Abstract
A systematic review and a meta-analysis were performed to quantify the accumulated information from genetic association studies investigating the impact of the CYP4F2 rs2108622 (p.V433M) polymorphism on coumarin dose requirement. An additional aim was to explore the contribution of the CYP4F2 variant in comparison with, as well as after stratification for, the VKORC1 and CYP2C9 variants. Thirty studies involving 9,470 participants met prespecified inclusion criteria. As compared with CC-homozygotes, T-allele carriers required an 8.3% (95% confidence interval (CI): 5.6–11.1%; P < 0.0001) higher mean daily coumarin dose than CC homozygotes to reach a stable international normalized ratio (INR). There was no evidence of publication bias. Heterogeneity among studies was present (I2 = 43%). Our results show that the CYP4F2 p.V433M polymorphism is associated with interindividual variability in response to coumarin drugs, but with a low effect size that is confirmed to be lower than those contributed by VKORC1 and CYP2C9 polymorphisms.
Coumarin drugs (warfarin, acenocoumarol, and phenprocoumon) are currently the most extensively used oral anticoagulants worldwide. Tey are commonly prescribed for the treatment and prevention of thromboembolic events.1 Te narrow therapeutic index of these drugs and the high interindividual variability in therapeutic doses make coumarin therapy difficult to manage, with adverse effects being likely to occur in a wide variety of patients.2
Several factors have been identified that may potentially influence the anticoagulation effect of coumarin drug therapy. These include genetic factors that modify the pharmacokinetics and pharmacodynamics of coumarins, thereby influencing their responsiveness depending on ethnic group.3
Single-nucleotide polymorphisms (SNPs) in the gene for cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase complex subunit 1 (VKORC1) have repeatedly been shown to be associated with the clinical response to warfarin. Regression models have been developed that incorporate both clinical and genetic factors to predict the stable dose so as to reduce the treatment duration within the therapeutic range as well as the risks of thrombotic and hemorrhagic events. However, because these algorithms collectively explain only 50–60% of dose variability, efforts are still being made to identify additional genetic and clinical factors that influence warfarin dose requirement.4
CYP4F2 is a primary vitamin K1 oxidase in the liver that catalyzes the metabolism of vitamin K1 to hydroxylated vitamin K1 and acts as a counterpart to VKORC1 in limiting excessive accumulation of vitamin K.5 A SNP of CYP4F2 (rs2108622, p.V433M) was recently found to be associated with warfarin dose variability.6 Carriers of the CYP4F2 p.V433M variant allele have a reduced capacity to metabolize vitamin K, secondary to an rs2108622-dependent decrease in steady-state hepatic concentrations of the enzyme.5 Therefore, patients with the rs2108622 polymorphism are likely to have elevated hepatic levels of vitamin K, necessitating a higher coumarin drug dose to achieve therapeutic anticoagulation. Interestingly, we and others have recently found that this nonsynonymous variant is also associated—only in men—with hypertension prevalence and ischemic stroke, suggesting that the SNP has a sex-specific effect.7,8 Although a large number of studies have investigated the association between CYP4F2 genotype and coumarin dose requirements in humans, few of these studies were sufficiently powered to give useful quantitative estimates of the genotype effect. The first aim of this study was therefore to conduct a systemic review followed by a meta-analysis in order to quantify the magnitude of the effect of the CYP4F2 p. V433M polymorphism on mean daily coumarin dose. A consistent effect size could prompt the routine incorporation of this allele into algorithms that estimate individualized coumarin dosage. In addition, we evaluated the results to determine whether the association varies by gender. To assess the effects of the CYP4F2 polymorphism in comparison with those of the known VKORC1 and CYP2C9 variants, we performed additional explorative meta-analyses to evaluate the relative impacts of CYP2C9 and VKORC1 variants on coumarin maintenance dose. We also tested the impact of the CYP4F2 polymorphism after stratification for the CYP2C9 and VKORC1 genetic variants.
RESULTS
Study selection
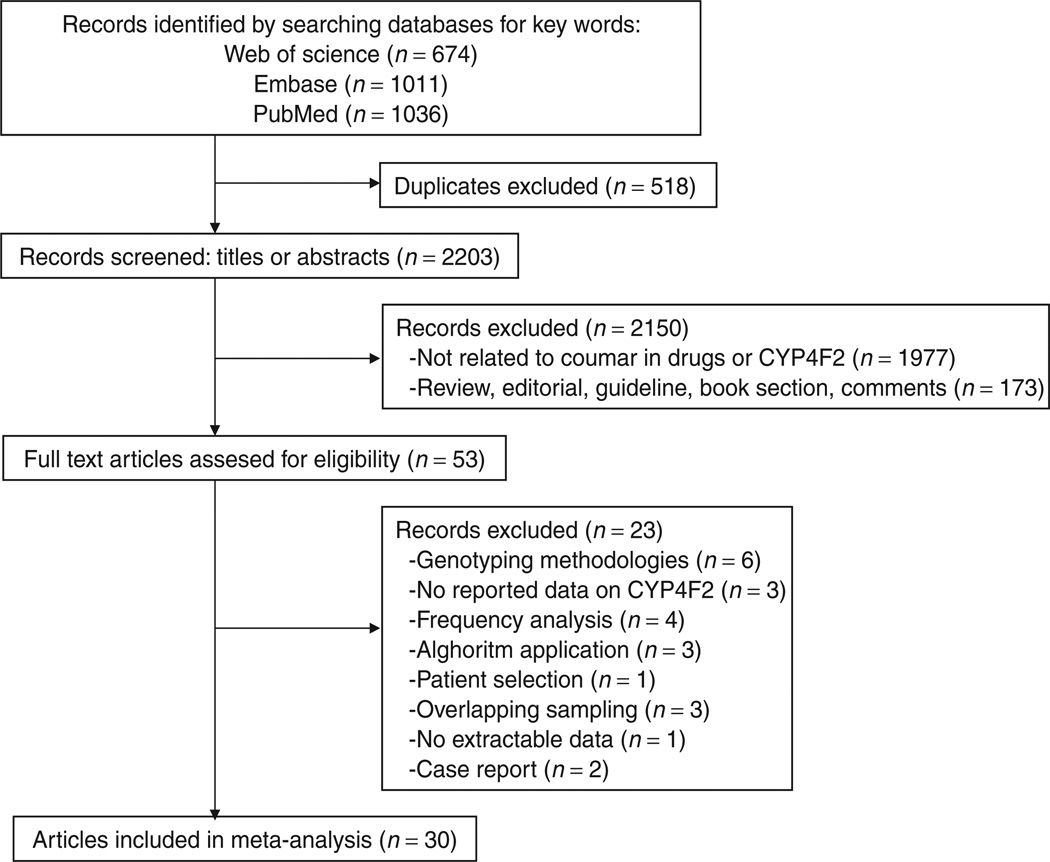
We identified a total of 2,721 reports in the initial search (Figure 1), of which we eliminated 518 duplicate publications. No further records were identified through other sources. We excluded 2,150 nonrelevant records on the basis of screens of titles or abstracts. Full-text articles were retrieved for 53 publications and assessed for eligibility. Of these, we excluded 17 articles because they did not meet the inclusion criteria. Three others were excluded because of overlapping samples,9–11 two because genotyping was performed in only a single patient,12,13 and one because it was not possible to contact the authors and to extract data from the article.14 Overall, we identified 30 articles that met the inclusion criteria, and these were included in the systematic review.
Figure 1.
Flow diagram showing the number of citations identified, retrieved, extracted, and included in the final analysis.
The κ statistic indicated a very good agreement between the abstracting investigators (κ 0.91 ± 0.04).
Characteristics of the included studies
The 30 studies involved a total of 9,470 participants and examined the association between the CYP4F2 polymorphism and coumarin drug maintenance dose. Of these, 26 studies5,6,15–38 evaluated this association for warfarin (7,313 patients), two for acenocoumarol (1,838 patients),39,40 and two for phenprocoumon (319 patients).41,42 The characteristics of the individual studies are summarized in Table 1. All the studies except two38,39 included both male and female participants, with a minimum of 23.9% male participants. The weighted mean age and body mass index (BMI) of participants were 64.2 ± 8.31 years and 27.1 ±8.31 kg/m2, respectively. One study28 selected very elderly patients (mean age 86.7 years). All but three studies21,22,39 included patients who were contemporarily on treatment with drugs known to interfere with coumarins. All the studies were published between 2008 and 2011.
Table 1.
Characteristics of included studies regarding the effect of CYP4F2 gene polymorphism on coumarin dose requirement
| Study name | Location | Predominant Population |
HWE | N | Male (%) |
Age (years) |
BMI (kg/m2) |
INR target | Indication | Data extracted from articles |
Data obtained from authors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Borgiani P et al., 200915 | Italy | Caucasian | 0.53 | 141 | 53.2 | 68.5 | n.a. | 2.0–4.0 | AF,DVT,VR, ictus | Yes | Yes |
| Botton MR et al., 201116 | Brazil | Caucasian | 0.02 | 279 | 55.6 | 62.6 | 27.1 | 2.0–3.5 | VR,AF,TE, CA | No | Yes |
| Caldwell MD et al., 2008 (Florida)6 | Florida (USA) | White (91%) | 0.62 | 343 | 87.2 | 69.1 | 29.9 | 2.0–4.0 | AF,TE,CA, TIA,VR,CM | Yes | Yes |
| Caldwell et al., 2008 (Marshfield)6 | Wisconsin (USA) | White | 0.77 | 490 | n.a. | n.a. | n.a. | 2.0–3.5 | AR,VR,DVT, PE, stroke | Yes | No |
| Caldwell et al., 2008 (Washington)6 | Missouri (USA) | White | 0.91 | 269 | n.a. | n.a. | n.a. | 2.6 | AF,DVT,PE, stroke, VR | Yes | No |
| Carlquist JF et al., 201017 | Utah (USA) | n.a. | 0.51 | 157 | 47.1 | 70.9 | 28.5 | 2.0–3.0 | DVT.AF | Yes | Yes |
| Cavallari LH et al., 201018 | Illinois (USA) | African Americans | 0.06 | 223 | 29.2 | 56.8 | 33.4 | 2.0–4.0 | DVT,PE,AF, stroke, TIA, VR | Yes | Yes |
| Cen HJ et al., 201019 | China | Asian | 0.55 | 222 | 46.9 | 45.1 | 21.2 | 1.5–3.0 | VR | Yes | Yes |
| Cha PC et al., 201020 | Japan | Asian | 0.91 | 440 | 66.6 | 67.6 | 23.9 | 1.5–3.0 | TE,VR,AF, stroke | Yes | Yes |
| Chan SL et al., 201121 | Singapore | Asian | 0.32 | 248 | n.a. | n.a. | n.a. | 2.0–3.0 | AF,VR,TE, stroke | Yes | No |
| Choi JR et al., 201122 | Korea | Asian | 0.61 | 403 | 54.3 | 63.2 | 24.5 | n.a. | AF, stroke, VR,DVT,PE | Yes | No |
| aGeisen C et al., 201141 | Germany | Caucasian (97.3%) | 0.05 | 75 | 49.3 | 64 | n.a. | 2.0–3.0 | TE,AF,VR | Yes | No |
| Gong IY et al., 201123 | Canada | White (95.2%) | 0.44 | 167 | 56.5 | 60 | 28.7 | 2.0–3.0 | AF,VR,DVT | Yesc | No |
| Harada T et al., 201024 | Japan | Asian | 0.79 | 97 | 52.6 | 63.2 | 22.3 | 2.0–3.0 | VR | Yes | No |
| Kringen MK et al., 201125 | Norway | n.a. | 0.98 | 105 | 82.9 | 60.1 | n.a. | 2.0–4.2 | Ml | Yes | Yes |
| Lee MT et al., 200926 | Taiwan | Asian | 0.97 | 235 | 55.7 | 63 | 28.9 | 1.7–3.0 | AF,VR, stroke, DVT.PE | No | Yes |
| Lubitz SA et al., 201027 | New York (USA) | Caucasian (57%) | 0.45 | 156 | 62.2 | 66.6 | 28.5 | 2.0–3.0 | AF,DVT,PE | Yes | Yes |
| Mc Donald MG et al 20095 | Washington (USA) | Caucasian (>90%) | 0.25 | 181 | 64.6 | 59.6 | n.a. | 2.0–3.0 | AF,CM,DVT, PE,VR | Yes | Yes |
| Pautas E et al., 201028 | France | Caucasian | 0.40 | 272 | 23.9 | 86.7 | n.a. | 2.0–3.0 | TE,AF | Yes | Yes |
| bPerez-Andreu V et al 200939 | Spain | Caucasian | 0.28 | 100 | 100 | 62.2 | 26.7 | 2.0–3.0 | AF | Yes | Yes |
| Perini JA et al., 201029 | Brazil | White (49.7%) | 0.02 | 387 | 48.1 | 54.1 | 25.3 | 2.0–3.5 | VR,AF,TE | Yes | Yes |
| Sagrieya H et al., 201030 | California (USA) | White (75%) | 0.72 | 101 | 57.4 | 63.9 | 27.5 | 2.0–3.0 | AF,DVT,VR,PE | Yes | Yes |
| Shahin MH et al., 201131 | Egypt | Africans | 0.34 | 192 | 45.3 | 47.7 | 28.1 | 1.5–3.5 | AF,VR,DVT,PE, CA,CM | Yes | Yes |
| Singh O et al., 201132 | Singapore | Asian | 0.28 | 124 | 56.5 | 60.5 | 25.4 | 2.0–3.0 | AF,DVT,VR | Yes | Yes |
| Suriapranata IM et al., 201133 | Indonesia | Asian | 0.32 | 85 | 55.3 | 57 | 23.7 | 1.5–3.0 | AF,VR,ATD | Yes | Yes |
| Takeuchi F et al., 200934 | Sweden | n.a. | n.a. | 1,053 | n.a. | 66 | n.a. | 2.0–3.0 | AF,DVT | Yesd | No |
| bTeichert M et al., 200940 | Netherlands | Caucasians | 0.02 | 1,738 | 44 | 74.7 | 27 | 2.0–4.5 | DVT,PE,AF, MI, stroke, TIA, AD,VR | No | Yes |
| aTeichert M et al., 201142 | Netherlands | Caucasians | 0.80 | 244 | 61.9 | 75.2 | 26.8 | 2.0–4.0 | DVT,PE,AF, MI, stroke, TIA, AD,VR | Yes | Yes |
| Wells PS et al., 201035 | Canada | Caucasian (94%) | 0.48 | 246 | 55.3 | 60.4 | 29.3 | 2.0–3.0 | DVT,PE,AT, AF,VR | Yes | Yes |
| Zambon CF et al., 201136 | Italy | Caucasian | 0.25 | 371 | 62.3 | 74 | 27.4 | 2.0–3.0 | AT, DVT, and other | Yes | Yes |
| Zhang JE et al., 200937 | UK | Caucasian (>90%) | 0.34 | 204 | 59.8 | 66.4 | 28.8 | n.a. | DVT,AF | Yes | Yes |
| Zhang X et al., 201138 | New York (USA) | White (94%) | 0.52 | 122 | 100 | 72 | 30.2 | 1.8–3.2 | AF,DVT,CA,PE | No | Yes |
AD, atrial disease; AF, atrial fibrillation; ATD, atherothrombotic disease; BMI, body mass index; CA, cerebrovascular accident; CM, cardiomyopathy; DVT, deep vein thrombosis; HWE, Hardy-Weinberg equilibrium; INR, international normalized ratio; Ml, myocardial infarction; n.a., data not available; PE, pulmonary embolism; TE, thromboembolism; TIA, transient ischemic attack; VR, valve replacement.
Studies in which the coumarin drug was different from warfarin are labeled as follows:
phenprocoumon;
acenocoumarol.
Only dominant genetic model applicable.
Data extracted from a published meta-analysis.43
Summary data for 24 articles were obtained from the auth ors. 5,6,15–20,25–33,35–40,42 for these studies, we performed a separate meta-analysis stratified by gender and exploratory meta-analyses to evaluate the association between polymorphisms of VKORC1 and CYP2C9 and coumarin maintenance dose in the same populations that had been genotyped for the CYP4F2 variant, as well as the impact of the CYP4F2 p.V433M variant, after stratification for different genotypes. Data extraction from published primary studies was possible for 24 articles.5,6,15,17–25,27–32,35–37,39,41,42 Data from one article34 were obtained from a published meta-analysis.43
Ethnic differences between populations included in different studies
Samples from 24 of the included studies were reported as being ethnically homogeneous (ethnic homogeneity >90%).5,6,15,16 18–24,26,28,31–33,35–42 The ethnicities of 1,315 subjects from three different studies were unknown according to the authors.17,34,25 For these studies, ethnicity was assigned according to the predominant ethnic group in each study’s specific geographic area (Caucasian for European studies and white Americans for the study performed in the United States). For the other three studies involving participants of varied ethnicity,27,29,30 the authors provided data stratified by ethnic group.
In total, there were 556 individuals of African descent (Africans, African Americans, and black Brazilians), 1,889 Asians, and 6,908 Caucasian/white Americans. One hundred and seventeen Brazilian patients, self-identified as brown, were excluded from these analyses because of the extensive admixture of European and African ancestors.29 The mean minor allele frequencies in the three groups were 0.15, 0.25, and 0.31 for African descendants, Asians, and Caucasians/white Americans, respectively.
Impact of the CYP4F2 gene on mean daily dose of coumarin anticoagulants
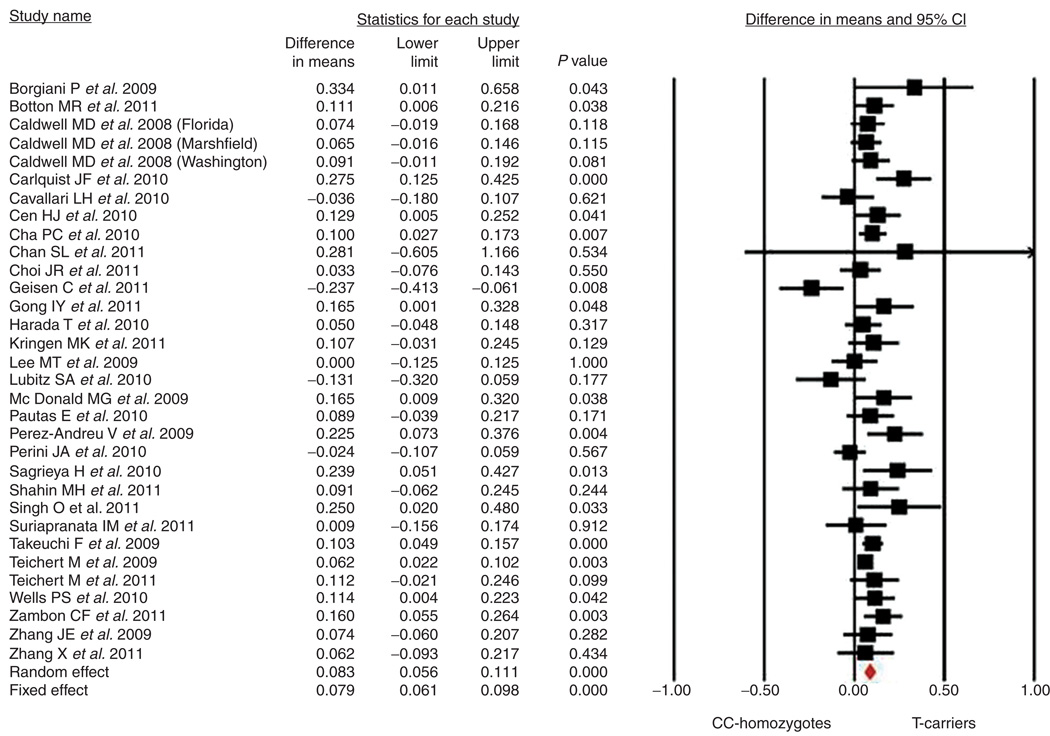
Thirty published studies fulfilled the prespecified inclusion criteria and constituted the basis for the main meta-analysis. We used data extracted from the full text of six studies21–24,34,41 and data received from the respective authors of the other 24 studies 5,6,15–20,25–33,35–40,42 The final number of cohorts analyzed was 32 because one of the studies (by Caldwell et al.) was divided into three cohorts according to study location. Hardy-Weinberg equilibrium was present in all but four studies,16,29,40,41 which showed a mild deviation (see Table 1). In the forest plot (Figure 2), the relative difference in mean coumarin daily dose for each study is presented, along with the overall results of the meta-analysis. As compared with homozygotes for the wild-type C allele, T-allele carriers required an 8.3% higher dose of coumarin (95% confidence interval (CI): 5.6–11.1%; P < 0.0001), with low to moderate heterogeneity (Q = 54.62; df= 31; P = 0.006; I2 = 43.25%; τ2 = 0.002± 0.001). The corresponding summary difference in mean values obtained using the recessive and additive models were 12.4% (95% CI: 7.4–17.4%) and 7.3% (95% CI: 5.3–9.4%), respectively, as shown in Supplementary Figures S1 and S2 online. We found that the CYP4F2 p.V433M polymorphism, regardless of the genetic model adopted, is highly significantly associated with increased daily dosage of coumarin anticoagulant. Finally, to be sure that there were no substantial differences between the published data and those sent by the authors, we carried out the same meta-analysis using data obtained directly from the published articles. Because the normalization procedure was not applicable in this meta-analysis, we used the standardized difference in means as the measure of effect size. Effect size and statistical significance were substantially similar in the two meta-analyses (0.183 ±0.029 vs. 0.169 ± 0.042; P< 0.0001 for both) (Supplementary Figure S3 online).
Figure 2.
Effect of the CYP4F2 rs2108622 C>T polymorphism on coumarin dosage requirement per the dominant genetic model (CC vs. CT&TT). CI, confidence interval.
Effects of VKORC1 and CYP2C9 polymorphisms on mean coumarin dose
From the 24 cohorts, we were able to obtain data directly from the authors of the primary studies. We used these data to calculate the impact of VKORC1 and CYP2C9 polymorphisms on mean coumarin dose and compared the result with that obtained for the CYP4F2 variant in the same subsamples. As presented in Table 2, carriers of the –1639 G>A variant allele for VKORC1 required a 31.5% lower coumarin dose as compared with homozygotes, whereas carriers of *2 and/or *3 variant alleles for CYP2C9 required a 24.1% lower coumarin dose as compared with *1*1 carriers. The forest plots of these meta-analyses are presented separately in Supplementary Figures S4–S7 online. The differences between the effects of the VKORC1 and CYP2C9 variants and CYP4F2 V433M were statistically significant. (Q > 178; df=1; P< 0.0001 for all).
Table 2.
Relative contribution of different polymorphisms on coumarin maintenance dose
| Gene variant | MAF | Difference in means |
Lower Limit |
upper limit | P value |
|---|---|---|---|---|---|
| CYP4F2 | 0.28 | 0.093 | 0.060 | 0.125 | P < 0.001 |
| VKORC1 | 0.48 | −0.315 | −0.345 | −0.285 | P < 0.001 |
| CYP2C9*2 | 0.10 | −0.185 | −0.218 | −0.147 | P < 0.001 |
| CYP2C9*3 | 0.05 | −0.272 | −0.312 | −0.232 | P < 0.001 |
| CYP2C9*2*3 | 0.14a | −0.241 | −0.278 | −0.204 | P < 0.001 |
Data calculated from a subgroup of 24 studies including over 6,000 participants. Because two studies19,20 did not have data for CYP2C9*2 genotyping, data from this polymorphism are derived from about 5,600 participants.
MAF, minor allele frequency.
MAF of the CYP2C9*2*3 refers to people who have at least a *2 or *3 variant.
Effect of CYP4F2 polymorphism on mean coumarin dose after stratification for the CVP2C9 and VKORC1 genotypes
Two meta-analyses were performed after stratification for the ClT2C9 *2/*3 and VKORC1 −1639 G>A polymorphisms. In homozygotes for the CYP2C9*1 variant (i.e., persons with no *2 or *3 alleles), the difference in the required doses of coumarin for CYP4F2 T carriers and CC homozygotes was 11.0% (95% CI: 6.8–15.2%), which is not significantly different from that for carriers of at least one CYP2C9 variant allele (either *2 or *3), 7.9% (95% CI: 1.9–13.9%; Q = 0.695; df= 1; P = 0.40) (Supplementary Figure S8 online).
In VKORC1 −1639 GG homozygotes, the difference in the required doses of coumarin for CYP4F2 T carriers and CC homozygotes was 6.9% (95% CI: 2.0–11.8%), whereas in carriers of at least one −1639 A variant allele the difference was 7.6% (95% CI: 3.5–11.7%; Q = 0.052; df= 1; P = 0.82) (Supplementary Figure S9 online).
Sensitivity analysis
In cumulative meta-analysis carried out according to time of publication (Supplementary Figure S10 online), statistical significance was present after a few studies were published, with stable effect sizes over time. In the cumulative meta-analysis carried out according to sample size, no clear trend was apparent with respect to sample size (Supplementary Figure S11 online).
Meta-regression
In meta-regression, none of the examined variables was significantly associated with a difference in the required dose of coumarin across studies (age: β ± SE = 0.0012 ± 0.0019, P = 0.531; BMI: β ± SE = −0.0016 ± 0.0061, P = 0.794; percentage of male participants: β ± SE = 0.0012 ± 0.001, P = 0.209; quality score: β ± SE = 0.0074 ± 0.0109, P = 0.499).
Meta-analysis in subgroups
Gender
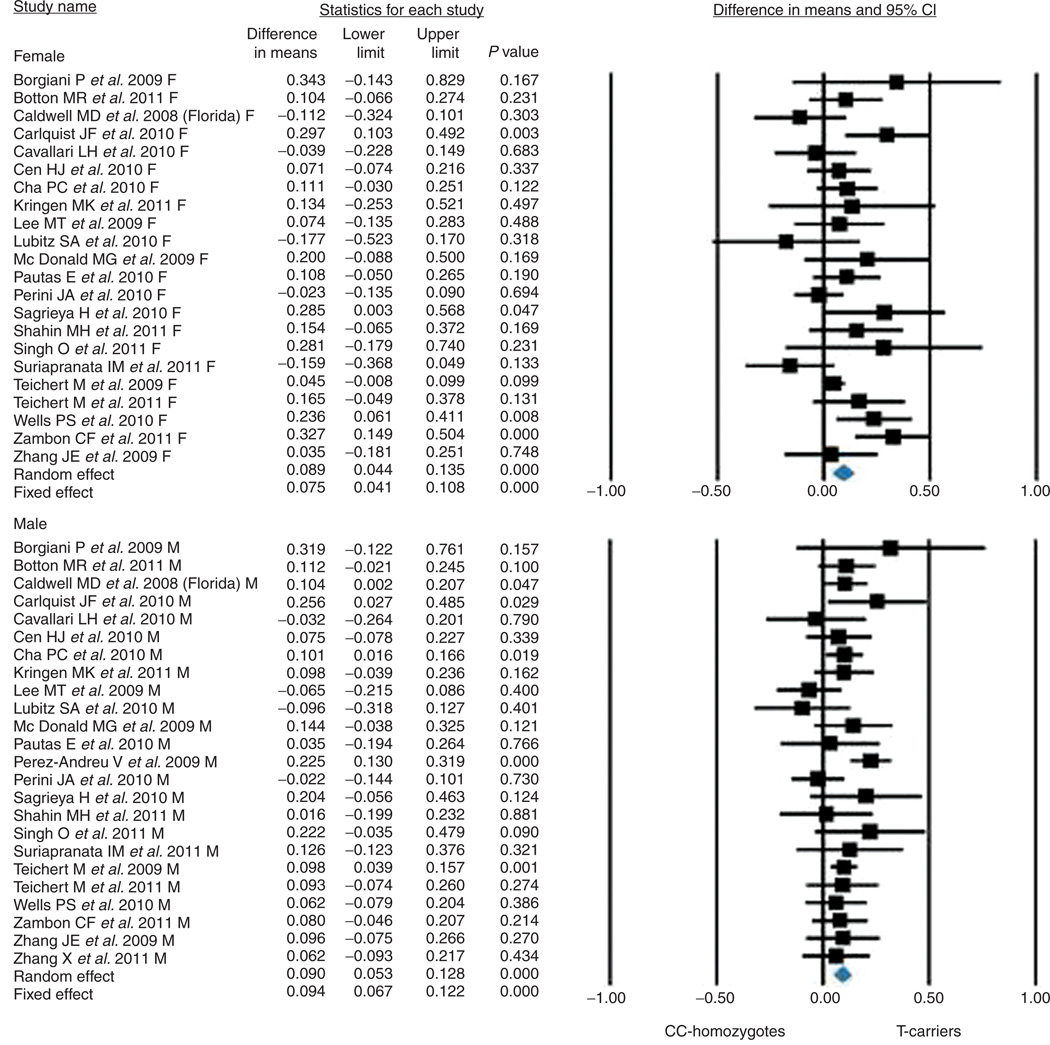
Because primary studies did not report data about coumarin drug dosage in male and female participants separately, this analysis was possible only in the 24 studies for which data were obtained directly from the authors. In total, these 24 studies involved 3,636 male participants and 3,032 female participants. As shown in Figure 3, the increase in coumarin daily dosage was 8.9 ± 4.4% in the women and 9.0 ± 5.3% in the men (P < 0.0001 for the comparison between CC homozygotes and T carriers in both subgroups; Q value = 0.001; df = 1; P = 0.978 for the comparison between male and female participants).
Figure 3.
Effect of the CYP4F2 rs2108622 C>T polymorphism on coumarin dose requirements in subgroups categorized by gender. CI, confidence interval.
Ethnicity
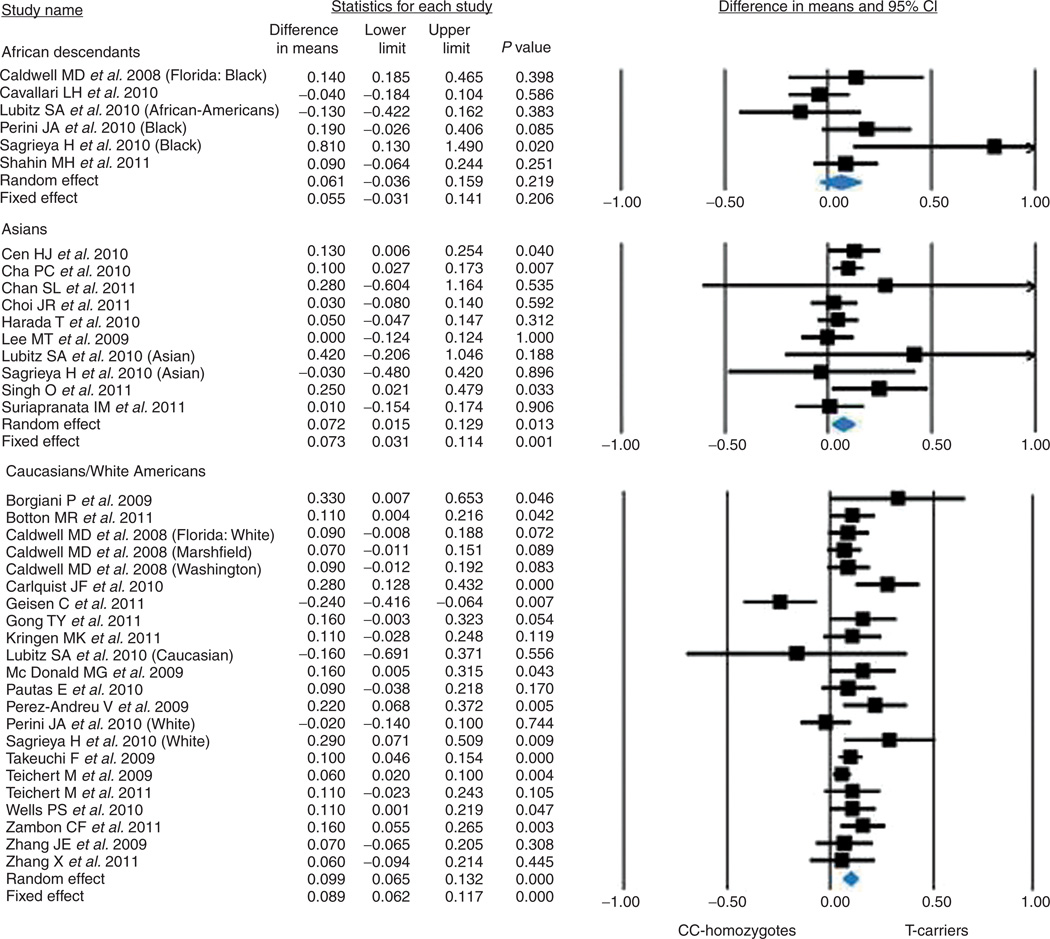
The summary effects as categorized by ethnicity yielded the following results: African descendants, 6 studies, 556 individuals, 6.1 ± 5.0%, P = 0.219; Asians, 10 studies, 1,889 individuals, 7.2 ± 2.9%, P = 0.013; Caucasian/white Americans, 23 studies, 6,908 individuals, 9.9 ± 1.7%, P < 0.0001 (Q value = 0.976; df = 2; P = 0.614) (Figure 4).
Figure 4.
Effect of the CYP4F2 rs2108622 C>T polymorphism on coumarin dose requirements in different ethnic subgroups. CI, confidence interval.
Target INR
No difference in coumarin drug dosage was detectable in the subgroups categorized according to target INR, either as assessed between groups based on the limits of INR (narrow limits of INR (2–3) vs. wider limits of INR (lower limit <2, upper limit >3); Q value = 2.252; df = 1; P = 0.133) or with varied permissible INR ranges (a decreased lower limit (1.5– 3.0), a narrow interval (2–3), or a higher upper limit (2–4); Q value = 1.944; df = 2; P = 0.378).
Quality score
A separate analysis, with studies categorized on the basis of quality score, showed no statistical differences between groups with high- and low-quality scores. The normalized value of the difference ± SE was 7.6 ± 1.7% (P < 0.0001) for studies with a quality score ≥5 (n = 21) and 10.4 ± 2.7% (P < 0.0001) for studies with a quality score <5 (n = 11; Q value = 0.789; df = 1, P = 0.375 for the comparison between high-quality and low-quality studies).
Coumarin drug
After categorizing the studies on the basis of the specific coumarin anticoagulant used, we found the following summary effects: 8.8 ± 1.6% (P < 0.0001; corresponding to a mean daily dose difference of 0.343 ± 0.057 mg/day) for studies involving warfarin (n = 28) and 5.9 ± 3.9% (P = 0.130; 6.2 ± 1.9%; P = 0.001 with a fixed effect) for studies involving other coumarins (n = 4) (Q value = 0.483; df = 1; P = 0.487).
Publication bias
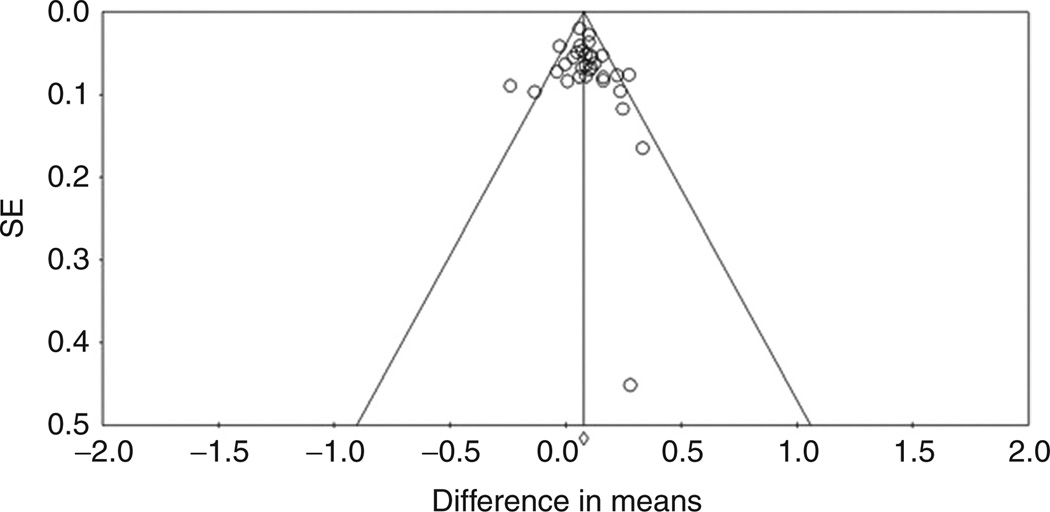
The funnel plot of the studies included in the main meta-analysis is presented in Figure 5. Five studies were outside the expected 95% confidence limits. This asymmetry indicates the possibility of heterogeneity in terms of small studies or selection bias. The modified Egger regression test indicated no significant asymmetry across studies (t = 0.912, P = 0.369), and neither did the Begg and Mazumdar rank correlation test (P = 0.322).
Figure 5.
Funnel plot for association studies of the CYP4F2 rs2108622 OT polymorphism on coumarin dose requirements. The solid vertical line represents the summary effect estimate, derived by using fixed-effects metaanalysis, for displaying the center of the plot in the absence of bias. The diagonal lines represent 95% confidence limits for the expected distribution of studies in the absence of heterogeneity between studies and absence of selection biases.
The trim-and-fill analysis suggested that five studies were missing. The adjusted normalized difference in means per the random-effects summary was 7.1% (95% CI: 4.2–10.0), obtained after symmetrically filling the funnel plot. This indicated that the strength of the association with mean coumarin drug maintenance dose may be overestimated (by 12%) because of unpublished studies.
DISCUSSION
The results of this meta-analysis indicate that the CYP4F2 p.V433M polymorphism is a statistically significant factor that affects daily maintenance coumarin dose but that it has a low effect size. Using a systematic search strategy, we retrieved 30 studies that met the prespecified eligibility criteria. The summary difference in mean values derived by adopting a dominant genetic model indicates that carriers of the T allele required an 8.3% higher mean daily coumarin dose than CC homozygotes did to achieve the target INR, and different genetic models gave significant estimates: 7.3% per the additive model and 12.4% per the recessive model. Our results appear to be robust, and no single study seems to be primarily responsible for the observed association between the CYP4F2 p.V433M polymorphism and coumarin dose requirements. It is important to note that 28 of the 32 studies included in our meta-analysis show a positive effect of the SNP. However, the funnel plot shows a certain asymmetry, estimated as nonsignificant when analyzed using Egger’s regression and a Begg and Mazumdar rank test. It is still possible that our results overestimate the effect size, as shown by the trim-and-fill analysis, suggesting that caution is necessary in interpreting the data.
In addition, there is some heterogeneity between mean differences in coumarin dose across studies, which is not explained by our moderating parameters, including age, BMI, percentage of male participants, and quality scores in different studies. We can only speculate that other genetic factors or environmental factors such as concomitant drugs are responsible for the observed differences.
As anticipated, two recent meta-analyses performed on the other well-known genetic polymorphisms related to coumarin dosage showed that these have an impact on daily warfarin dose requirement. When expressed as a percentage change in dose, the effects range from 19.6 to 78.1% and from 32 to 63% for CYP2C944 and VKORC1 SNPs,49 respectively. Another recent meta-analysis that involved only 13 studies for the CYP4F2 p.V433M polymorphism (as compared with the 30 analyzed here) found that T carriers of the CYP4F2 variant allele required an 11.0% (95% CI: 6.0–17.0) higher warfarin dose than individuals with the CC genotype.43 Although results concerning effect size in this study and in previous meta-analyses are not directly comparable, it seems clear that the CYP4F2 polymorphism plays a smaller role than CYP2C944 and VKORC1 in determining the warfarin maintenance dose.49 These data have also been confirmed by our results in a subsample of approximately 6,000 patients. Nonetheless, our data support the notion that CYP4F2 is the third most influential genetic locus with respect to coumarin drug maintenance dose. There are ongoing efforts aimed at implementing algorithms to estimate individualized coumarin dosage based on CYP2C9 and VKORC1 genotypes.45 The inclusion of CYP4F2 in such studies has often been proposed. Given the highly significant positive result related to CYP4F2 in our meta-analysis, our data could support the inclusion of CYP4F2 in pharmacogenetics dosing algorithms; however, the low effect size of this variant makes its actual clinical utility unclear. When dissecting this issue, several considerations should be kept in mind. The results of meta-analyses could yield an estimate of the mean effect of the polymorphism on an “average population” but cannot exclude the possibility that the influence of the polymorphism is greater in selected subgroups (e.g., people requiring very high or very low doses of coumarin drugs, people with very high or very low vitamin K intake). Indeed, a significant interaction with either genetic (mostly the CYP2C9 and VKORC1 loci) or environmental/demographic factors (already included in clinical algorithms) could prove to be of importance, even though our exploratory meta-analyses after stratification for the most common CYP2C9 and VKORC1 polymorphisms do not support this thesis. Finally, ongoing randomized controlled trials are comparing results related to daily coumarin doses as obtained using standard (i.e., without genetic data) algorithms with those obtained using complex (with the inclusion of genetic data) algorithms. The data from these studies will clarify the clinical utility of pharmacogenetics-guided coumarin dosing.46–48
Because sensitivity analyses found no significant changes and reversal results, we consider the results of our meta-analysis to be stable and reliable.
Recent data associating the rs2108622 polymorphism with cardiovascular-related end points suggested a sex-related difference in its effect. However, our study shows no significant gender-related difference in the effect size of this polymorphism on coumarin anticoagulant dose.
We found that the prevalence of the rs2108622 polymorphism varies across ethnic groups. We therefore performed an analysis across ethnic groups. This analysis should be taken as exploratory because the available data for ethnicity were not of high quality, especially for studies in countries with mixed populations. Keeping these considerations in mind, we found no substantial differences between these somewhat arbitrary ethnic groups obtained by pooling together (i) Africans plus African Americans and black Brazilians, (ii) Caucasians plus white Americans, and (iii) Asians; in the ethnic group composed of Africans plus African Americans, the influence of the polymorphism was not significant. Finally, the quality of the included studies does not seem to play a major role in determining significant differences in the effect size.
Limitation of the study
Our systematic meta-analysis has several limitations. First, although we applied a highly sensitive search strategy for the retrieval of potentially eligible studies, we cannot rule out the possibility that some relevant studies might have been overlooked. Second, we excluded results from association studies existing only as abstracts. This could have caused a disproportionate exclusion of significant or nonsignificant findings, thereby resulting in publication bias. However, incomplete data from abstracts results in uncertainty and thus do not allow validity assessments. Finally, data related to mean maintenance dose were not adjusted for nongenetic predictors such as vitamin K intake and interacting drugs.
Conclusion
In conclusion, our meta-analysis provides substantial evidence that the rs2108622 polymorphism in CYP4F2 is significantly associated with altered dose requirements for coumarin anticoagulants. On the other hand, given the small effect size, the need to include the CYP4F2 p.V433M genotype in pharmacogenetic algorithms created to optimize the maintenance dose of coumarin remains under debate. Well-performed randomized clinical trials are needed, comparing starting and maintenance coumarin doses and chosen on the basis of different algorithms that either do or do not take into account genetic information for the CYP4F2 rs2108622 polymorphism.
METHODS
We conducted the meta-analysis in accordance with the general guidelines of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.2; the PRISMA guidelines for reporting on systematic reviews; and the specific recommendations for genetic meta-analysis in the HuGE Review Handbook, version 1.0. (See Supplementary Materials and Methods online for the extended version of the Methods section.)
Information sources and search strategy
We searched Medline, Embase, and Web of Science from their inception to 31 August 2011, without language restrictions. The search algorithm combined the categories for “drug,” “cytochrome,” and “gene” by the Boolean operator “AND.” The search terms (medical subject headings and text words) in each category were combined with the operator “OR.”
The following search strategy was applied: (warfarin OR coumarin OR Coumadin OR acenocoumarol OR phenprocoumon) AND (CYP4F2 OR 4F2 OR cytochrome) AND (gene OR genetic* OR genomic* OR pharmacogenet* OR pharmacogenom* OR polymorph*).
In addition, in order to identify advance online publications, we searched the online databases of the 10 journals with the highest frequency of eligible publications as indexed by ISI Web of Science.
We manually searched the tables of contents of the issues of these journals for 2005–2011, along with the bibliographies of relevant articles to retrieve further potential publications.
Study selection and eligibility criteria
We included original papers of observational studies and clinical trials published in full text and those for which we had full access to all original data and protocols. We excluded studies that were published only as abstracts or conference reports. We also excluded case reports, reviews, meeting abstracts, and notes.
For unambiguous determination, the polymorphism of CYP4F2 needed to be designated by its NCBI dbSNP identifier (rs2108622), its mRNA nucleotide exchange (1347C>T), or its HGVS name (c.1297G>A and p.V433M).50
The studies selected had to meet the following inclusion criteria: (i) clinical cohort or cross-sectional study in coumarin-treated patients, (ii) CYP4F2 genotyping performed in all patients or in a random selection of patients (studies that selected participants on the basis of coumarin dose were eliminated), and (iii) coumarin maintenance dose data available separately for CC, CT, and TT genotypes or for the dominant model. There were no restrictions in the inclusion criteria with respect to patient demographic information, including mean age, body weight, height, use of interacting drugs, indication for coumarin use, and target INR range.
Data-collection process and extracted items
For standardization of data extraction, we adopted the Cochrane Consumers and Communication Review extraction template, modified it according to the recommendations in the HuGE Review Handbook for abstracting genetic information, pilot-tested five randomly selected studies among those included, and refined it accordingly.
With the aim of comparing the contribution of the CYP4F2 polymorphism as compared with the VKORC1 and CYP2C9 variants in determining the dose variability of coumarin, we asked the first/last authors of the primary studies to participate in a collaborative meta-analysis collecting data for these additional genotypes. We thus performed separate meta-analyses evaluating the impact of the following polymorphisms on coumarin maintenance dose: rsl799853 (430C>T) and rsl057910 (1075A>C) for CYP2C9 (known as CYP2C9 *2 and CYP2C9*3, respectively); and rs9923231 (−1639G>A) for VKORC1.
For the latter gene, we also included data from studies that used two alternative polymorphisms: rs9934438 (1173C>T) in the VKORC1 gene, which is in complete linkage disequilibrium with the reference polymorphism,15,25 and rsl0871454−1168C>T) located in the Syntaxin 4 A-placental (STX4A) gene, flanking the VKORC1 gene, which showed a linkage disequilibrium of 0.99 with the reference polymorphism.40,42
Successively, in order to evaluate the impact of the CYP4F2 polymorphism in carriers of different VKORC1 and CYP2C9 genotypes, we performed explorative meta-analyses after stratification for CYP2C9 and VKORC1 variants (the autosomal dominant model was applied).
In addition to these records, the authors also provided sex-stratified data, and these were used to investigate whether there is a sex-specific effect of the CYP4F2 p.V433M polymorphism.
To remove sources of heterogeneity caused by differences in coumarin types among the populations studied, we used normalized data in each of the meta-analyses. We emphasized results from dominant genetic models to quantify the effect size in each study. Data on additive and autosomal recessive models are reported in the Supplementary Materials and Methods online.
Methods for assessing the risk of bias in individual studies
To explore the risk of bias in primary studies, we investigated indicators of the quality of epidemiologic studies in general, applying items taken from the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies, indicators specific to the quality of genetic association studies, and indicators specific for coumarin (e.g., stable anticoagulation).
We checked for departure from Hardy–Weinberg equilibrium using Michael H. Court’s (2005–2008) online calculator (http://www.tufts.edu/~mcourt01/Documents/Court%20lab%20-%20HW%20calculator.xls).
We graded the methodologic quality of the selected studies by applying a scale with a maximum score of 8 points (see Supplementary Materials and Methods online for details). The score was plotted in meta-regression against the daily mean dose of the coumarin drug. In subgroup analyses, studies with a median scores <5 were compared against studies with median scores ≥5.
Summary-effect measures
We calculated crude differences in means and SE for each study on the basis of genotype contrasts of a dominant model that compared the following two groups: heterozygous genotypes and homozygous genotypes of the minor allele vs. homozygous genotypes of the major allele. Where the normalization procedure was not applicable, we calculated the standardized difference in means. Anticipating heterogeneity between studies in meta-analyses of genetic association studies, we decided to calculate, as a primary summary-effect estimate, the difference in means according to the DerSimonian and Laird random-effects model, which is the generally preferred approach adopted in these cases. Nevertheless, because random-effects models give more weight to smaller studies, thereby introducing the risk of generating higher summary estimates in the presence of bias from small study effects, we also calculated the fixed-effects summary estimates according to the Mantel-Haenszel method (presented only in the figures). A two-sided P test value <0.05 indicates a nominally significant overall association.
Heterogeneity measures
Heterogeneity among studies was tested using Cochran’s Q test (Mantel-Haenszel χ2 test), with P < 0.10 indicating significant heterogeneity. The extent of variance between studies was estimated using the τ2 metric. The percentage of total variance attributable to heterogeneity between studies was quantified using the I2 metric.
Sensitivity analyses, meta-regression, and subgroup analyses
Sensitivity analyses were performed to assess sources of heterogeneity. We first ran a cumulative meta-analysis by adding studies one at a time, in chronological order, to explore the temporal evolution of the effect size. Next, we repeated this procedure according to sample size to test whether bias related to publication could affect the results. Meta-regressions testing age, BMI, percentage of male participants, and the quality score as moderators were performed using the random effect “method of moments.” Subgroup meta-analyses comparing the effects of the polymorphism in male participants relative to female participants, in subjects belonging to different ethnicities, in studies with high- vs. low-quality scores, in studies with different INR targets, and in studies using different types of coumarin drugs were performed using Cochran’s Q test (Mantel–Haenszelχ2 test).
Assessment of bias across studies
To assess potential bias from small study effects, we constructed funnel plots displaying the log difference in mean values from individual studies on the horizontal axis and the standard errors of the log difference in mean values (precision) on the vertical axis. Methods used for assessing publication bias are presented in the Supplementary Materials and Methods online. All statistical analyses were performed using the Comprehensive Meta-Analysis package, version 2.2.064 (Biostat, Englewood, NJ).
Supplementary Material
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
To date, a large number of studies have investigated the association between CYP4F2 genotype and coumarin dose requirements in humans; many of these studies were not sufficiently powered to give useful quantitative estimates of the genotype effect.
WHAT QUESTION DID THIS STUDY ADDRESS?
The aims of this study were (i) to conduct a systemic review and meta-analysis in order to quantify the magnitude of the effect of the CYP4F2 p.V433M polymorphism on mean daily coumarin dose and (ii) to evaluate whether the association is gender related.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
Our results show that the CYP4F2 p.V433M polymorphism is highly associated with the interindividual variability in response to coumarin drugs, albeit with a low effect size.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS
The clinical utility of including this variant allele in current pharmacogenetic coumarin dosing algorithms remains unclear.
ACKNOWLEDGMENTS
We thank Rocio Gonzalez-Conejero, who kindly provided data necessary for our analyses. The efforts of J.AJ., A.E.R., and N.T.A. were supported, in part, by National Institutes of Health research grants U01GM074492, U01GM092676, R01GM068797, and TLI RR025016. J.E.Z. and MP. were supported by the UK Department of Health NHS Chair of Pharmacogenetics programme.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
AUTHOR CONTRIBUTIONS, C.F., E.D., M.M., J.A.J., A.E.R., C.F.Z., S.A.L., G.S.-K., L.H.C., L.Z., Y.N.J.M., M.K.K., P.B., C.C., N.T.A.,T.L,V.S., M.A.L., H.S., M.H.A.S., S.A.S., S.I.K., B.C., I.M.S., M.T, B.H.S., M.X, M.R.B., J.E.Z., M.P., X.Z., J.F.C., B.D.H., M.T.M.L, V.P., R.B.A., and M.H. wrote the manuscript. C.F., E.D., G.C.G., and P.M. designed the research. E.D. and M.M. performed the research. C.F. and E.D. analyzed the data. J .A J., A.E.R., C.F.Z., S.A.L., G.S.-K., L.H.C., L.Z., Y.N.J.M., M.K.K., P.B., C.C., N.T.A.J.L., V.S., M.A.L., H.S., M.H.A.S., S.A.S., S.I.K., B.C., I.M.S., M.T, B.H.S., M.T, M.R.B., J.E.Z., M.R,X.Z., J.F.C., B.D.H., M.T.M.L., V.R, R.B.A., and M.H. provided sex-stratified and non-stratified data.
CONFLICT OF INTEREST
J.A.J. is a member of the Clarification of Optimal Anticoagulation Through Genetics (COAG) clinical trial executive committee. S.A.S.,T.L, and J.F.C. are members of the COAG genotyping committee. The other authors declared no conflict of interest.
References
- 1.Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:204S–233S. doi: 10.1378/chest.126.3_suppl.204S. [DOI] [PubMed] [Google Scholar]
- 2.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 3.Lurie Y, Loebstein R, Kurnik D, Almog S, Halkin H. Warfarin vitamin Kintake in the era of pharmacogenetics. Br. J. Clin. Pharmacol. 2010;70:164–170. doi: 10.1111/j.1365-2125.2010.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavallari LH, Shin J, Perera MA. Role of pharmacogenomics in the management of traditional and novel oral anticoagulants. Pharmacotherapy. 2011;31:1192–1207. doi: 10.1592/phco.31.12.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 is a vitamin K1 oxidase: An explanation for altered warfarin dose in carriers of the V433M variant. Mol. Pharmacol. 2009;75:1337–1346. doi: 10.1124/mol.109.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell MD, et al. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008;111:4106–4112. doi: 10.1182/blood-2007-11-122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fava C, et al. The V433M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension. 2008;52:373–380. doi: 10.1161/HYPERTENSIONAHA.108.114199. [DOI] [PubMed] [Google Scholar]
- 8.Fava C, Ricci M, Melander O, Minuz P. Hypertension, cardiovascular risk and polymorphisms in genes controlling the cytochrome P450 pathway of arachidonic acid: A sex-specific relation. Prostaglandins Other Lipid Mediat. 2012;98:75–85. doi: 10.1016/j.prostaglandins.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Cavallari LH, et al. Association of apolipoprotein E genotype with duration of time to achieve a stable warfarin dose in African-American patients. Pharmacotherapy. 2011;31:785–792. doi: 10.1592/phco.31.8.785. [DOI] [PubMed] [Google Scholar]
- 10.Chan SL, et al. Effects of CYP4F2 GGCX genetic variants on maintenance warfarin dose in a multi-ethnic Asian population. Thromb. Haemost. 2011;105:1100–1102. doi: 10.1160/TH11-01-0018. [DOI] [PubMed] [Google Scholar]
- 11.Cooper GM, et al. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112:1022–1027. doi: 10.1182/blood-2008-01-134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashley EA, et al. Clinical assessment incorporating a personal genome. Lancet. 2010;375:1525–1535. doi: 10.1016/S0140-6736(10)60452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciccacci C, et al. Characterization of a novel CYP2C9 gene mutation and structural bioinformatic protein analysis in a warfarin hypersensitive patient. Pharmacogenet. Genomics. 2011;21:344–346. doi: 10.1097/FPC.0b013e328344c340. [DOI] [PubMed] [Google Scholar]
- 14.Isaza C, et al. [Genetic and bioenvironmental factors associated with warfarin response in Colombian patients] Biomedica. 2010;30:410–420. [PubMed] [Google Scholar]
- 15.Borgiani P, et al. CYP4F2 genetic variant (rs2108622) significantly contributes to warfarin dosing variability in the Italian population. Pharmacogenomics. 2009;10:261–266. doi: 10.2217/14622416.10.2.261. [DOI] [PubMed] [Google Scholar]
- 16.Botton MR, Bandinelli E, Rohde LE, Amon LC, Hutz MH. Influence of genetic, biological and pharmacological factors on warfarin dose in a Southern Brazilian population of European ancestry. Br. J. Clin. Pharmacol. 2011;72:442–450. doi: 10.1111/j.1365-2125.2011.03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlquist JF, et al. An evaluation of nine genetic variants related to metabolism and mechanism of action of warfarin as applied to stable dose prediction. J. Thromb. Thrombolysis. 2010;30:358–364. doi: 10.1007/s11239-010-0467-3. [DOI] [PubMed] [Google Scholar]
- 18.Cavallari LH, et al. Genetic clinical predictors of warfarin dose requirements in African Americans. Clin. Pharmacol. Ther. 2010;87:459–464. doi: 10.1038/clpt.2009.223. [DOI] [PubMed] [Google Scholar]
- 19.Cen HJ, et al. CYP4F2 rs2108622: a minor significant genetic factor of warfarin dose in Han Chinese patients with mechanical heart valve replacement. Br. J. Clin. Pharmacol. 2010;70:234–240. doi: 10.1111/j.1365-2125.2010.03698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha PC, et al. Genome-wide association study identifies genetic determinants of warfarin responsiveness for Japanese. Hum. Mol. Genet. 2010;19:4735–4744. doi: 10.1093/hmg/ddq389. [DOI] [PubMed] [Google Scholar]
- 21.Chan SL, Suo C, Lee SC, Goh BC, Chia KS, Teo YY. Translational aspects of genetic factors in the prediction of drug response variability: a case study of warfarin pharmacogenomics in a multi-ethnic cohort from Asia. Pharmacogenomics J. 2012;12:312–318. doi: 10.1038/tpj.2011.7. [DOI] [PubMed] [Google Scholar]
- 22.Choi JR, et al. Proposal of pharmacogenetics-based warfarin dosing algorithm in Korean patients. J. Hum. Genet. 2011;56:290–295. doi: 10.1038/jhg.2011.4. [DOI] [PubMed] [Google Scholar]
- 23.Gong IY, et al. Clinical and genetic determinants of warfarin pharmacokinetics and pharmacodynamics during treatment initiation. PLoS ONE. 2011;6:e27808. doi: 10.1371/journal.pone.0027808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada T, et al. Application of Akaike information criterion to evaluate warfarin dosing algorithm. Thromb. Res. 2010;126:183–190. doi: 10.1016/j.thromres.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Kringen MK, et al. Genetic variation of VKORC1 and CYP4F2 genes related to warfarin maintenance dose in patients with myocardial infarction. J. Biomed. Biotechnol. 2011;2011:739751. doi: 10.1155/2011/739751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MT, et al. Genetic determinants of warfarin dosing in the Han-Chinese population. Pharmacogenomics. 2009;10:1905–1913. doi: 10.2217/pgs.09.106. [DOI] [PubMed] [Google Scholar]
- 27.Lubitz SA, et al. Comparative performance of gene-based warfarin dosing algorithms in a multiethnic population. J. Thromb. Haemost. 2010;8:1018–1026. doi: 10.1111/j.1538-7836.2010.03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pautas E, et al. Genetic factors (VKORC1 CYP2C9 EPHX1 and, CYP4F2) are predictor variables for warfarin response in very elderly, frail inpatients. Clin. Pharmacol. Ther. 2010;87:57–64. doi: 10.1038/clpt.2009.178. [DOI] [PubMed] [Google Scholar]
- 29.Perini JA, Struchiner CJ, Silva-Assunção E, Suarez-Kurtz G. Impact of CYP4F2 rs2108622 on the stable warfarin dose in an admixed patient cohort. Clin. Pharmacol. Ther. 2010;87:417–420. doi: 10.1038/clpt.2009.307. [DOI] [PubMed] [Google Scholar]
- 30.Sagreiya HE, et al. xtending evaluating a warfarin dosing algorithm that includes CYP4F2 and pooled rare variants of CYP2C9. Pharmacogenet. Genomics. 2010;20:407–413. doi: 10.1097/FPC.0b013e328338bac2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahin MH, et al. Genetic nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet. Genomics. 2011;21:130–135. doi: 10.1097/FPC.0b013e3283436b86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh O, Sandanaraj E, Subramanian K, Lee LH, Chowbay B. Influence of CYP4F2 rs2108622 (V433M) on warfarin dose requirement in Asian patients. Drug. Metab. Pharmacokinet. 2011;26:130–136. doi: 10.2133/dmpk.dmpk-10-rg-080. [DOI] [PubMed] [Google Scholar]
- 33.Suriapranata IM, et al. Genetic factors associated with patient-specific warfarin dose in ethnic Indonesians. BMC. Med. Genet. 2011;12:80. doi: 10.1186/1471-2350-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi F, et al. A genome-wide association study confirms VKORC1 CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5:e1000433. doi: 10.1371/journal.pgen.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells PS, et al. A regression model to predict warfarin dose from clinical variables and polymorphisms in CYP2C9, CYP4F2 and VKORC1: derivation in a sample with predominantly a history of venous thromboembolism. Thromb. Res. 2010;125:e259–e264. doi: 10.1016/j.thromres.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Zambon CF, et al. VKORC1, CYP2C9 and CYP4F2 genetic-based algorithm for warfarin dosing: an Italian retrospective study. Pharmacogenomics. 2011;12:15–25. doi: 10.2217/pgs.10.162. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JE, et al. Effects of CYP4F2 genetic polymorphisms and haplotypes on clinical outcomes in patients initiated on warfarin therapy. Pharmacogenet. Genomics. 2009;19:781–789. doi: 10.1097/FPC.0b013e3283311347. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Li L, Ding X, Kaminsky LS. Identification of cytochrome P450 oxidoreductase gene variants that are significantly associated with the interindividual variations in warfarin maintenance dose. Drug. Metab. Dispos. 2011;39:1433–1439. doi: 10.1124/dmd.111.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pérez-Andreu V, et al. Pharmacogenetic relevance of CYP4F2 V433M polymorphism on acenocoumarol therapy. Blood. 2009;113:4977–4979. doi: 10.1182/blood-2008-09-176222. [DOI] [PubMed] [Google Scholar]
- 40.Teichert M, et al. A genome-wide association study of acenocoumarol maintenance dosage. Hum. Mol. Genet. 2009;18:3758–3768. doi: 10.1093/hmg/ddp309. [DOI] [PubMed] [Google Scholar]
- 41.Geisen C, et al. Prediction of phenprocoumon maintenance dose and phenprocoumon plasma concentration by genetic and non-genetic parameters. Eur. J. Clin. Pharmacol. 2011;67:371–381. doi: 10.1007/s00228-010-0950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teichert M, et al. Dependency of phenprocoumon dosage on polymorphisms in the VKORC1, CYP2C9 and CYP4F2 genes. Pharmacogenet. Genomics. 2011;21:26–34. doi: 10.1097/FPC.0b013e32834154fb. [DOI] [PubMed] [Google Scholar]
- 43.Liang R, Wang C, Zhao H, Huang J, Hu D, Sun Y. Influence of CYP4F2 genotype on warfarin dose requirement-a systematic review and metaanalysis. Thromb. Res. 2012;130:38–44. doi: 10.1016/j.thromres.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 44.Lindh JD, Holm L, Andersson ML, Rane A. Influence of CYP2C9 genotype on warfarin dose requirements-a systematic and meta-analysis. Eur. J. Clin. Pharmacol. 2009;65:365–375. doi: 10.1007/s00228-008-0584-5. [DOI] [PubMed] [Google Scholar]
- 45.Johnson JA, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes warfarin dosing. Clin. Pharmacol. Ther. 2011;90:625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.French B, et al. Statistical design of personalized medicine interventions: the Clarification of Optimal Anticoagulation through Genetics (COAG) trial. Trials. 2010;11:108. doi: 10.1186/1745-6215-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Schie RM, et al. Genotype-guided dosing of coumarin derivatives: the European pharmacogenetics of anticoagulant therapy (EU-PACT) trial design. Pharmacogenomics. 2009;10:1687–1695. doi: 10.2217/pgs.09.125. [DOI] [PubMed] [Google Scholar]
- 48.Anderson JL, et al. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II) Circulation. 2012;125:1997–2005. doi: 10.1161/CIRCULATIONAHA.111.070920. [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Ge W, Yu F, Zhu H. Impact of VKORC1 gene polymorphism on interindividual and interethnic warfarin dosage requirement-a systematic review and meta analysis. Thromb. Res. 2010;125:e159–e166. doi: 10.1016/j.thromres.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Stec DE, Roman RJ, Flasch A, Rieder MJ. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol. Genomics. 2007;30:74–81. doi: 10.1152/physiolgenomics.00003.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.