1X TTB with 1% (50 μL) β-mercaptoethanol. Incubate at 37°C rocking on nutator for 2 hrs (see Note 16).
Spin 282xg for 1 minute and aspirate off supernatant. Resuspend in 5 mL 1X BO3 buffer (see Note 17). Respin and remove supernatant.
Re-suspend in 6.3 mL 1X BO3 and 700 μL 0.1 M DTT (this DTT solution needs to be made fresh). Rock on nutator for 15 minutes. Spin at 282xg for 1 minute and aspirate off supernatant.
Re-suspend in 7 mL 1X BO3, invert 3X, respend, and aspirate off supernatant.
Re-suspend in 7 mL 1X BO3 and 70 μL 30% H2O2 (see Note 18). Incubate 15 minutes while inverting every 5 minutes (see Note 19).
Spin 282xg for 1 minute and aspirate off supernatant. Re-suspend in 7 mL 1X BO3.
Spin 282xg for 1 minute and aspirate off supernatant. Re-suspend in 5 mL AbB and rotate on nutator for 20 minutes.
Spin 282xg for 1 minute and aspirate off supernatant. Resuspend in 1 mL AbA and transfer to Eppendorf 1.5 mL tube.
Centrifuge in microfuge for ~12 seconds and remove all but ~100 μL buffer. Fixed worms can be kept for up to a month at 4°C.
3.3 Brief description of immunostaining using either Nonet or Constant Spring fixed worms
Using a cut down p200 tip with a p20 pipetteman, dispense 5 μL of packed fixed worms into a 1.5 mL Eppendorf tube (see Note 20).
Add at least 20 μL primary antibody in AbA to the worms and incubate overnight at room temperature. Either rocking horizontally on a flat bed orbital shaker, or rocking in a tube rack sitting on the top of a moving nutator are effective.
Wash 4X by adding 1 mL 1X PBS, 0.5% Trition X-100, rocking on nutator for 15 minutes, centrifuge in microfuge for ~12 seconds, and pipette off supernantant (see Note 21).
After last wash remove as much buffer as possible then add at least 20 μL secondary antibody in AbA for 2 hours without azide at room temperature in the dark (see Note 22).
Repeat washing step detained in step 3.
After the last wash leave some buffer with the worms and with a cut down p200 tip using a p20 pipetteman, dispense 5 μL slightly suspended (by re-pipetting)(see Note 23) stained worms onto a glass slide. Apply on top of a 5 μL drop of DABCO on a glass coverslip then gently place coverslip onto slide (such that the DABCO and worms touch). Then seal with nail polish. Alternatively, apply 5 μL of worms directly into a 50 μL drop of Prolong (cat. # P36930, Invitrogen, Carlsbad, California), obviating the need for nail polish.
Evaluate body wall muscle using confocal microscopy (see Note 24).
Acknowledgments
We would like to thank the NIH for research support (grant AR052133), and for a Fellowship in Research and Science Teaching (FIRST) post-doctoral fellowship (K12GM000680), also from the NIH, for supporting K.J.W.
Footnotes
Saturated picric acid solution can be made from the solid but we do not recommend this as the powder can be explosive. Also wear gloves anytime handling solutions containing picric acid.
Preparing this solution as 20% weight per volume is critical because the high viscosity of Triton X-100 makes it extremely difficult to be accurate in measuring out a volume.
The pH of the Borate buffer is critical for both fixation methods but especially constant spring.
Sodium azide (NaN3) is a poison so wear gloves.
Most important factor in good fixation (and thus good immunostaining) is that of the worm stage when the worms are fixed. The worms ideally should be young adults. Worm growth and stage is highly variable upon strain, temperature, etc.
Worms stick to plastic tips and tubes so it is best to use glass whenever possible.
Letting the worms settle by gravity is meant to remove bacteria from the solution and from inside the worm to reduce background when staining. Thus, it is important for this to be done at room temperature so the worms can swim around. Some of the worms may not settle during the 15 minutes. These worms could be immature/larval worms that do not stain well anyway, so aspirate them off.
Ice makes the worms stop moving allowing for a better pack of the worms.
Once the worms are in fixative they will no longer significantly stick to plastic.
The worms can settle quickly so mix the worms before dipping, then every couple of seconds take out and invert a couple of times until frozen. If the worms settle the fixation will not be as good.
Be careful to wear gloves because tubes tend to leak at this point. After thawed, open lid, and wipe off to prevent leaking during next step.
The goal is to have the solution be relatively clear of yellow (picric acid) and the worms should be bright yellow. Bright yellow worms indicate that the fixation went well.
The purpose of this step and subsequent washing steps is to remove picric acid from the worms. If the solution is still yellow it is necessary to do additional washes with BTB solution.
It is very important to use polypropylene not polystyrene because during freezing a polystyrene tube will crack and break.
Thawing worms only until glacial stage reduces worm fragmentation during fixation that can be very significant if thawing all the way to the liquid stage.
This step is critical step for the permeabilization of the cuticle (by breaking down disulfide bonds of the cysteines in the cuticular collagens), and the elevated temperature aids the kinetics.
During these washes without detergent it is normal for many of the worms to become attached to the plastic conical. Upon the addition of detergent in later steps some will detach.
The H2O2 solution needs to be fresh (less than one year since opening). The H2O2 solution can burn badly, so don’t touch without gloves.
Oxidation by H2O2 prevents re-formation of disulfide bonds among cysteines, and consequent collagen cross-linking, thus maintaining the “holes” in the cuticle. During the 15 minute incubation one should observe small bubbles, so it is important to keep the cap ajar between mixing, otherwise the tube might explode. Please use caution when handling H2O2, as it can cause severe burns upon skin exposure, so it is important to always wear gloves!
The cut-down tip prevents worm lysis.
Pipetting off the wash prevents the loss of worms that could occur if using aspiration because if worms are taken up into the tip they can be expelled and centrifuged again.
We have fond that Alexa 488 conjugated secondary antibodies produce more stable signals than FITC conjugated secondary antibodies. Also, non-specific signals from secondary antibodies can be eliminated using pre-absorption with wild type worm acetone powder overnight at 4°C. For specific details see (10).
The worms need to be slightly suspended to allow worms to be spread out on slide.
We have found that confocal microscopy tends to provide better images than deconvolution microscopy for adult body wall muscle.
References
- 1.Xiong G, Qadota H, Mercer KB, McGaha LA, Oberhauser AF, Benian GM. A LIM-9 (FHL)/SCPL-1 (SCP) complex interacts with the C-terminal protein kinase regions of UNC-89 (obscurin) in Caenorhabditis elegans muscle. J Mol Biol. 2009;386:976–88. doi: 10.1016/j.jmb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RK, Qadota H, Stark TJ, Mercer KB, Wortham TS, Anyanful A, et al. CSN-5, a component of the COP9 signalosome complex, regulates the levels of UNC-96 and UNC-98, two components of M-lines in Caenorhabditis elegans muscle. Mol Biol Cell. 2009;20:3608–16. doi: 10.1091/mbc.E09-03-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qadota H, McGaha LA, Mercer KB, Stark TJ, Ferrara TM, Benian GM. A novel protein phosphatase is a binding partner for the protein kinase domains of UNC-89 (Obscurin) in Caenorhabditis elegans. Mol Biol Cell. 2008;19:2424–32. doi: 10.1091/mbc.E08-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevenson TO, Mercer KB, Cox EA, Szewczyk NJ, Conley CA, Hardin JD, et al. unc-94 encodes a tropomodulin in Caenorhabditis elegans. J Mol Biol. 2007;374:936–50. doi: 10.1016/j.jmb.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qadota H, Mercer KB, Miller RK, Kaibuchi K, Benian GM. Two LIM domain proteins and UNC-96 link UNC-97/pinch to myosin thick filaments in Caenorhabditis elegans muscle. Mol Biol Cell. 2007;18:4317–26. doi: 10.1091/mbc.E07-03-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller RK, Qadota H, Landsverk ML, Mercer KB, Epstein HF, Benian GM. UNC-98 links an integrin-associated complex to thick filaments in Caenorhabditis elegans muscle. J Cell Biol. 2006;175:853–9. doi: 10.1083/jcb.200608043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercer KB, Miller RK, Tinley TL, Sheth S, Qadota H, Benian GM. Caenorhabditis elegans UNC-96 is a new component of M-lines that interacts with UNC-98 and paramyosin and is required in adult muscle for assembly and/or maintenance of thick filaments. Mol Biol Cell. 2006;17:3832–47. doi: 10.1091/mbc.E06-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small TM, Gernert KM, Flaherty DB, Mercer KB, Borodovsky M, Benian GM. Three new isoforms of Caenorhabditis elegans UNC-89 containing MLCK-like protein kinase domains. J Mol Biol. 2004;342:91–108. doi: 10.1016/j.jmb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Mercer KB, Flaherty DB, Miller RK, Qadota H, Tinley TL, Moerman DG, et al. Caenorhabditis elegans UNC-98, a C2H2 Zn finger protein, is a novel partner of UNC-97/PINCH in muscle adhesion complexes. Mol Biol Cell. 2003;14:2492–507. doi: 10.1091/mbc.E02-10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DM, Shakes DC. Immunofluorescence microscopy. Methods Cell Biol. 1995;48:365–94. [PubMed] [Google Scholar]
- 11.Nonet ML, Grundahl K, Meyer BJ, Rand JB. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993;73:1291–305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- 12.Benian GM, Tinley TL, Tang X, Borodovsky M. The Caenorhabditis elegans gene unc-89, required fpr muscle M-line assembly, encodes a giant modular protein composed of Ig and signal transduction domains. J Cell Biol. 1996;132:835–48. doi: 10.1083/jcb.132.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finney M, Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;63:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Watanabe C, Qadota H, Hanazawa M, Sugimoto A. Efficient production of monoclonal antibodies recognizing specific structures in Caenorhabditis elegans embryos using an antigen subtraction method. Genes Cells. 2008;13:653–65. doi: 10.1111/j.1365-2443.2008.01195.x. [DOI] [PubMed] [Google Scholar]