Abstract
Interpersonal violence (IPV) is major public health concern with wide-ranging sequelae including depression, posttraumatic stress disorder (PTSD), and possible alterations of immune and inflammation processes. There is a need to identify the psycho-biological pathways through which IPV may translate to altered inflammatory processes since both PTSD and inflammation are associated with serious physical health conditions such as obesity, diabetes, and cardiovascular disease. This study investigated the relationships between IPV, psychological distress, and the inflammatory marker C-reactive protein (CRP), in a sample of 139 urban women who have a high likelihood for having experienced IPV. Participants were recruited from an outpatient gynecology clinic to complete self-report measures about their IPV histories and psychological symptoms, as well as to have their blood sampled using a finger stick. Results indicated that exposure to IPV predicted the presence of probable depression and PTSD diagnoses. Individuals who experience clinical levels of PTSD exhibited higher CRP levels, and this relationship held after adjusting for comorbid depression. Correlational analyses suggested that reexperiencing symptoms may explain the link between PTSD diagnosis and higher levels of CRP. Follow-up path analytic models provided good fit to the overall data, and indicated that the relationship between probable PTSD status and CRP is not explained by higher BMI. Overall, these findings call for increased attention to the role of PTSD in explaining links between trauma and diminished health.
Keywords: interpersonal violence, PTSD, depression, inflammation, C-reactive protein
1. Introduction
Interpersonal violence (IPV) trauma is recognized as a major public health concern as research has consistently demonstrated its deleterious effects on mental and physical health [1–5]. As such, it is critical to attend to populations in which IPV prevalence rates are dramatically higher, such as inner-city and ethnic minority women living in lower socioeconomic communities [6,7]. Such populations are not only at greater risk for exposure to IPV, but they also have an increased likelihood of developing physical health conditions such as diabetes and cardiovascular disease [1,8], in addition to psychological difficulties such as depression and posttraumatic stress disorder (PTSD) [2,4].
Lifetime prevalence of physical assault for women in the general population is approximately 51%, and lifetime prevalence for rape (completed or attempted) is 17.6% [9]. A 2010 report on intimate partner violence estimates that, in their lifetimes, more than one in three U.S. women (35.6%, or approximately 42.4 million) have experienced at least one form of IPV (e.g., sexual or physical assault) and 5.9% of these women report having experienced these forms of violence within the prior 12 months [10]. Inner-city women living in urban communities characterized by lower socioeconomic status face even higher rates of IPV than women who do not live in these communities. In fact, up to 68% of inner-city women experience IPV or another traumatic event at least once during their lifetime [11,12].
The experience of trauma and violence is a known risk factor for an array of psychological symptoms and disorders, most commonly PTSD and depression [4,5,13,14]. Women who experienced physical and/or sexual abuse have been found to be four times more likely to report severe depressive symptoms as compared to women without a history of violence [13], and those who experienced sexual abuse during both childhood and adulthood were 17 times more likely to develop psychological symptoms than women with no history of abuse [12].
In addition to the psychological consequences, IPV has been found to be related to chronic physical health concerns such as weight gain, higher body mass index (BMI), immune system dysregulation, and cardiovascular diseases (CVD) [1,2,15,16]. Women with IPV histories are six times more likely to report feeling in poor health when compared to those without IPV histories [14]. In addition to having experienced trauma, individuals who go on to develop PTSD and depressive symptoms have even higher rates of obesity, higher BMI, more physical health problems, and also exhibit altered neuroendocrine and immune responses, such as activation of the hypothalamic-pituitary-adrenal axis and inflammation [17,18].
The immune system releases acute-phase proteins such as C-reactive protein (CRP), not only in response to infection, but also in response to exposure to stressful experiences [19]. In the context of acute stressors, alterations in immune responses, and the associated release of CRP, are adaptive and do not impair physical health [15,16,20]. However, chronic experiences of stress and depression can lead to longer-term inflammation [15,20,21], resulting in a greater presence of CRP in the blood. CRP is a well-studied biomarker of low-grade systemic inflammation, and has also been shown to indicate an increased risk for the development of CVD [22,23]. Studies have consistently shown that systemic elevation of CRP is also a hallmark of obesity, given the strong correlations between CRP and body mass index (BMI) [24]. Specific populations may be at even higher risk for health complications such as obesity and CVD, as CRP is also significantly increased in sample populations such as African-American and Hispanic women enrolled in the multi-site Study of Women’s Health Across the Nation [25], and among African-American women living in urban environments [26,27].
Past studies have consistently demonstrated the critical connections between depression and increased levels of CRP [28,29], as well as increased risk for CVD and related complications [30,31]. However, emerging evidence suggests that PTSD may be the more potent predictor of diminished health [32,33]. Many prior studies have focused on the general effects of stress on health; however, there is much heterogeneity in the stress response and little is known about how PTSD symptomatology and inflammation are related. Among the few studies investigating the relationship between inflammatory markers and PTSD, results have been mixed [34–38]. It is possible that these mixed results are found as researchers have not utilized methods to disentangle the unique associations depression and PTSD may have with biological variables [39]. Although the deleterious impact of IPV on general health outcomes has been found to be especially pronounced in low-income women [7], no studies to date have elucidated specific pathways through which IPV can lead to inflammation (e.g., via PTSD and depression), especially in a population of inner-city women subjected to high rates of traumatic experiences.
1.1. The Current Research
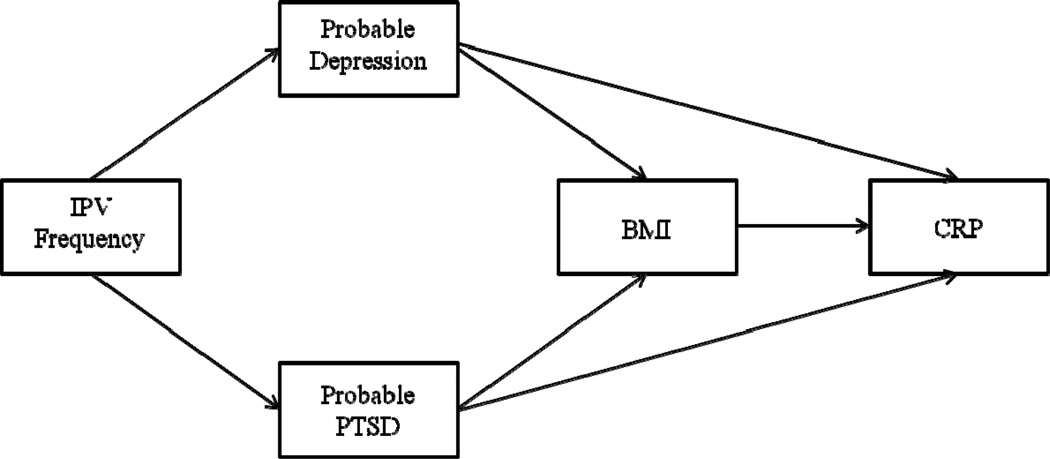
We investigated the relationships among IPV, PTSD, depression, and CRP in a sample of women with a high frequency of IPV. First, we hypothesized that PTSD and depression symptoms would be positively associated with CRP levels. Second, we hypothesized that PTSD symptoms would still be positively associated with greater CRP levels after controlling for depression symptoms. The significance of distinguishing the effects of PTSD from those of depression is critical because although many people with PTSD also have depression, many people with depression may not necessarily have PTSD [40]. Therefore, the finding that PTSD symptoms, independent of depression, predict higher CRP levels would suggest that reports of the links between depression and illness may be overestimated due to confounds with trauma exposure and PTSD. Finally, we hypothesized an exploratory path model whereby the impact of exposure to IPV on BMI and subsequent CRP inflammation is mediated by depression and PTSD symptoms (see Figure 1). BMI is included as a mediator in this model given that it is a known predictor of higher CRP levels, and is correlated with IPV and PTSD.
Figure 1.
Proposed path model.
2. Materials and Methods
2.1. Participants
Participants in this study were 139 female patients presenting for routine gynecologic care at a major urban medical center in the Midwestern United States and were recruited via posters and brochures. Gynecologic providers were also informed of the study and encouraged to invite patients meeting inclusion criteria to participate in the study. Women who called to schedule an appointment were screened over the phone for various medical illnesses to determine eligibility. Inclusion criteria were: between the ages of 18 and 45; free of major illnesses (e.g., cancer, immune conditions, seizures, multiple sclerosis, stroke, diabetes, rheumatoid arthritis, and HIV/AIDS); free of acute infections (e.g., cold, flu, bacterial vaginosis) for the previous two weeks; not currently taking antibiotics, immunosuppressants, or steroids; have not given birth in the previous two months, and not currently breastfeeding. The goal of such criteria was to obtain a sample of generally healthy participants to identify potential predisease factors affecting CRP levels. A total of 153 women volunteered for this study; however, 14 women were excluded based on the criteria stated above, yielding a final sample size of 139.
Participants who met study criteria met individually with a trained research assistant who reviewed the consent form and explained the study. The research assistant measured each participant’s height and weight to calculate BMI and subsequently obtained five drops of blood using a finger stick procedure to assess biological markers. Following the blood spot collection, participants completed all questionnaire measures online via SurveyMonkey.com. The research assistant remained present while participants completed measures to answer any questions they had about technical issues, navigating the website, or to clarify item content. This study was approved by the Institutional Review Board of Rush University Medical Center.
2.2. Outcome Measures
2.2.1. Demographics Questionnaire
Basic demographic information was collected from every participant, including age, ethnicity, education, income, and number of dependent children.
2.2.2. Trauma History Questionnaire (THQ) [41]
The THQ is a 24-item measure that is designed to screen for exposure to various types of trauma in the content areas of crime related events, general disaster and trauma, and physical and sexual experiences. Interpersonal violence was measured by three physical assault items (e.g., “Has anyone, including family members or friends, ever attacked you with a gun, knife or some other weapon?”) and three sexual assault items (e.g., “Has anyone ever made you have intercourse, oral or anal sex against your will?”). Participants were asked how many times each type of interpersonal violence happened to them on a scale from 0 (never) to 3 (many times) and these items were summed to create a total frequency score.
2.2.3. PTSD Symptom Scale (PSS) [42]
The PSS is a 17-item measure that assesses participants’ levels of re-experiencing, avoidance/numbing, and hyperarousal symptoms associated with PTSD. Participants are instructed to answer the extent to which they have been distressed by each symptom in the past month, using a Likert-type scale from 0 (not at all) to 3 (very much). Summing these items yields total scores in which higher scores reflect more severe posttraumatic stress symptoms. Probable PTSD diagnosis was determined using DSM-IV [43] criteria of the presence at least one re-experiencing symptom, three avoidance/numbing symptoms, and two hyperarousal symptoms; non-zero item endorsement was considered to indicate the presence of each symptom. This measure demonstrated good internal consistency in this study (Cronbach’s α = .947).
2.2.4. Patient Health Questionnaire (PHQ-9) [44]
The PHQ-9 is a 9-item measure which assesses depressive symptom severity and has been demonstrated to be highly sensitive in identifying these symptoms. Participants are asked the extent to which they have experienced each symptom during the previous two weeks on a Likert-type scale ranging from 0 (not at all) to 3 (nearly every day). Probable depression diagnosis was determined using DSM-IV [43] criteria for a major depressive episode. A symptom was considered present if participants provided a non-zero response to a given item. Cronbach’s α for the current study was .875.
2.3. Blood Samples
Dried blood spots were collected on filter paper using the following procedure: 1) the interviewer sterilized the participant’s finger using an isopropyl alcohol pad; 2) the middle or index finger was pricked with a sterile, disposable lancet (Microtainer, Franklin Lakes, NJ); and 3) five drops of blood (~50uL per drop) were absorbed onto filter paper (Schleicher and Schuell #903, Keene, NH) that is certified to meet performance standards for sample absorption and lotto-lot consistency set by the National Committee on Clinical Laboratory Standards (NCCLS), and by the Food and Drug Administration regulations for Class II Medical Devices.
2.4. CRP Assay
The samples were air dried for at least 4 hours after the interview, then placed in low gaspermeable zip-closure bags. Samples were then placed in a freezer where it was stored at −17.2°C for no more than one week before being delivered to the Proteomics and Biomarkers Core laboratory at Rush University Medical Center, where it was stored at −20°C. Blood spot samples were analyzed for CRP levels using an HsCRP ELISA CRP kit (DRG International, Inc. USA). The lower limit of detection according to the manufacturer was 0.1 mg/L and our value was similar at 0.13 mg/L. Every assay plate contained control sera with low, medium and high values of CRP; the inter-assay coefficient of variation ranged from 8.4 to 9.6% for medium and high levels and was 15% for the very low CRP concentration. The obtained CRP values in this sample ranged from .10–26.84 mg/L (M = 2.85, SD = 3.93).
2.5. Statistical Analyses
Zero-order correlations were computed to assess for potential covariates between key study variables and demographic variables. Based on the data obtained from these bivariate correlation analyses, age and education were included in the proposed model as covariates as they were found to be significantly related to key study variables. CRP (in mg/L units) was the only study variable to demonstrate significant skew and kurtosis in its raw form; thus we used the square root transformation of this variable [45] for the analyses presented in sections 3.2. and 3.3. All other variables were normally distributed. Partial correlations, point-biserial correlations, and hierarchical multiple regression analyses were conducted in SPSS to test the first two study hypotheses.
Path analysis was used to test the exploratory hypothesis that IPV would significantly impact CRP levels via depression, PTSD, and BMI (see Figure 1). Path analysis is a useful statistical technique to test predictive relationships among multiple variables at one time without unduly inflating Type I Error. It functions similar to multiple regression but is more powerful because it allows for the inclusion of multiple dependent variables simultaneously. Path analysis also takes a confirmatory approach to data analyses by analyzing the fit between the proposed model (e.g., Figure 1) and the actual variance-covariance matrix of the data. The extent to which a proposed model fits the data is assessed with a variety of specific fit indices [45]. Model goodness of fit was assessed using the root mean squared error of approximation (RMSEA) with values below .08 and a lower bound of the 95% confidence interval (CI) less than .05 [46] and the Comparative Fit Index (CFI) with values greater than .95 indicating excellent fit [47]. Path modeling was analyzed in the AMOS 18 statistical software program. Age and education were included in the model as covariates due to their significant relationships with key study variables.
3. Results
3.1. Descriptive Statistics and Zero-Order Correlations
Of the 139 participants in this study, 83.50% were African American, 4.3% were Hispanic/Latina, 5.8% were white, and the remaining 6.40% identified their ethnicity as “other.” The mean age for this sample was 28.46 (SD = 7.76) and 66.90% reported having attained some post-high school education or career training (i.e., they were currently in college, in a career training program, or had obtained a college degree). Despite their educational achievement, nearly half (48.20%) of participants were unemployed at the time of the survey and the mean annual income was $13,202.88 (SD = 4107.00). Over half (61.90%) of these women were not in relationships at the time of the study and 71.20% had at least one dependent child (M = 1.24, SD = 1.09; range = 0–5). The majority of this sample (76.3%) fell in the overweight or higher categories of BMI (M = 31.90, SD = 9.26) and the mean CRP level among participants was 2.85 mg/L (SD = 3.93; see Table 1).
Table 1.
Descriptive Statistics
| Demographics | % | M (SD) | Range |
|---|---|---|---|
| Age | - | 28.46 (7.76) | 18 – 45 |
| Education | |||
| High School or Less | 33.1 | - | - |
| Some College or Higher | 66.9 | - | - |
| Employment | |||
| Full-time | 23.7 | - | - |
| Part-time | 18.7 | - | - |
| Unemployed | 48.2 | - | - |
| Income | - | 13,202.88 (4107.00) | 0 – 90,000 |
| Ethnicity | |||
| Black | 83.5 | - | - |
| Hispanic/Latina | 4.3 | - | - |
| White | 5.8 | - | - |
| Other | 6.4 | - | - |
| Relationship Status | |||
| Single/Divorced/Widowed | 61.9 | - | - |
| Partnered/Married/Cohabiting | 33.1 | - | - |
| Number of Children | - | 1.24 (1.09) | 0 – 5 |
| Study Variables | % | M (SD) | Range |
| IPV | |||
| At least one IPV event | 70.5 | - | - |
| Sub-sample with 1+ IPV event (n=97) | - | 3.97 (2.99) | 1 – 15 |
| PTSD | |||
| Scores | - | 10.06 (11.12) | 0 – 51 |
| Probable Diagnosis | 12.2 | - | - |
| Depression | |||
| Scores | - | 8.51 (6.31) | 0 – 27 |
| Probable Diagnosis | 22.3 | - | - |
| BMI | 31.90 (9.26) | 17.47 – 66.89 | |
| CRP mg/L | 2.85 (3.93) | .10 – 26.84 | |
Exposure to IPV was high, with 43.9% of participants experiencing at least one type of sexual assault (i.e., forced intercourse/penetration, molestation, or attempted sexual assault) and 51.8% experiencing at least one type of physical assault (i.e., attacked with a weapon, attacked without a weapon, or being physically assaulted by a family member enough to result in injury) in their lifetime. Of those with a physical assault history (n = 72), participants reported a mean of 2.63 (SD = 1.79) physical assaults, and of those with a sexual assault history (n = 61), participants experienced a mean of 3.21 (SD = 1.96) sexual assaults. In combination, 70.5% of this sample (n = 97) reported at least one IPV event during their lifetimes and, of those, participants experienced a mean of 3.97 (SD = 2.99) violent events. The full sample demonstrated a mean depression score of 8.51 (SD = 6.31) and a mean PTSD score of 10.06 (SD = 11.12), with 22.3% and 12.2% meeting criteria for probable diagnoses of depression and PTSD, respectively (see Table 1).
Significant correlations emerged between the demographic variables of age and education and several study variables. Age was significantly positively correlated with frequency of IPV (r = .17, p = .04). Education level was significantly negatively correlated with PTSD total scores (r = −.24, p < .01) and with each of the PTSD subscales (intrusions: r = −.20, p = .02; avoidance: r = −.26, p < .01; hyperarousal: r = −.21, p = .01; see Table 2).
Table 2.
Zero-Order Correlations among Demographic Variables and Study Variables (N = 139)
| A | B | C | D | E | F | G | H | I | J | |
|---|---|---|---|---|---|---|---|---|---|---|
| (A) Age | -- | .097 | −.076 | −.001 | .173** | −.053 | −.057 | −.058 | −.060 | −.045 |
| (B) Education | -- | −.076 | −.041 | −.015 | −.153* | .241*** | −.195** | −.255*** | −.211** | |
| (C) BMI | -- | .495*** | −.070 | .060 | .146* | .150* | .171** | .071 | ||
| (D) CRP | -- | −.094 | −.035. | .100 | .149* | .081 | .052 | |||
| (E) IPV frequency | -- | .416*** | .455*** | .338*** | .437*** | .421*** | ||||
| (F) Depression | -- | .729*** | .613*** | .670*** | .734*** | |||||
| (G) PTSD total scores | -- | .900*** | .958*** | .897*** | ||||||
| (H) PTSD intrusion subscale | -- | .811*** | .704*** | |||||||
| (I) PTSD avoidance subscale | -- | .792*** | ||||||||
| (J) PTSD hyperarousal subscale | -- |
Note. CRP = square root transformation of CRP.
p < .10.
p < .05.
p < .01.
3.2. Primary Analyses
We calculated bivariate correlations to test the first hypothesis that greater depression and PTSD symptoms would correlate with higher levels of the square-root transformation of CRP. Contrary to predictions, the only correlation to emerge from this analysis that approached significance was that CRP was positively correlated with the intrusion subscale of PTSD (r = .15, p = .08). To further explore potential relationships, PTSD and depression were dichotomized into probable diagnoses to determine if CRP levels differed as a function of having a PTSD or depression diagnosis. T-tests indicate that CRP levels did not significantly differ between participants with probable depression (M = 1.41, SD = 1.16) and those without probable depression (M = 1.37, SD = .92), t(137) = −.19, p = .86. However, participants with a probable PTSD diagnosis had higher CRP levels (M = 1.81, SD = 1.39) than participants without probable PTSD (M = 1.32, SD = .89), t(137) = 1.94, p = .05. This pattern of results remained, even after controlling for BMI using partial point-biserial correlations. Probable depression diagnosis was not related to CRP levels (rpb = −.07, p = .22) while participants with probable PTSD had higher levels of CRP, an association that approached significance (rpb = .14, p = .057).
We used hierarchical multiple regression analyses to test the second hypothesis that probable PTSD diagnosis would predict significantly higher levels of CRP beyond the effects of probable depression. BMI was entered in the first block of analyses as a covariate, followed by probable depression, and finally probable PTSD. After adjusting for the effect of BMI, depression did not significantly predict CRP levels (β = −.06, p = .48) and did not significantly explain additional variance in CRP above and beyond the effects of BMI, ΔR2 = .003. As expected, probable PTSD was a significant predictor of higher CRP levels (β = .23, p = .01, ΔR2 = .04). Interestingly, probable depression became a significant predictor of lower CRP levels (β = −.193, p = .04) when probable PTSD was added to the model. This final regression model predicted a significant amount of the variance of CRP (F(3, 135) = 17.70, p < .001, R2 = .282).
3.3. Exploratory Path Analyses
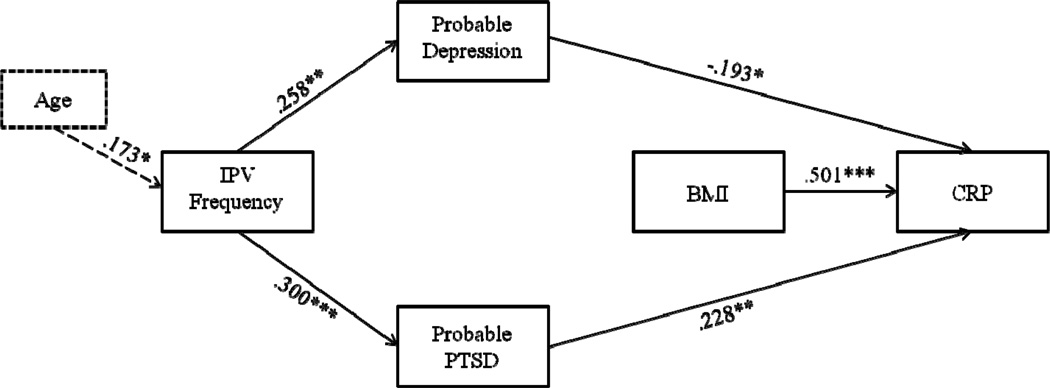
We used path analysis to test the third hypothesis that IPV frequency would predict depression and PTSD, which would, in turn, predict BMI and CRP levels (see Figure 1). Due to significant correlations at the bivariate level, age and education were added to the hypothesized model as covariates. This model demonstrated poor fit to the underlying data, χ2(11) = 57.645, p < .001, CFI = .611, RMSEA = .175 (90% CI = .132 – .221). However, modification indices suggested that correlating the errors between depression and PTSD would significantly improve model fit (MI = 38.560). After adding this pathway, model fit significantly improved, suggesting excellent fit to the data, χ2(10) = 10.339, p = .411, CFI = .997, RMSEA = .016 (90% CI = .000 − .094). Among demographic variables, only age had a significant effect in the model, such that being older significantly predicted greater frequency of having experienced IPV (β = .173, p = .039). Consistent with our hypothesis, higher levels of IPV significantly predicted probable depression (β = .258, p = .002) and probable PTSD (β = .300, p < .001). Neither probable depression nor probable PTSD significantly predicted to BMI. However, probable depression significantly predicted lower CRP levels (β = −.193, p = .030), and probable PTSD significantly predicted higher CRP levels (β = .228, p = .010). Additionally, BMI predicted higher CRP levels (β = .501, p < .001). No indirect (i.e., mediated) effects were significant. See Figure 2 for the final model.
Figure 2.
Final path model. Note: *p < .05, **p < .01, ***p < .001.
4. Discussion
Results of this study emphasize the complex relationships between exposure to interpersonal violence, psychological distress, and inflammatory processes. As hypothesized, exposure to interpersonal violence was predictive of probable depression and PTSD. Women who met clinical criteria for PTSD exhibited significantly higher CRP levels compared to women who did not meet clinical criteria for PTSD. This relationship held after adjusting for cooccurring depression diagnoses. Correlational analyses suggested that the link between PTSD diagnosis and greater CRP may be driven by the intrusive reexperiencing of trauma that is both a hallmark and unique symptom of PTSD. Follow-up path analytic models provided good fit to the overall data, and indicated that the relationship between probable PTSD status and CRP was not entirely explained by higher BMI. This suggests that PTSD may independently activate inflammatory processes and may be distinct from obesity-associated inflammation, as BMI was not a significant mediator between probable PTSD and CRP.
Overall, our findings are consistent with the emerging literature on stress, trauma, and inflammatory processes [21,37]. PTSD diagnosis, and in particular the characteristic intrusive reexperiencing of traumatic events, was associated with higher levels of CRP. This result concurs with those reported by Miller and colleagues [35] that trauma-related intrusions were associated with higher CRP. Taken together, these findings suggest that the recurrent and intrusive reliving of trauma, a symptom that is both hallmark of and unique to PTSD, may be a potential mechanism by which traumatic stress translates into chronic inflammation. One possibility is that immune and inflammatory processes are continually elicited by the vivid reliving of trauma and associated emotional responses on a frequent and regular basis, as if these actual threatening events were repeatedly occurring. Future studies employing momentary timesampling methods will be critical for investigating this possibility.
Less anticipated was the finding that depression was associated with decreased CRP, after adjusting for BMI and PTSD. There is considerable overlap between the constructs of PTSD and depression, but our results suggested that the unique aspects of PTSD are the more potent predictor of higher levels of CRP. This unanticipated finding contrasts with prior research [28,29] and calls for greater specificity in future research to identify psychophysiological mechanisms linking stress and inflammation. It is interesting to note that the construct of PTSD contains symptoms that encapsulate many symptoms of depression, and also has unique features of hyperarousal and intrusive thoughts. Thus, in a traumatized population, PTSD may be much more predictive of inflammatory processes than depression alone, as the path coefficient between PTSD and CRP was stronger and more significant than that of the path between depression and CRP. Because PTSD is less often investigated in psychoneuroimmunology research than is depression (and thus the unique effects of these two disorders are often merged), there is a need for larger, prospective designs that incorporate measures of trauma, depression, and PTSD to disentangle these effects, and identify targets for intervention.
Although we employed well-validated measures of trauma, and distress, and collected biological data from a sample with high rates of IPV, the present study had several limitations. For instance, IPV frequencies reported in this sample likely underestimate the actual rate of IPV. Reasons for this may be that participants underreported their exposure to IPV [48] or because these data were ordinally coded. Despite this potential attenuation, we were still able to detect meaningful effects of IPV on psychological and biological outcomes. Another potential limitation of this study was the use of a somewhat small sample of community women and a methodology with few exclusionary criteria. However, significant findings were detected in a manner that converged with prior literature. Moreover, studying the relationships among IPV, PTSD, and inflammation in an externally valid sample provides valuable information to the field of health disparities research. Future studies quantifying IPV exposure in different ways (e.g., using continuous rather than ordinally-coded data) will help to clarify how IPV impacts CRP levels. Finally, given that PTSD is associated with higher alcohol consumption [49], which in turn is associated with lower CRP [50], future work should investigate the whether the association between PTSD and CRP is attenuated among individuals who drink.
4.1. Conclusions
In summary, our findings have important implications for theory, research, and intervention regarding stress-related inflammation. We identified higher levels of CRP among participants with clinical levels of PTSD symptoms. In contrast, participants with probable depression evidenced lower CRP levels after accounting for probable PTSD diagnosis. This suggests that in highly traumatized populations, PTSD symptoms should be ongoing targets of basic and applied psychoneuroimmunology research.
Participants with PTSD had higher CRP levels even after controlling for depression.
Reexperiencing symptoms of PTSD were the most strongly related to CRP.
The relationship between PTSD and CRP is not explained by body mass index.
Acknowledgements
The authors would like to thank Seema Desai, Ph.D. for her insight and contributions to this manuscript. This research was made possible in part by the Charles J. and Margaret Roberts Fund and a grant from the NIH-NHLBI 1P50HL105189 Rush Center for Urban Health Equity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Black P. The inflammatory consequences of psychologic stress: Relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JC. Health consequences of intimate partner violence. Lancet. 2002;359:1331–1336. doi: 10.1016/S0140-6736(02)08336-8. [DOI] [PubMed] [Google Scholar]
- 3.Dutton MA, Green BL, Kaltman SI, Roesch DM, Zeffiro TA, Krause ED. Intimate partner violence, PTSD, and adverse health outcomes. J Interpers Violence. 2006;21:955–968. doi: 10.1177/0886260506289178. [DOI] [PubMed] [Google Scholar]
- 4.Golding JM. Intimate partner violence as a risk factor for mental disorders: A meta-analysis. J Fam Violence. 1999;14:99–132. [Google Scholar]
- 5.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EB. Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- 6.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit area survey of trauma. Arch Gen Psychiat. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland CA, Sullivan CM, Bybee DI. Effects of intimate partner violence versus poverty on women's health. Violence Against Wom. 2001;7:1122–1143. [Google Scholar]
- 8.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 9.Tjaden P, Thoennes N. N. Full report on prevalence, incidence, and consequences of violence against women: Findings from the national violence against women survey. (Research Report No. NCJ183781) Washington DC: National Institute of Justice; 2000. [Google Scholar]
- 10.Black MC, Basile KC, Breiding MJ, Smith SG, Walters ML, Merrick MT, Chen J, Stevens MR. The national intimate partner and sexual violence survey (NISVS): 2010 summary report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 11.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psych. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 12.Schumm JA, Stines LR, Hobfoll SE, Jackson AP. The double-barreled burden of child abuse and current stressful circumstances on adult women: The kindling effect of early traumatic experience. J Trauma Stress. 2005;18:467–476. doi: 10.1002/jts.20054. [DOI] [PubMed] [Google Scholar]
- 13.Bonomi AE, Thompson RS, Anderson M, Reid RJ, Carrell D, Dimer JA, Rivara FP. Intimate partner violence and Women’s physical, mental, and social functioning. Am J Prev Med. 2006;30:458–466. doi: 10.1016/j.amepre.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Romitoa P, Turan JM, de Marchi M. The impact of current and past interpersonal violence on women's mental health. Soc Sci Med. 2005;60:1717–1727. doi: 10.1016/j.socscimed.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP, P G. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulat. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 16.McEwen B. Mood disorders and allostatic load. Biol Psychiat. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 17.Boscarino JA. Posttraumatic stress disorder and physical illness: Results from clinical and epidemiologic studies. Ann NY Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 18.Wong CM. Post-traumatic stress disorder: Advances in psychoneuroimmunology. Psychiat Clin N Am. 2002;25:369–383. doi: 10.1016/s0193-953x(01)00006-5. [DOI] [PubMed] [Google Scholar]
- 19.Robles TF. Out of balance: A new look at chronic stress, depression, and immunity. Curr Dir Psychol Sci. 2005;14:111–115. [Google Scholar]
- 20.Du Clos TW, Mold C. C. C-reactive protein: An activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30:261–277. doi: 10.1385/IR:30:3:261. [DOI] [PubMed] [Google Scholar]
- 21.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of proinflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 22.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. Brit Med J. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 25.Matthews KA, Sowers MF, Derby CA, Stein E, Miracle-McMahill H, Crawford SL, Pasternak RC. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women's Health Across the Nation (SWAN) Am Heart J. 2005;149:1066–1073. doi: 10.1016/j.ahj.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 26.McDade T, Hawkley L, Cacioppo J. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The Chicago health, aging, and social relations study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 27.Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav Immun. 2003;17:286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 28.Howren MB, Lamkin DM, Suls J. Associations of depression with c-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 29.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: The national heart, lung, and blood institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 30.Czajkowski SM. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The enhancing recovery in coronary heart disease patients (ENRICHD) randomized trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 31.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: Epidemiology, biology, and treatment. Arch Gen Psychiat. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 32.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: The problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 33.Sawchuk CN, Roy-Byrne P, Goldberg J, Manson S, Noonan C, Beals J, Buchwald D. The relationship between post-traumatic stress disorder, depression and cardiovascular disease in an American Indian tribe. Psychol Med. 2005;35:1785–1794. doi: 10.1017/S0033291705005751. [DOI] [PubMed] [Google Scholar]
- 34.McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM, Violanti JM. C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55:74–78. doi: 10.1016/j.cyto.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Miller RJ, Sutherland AG, Hutchison JD, Alexander DA. C-reactive protein and interleukin 6 receptor in posttraumatic stress disorder: A pilot study. Cytokine. 2001;13:253–255. doi: 10.1006/cyto.2000.0825. [DOI] [PubMed] [Google Scholar]
- 36.Sondergaard HP, Hansson LO, Theorell T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta. 2004;342:93–98. doi: 10.1016/j.cccn.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, Löwe B, Grabe HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: Evidence from the general population. J Psychiatr Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 38.von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Yehuda R, Siever RJ, Teicher MH, Levengood RA, Gerber DK, Schmeidler J, Yang RK. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiat. 1998;44:56–63. doi: 10.1016/s0006-3223(98)80007-3. [DOI] [PubMed] [Google Scholar]
- 40.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. C. B. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiat. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 41.Green BL. Psychometric review of the Trauma History Questionnaire (Self-Report) In: Stamm BH, Varra EM, editors. Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sidran Press; 1996. [Google Scholar]
- 42.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459–473. [Google Scholar]
- 43.Diagnostic and Statistical Manual of Mental Disorders: Text Revision. fourth. Washington, DC: American Psychiatric Assocation; 2000. [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabachnick BG, Fidell LS. Using Multivariate Statistics. fifth. Boston, MA: Pearson Education Inc.; 2007. [Google Scholar]
- 46.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- 47.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 48.Marshall AD, Panuzio J, Makin-Byrd KN, Taft CT, Holtzworth-Munroe A. A multilevel examination of interpartner intimate partner violence and psychological aggression reporting concordance. Behav Ther. 2011;42:364–377. doi: 10.1016/j.beth.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. Am J Psychiat. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- 50.Stewart SH, Mainous AG, Gilbert G. Relation between alcohol consumption and C-reactive protein levels in the adult US population. J Am Board Fam Pract. 2002;15:437–442. [PubMed] [Google Scholar]