Abstract
Background
Since the Diabetes Prevention Project (DPP) demonstrated that lifestyle weight-loss interventions can reduce the incidence of diabetes by 58%, several studies have translated the DPP methods to public health–friendly contexts. Although these studies have demonstrated short-term effects, no study to date has examined the impact of a translated DPP intervention on blood glucose and adiposity beyond 12 months of follow-up.
Purpose
To examine the impact of a 24-month, community-based diabetes prevention program on fasting blood glucose, insulin, insulin resistance as well as body weight, waist circumference, and BMI in the second year of follow-up.
Design
An RCT comparing a 24-month lifestyle weight-loss program (LWL) to an enhanced usual care condition (UCC) in participants with prediabetes (fasting blood glucose=95–125 mg/dL). Data were collected in 2007–2011; analyses were conducted in 2011–2012.
Setting/participants
301 participants with prediabetes were randomized; 261 completed the study. The intervention was held in community-based sites.
Intervention
The LWL program was led by community health workers and sought to induce 7% weight loss at 6 months that would be maintained over time through decreased caloric intake and increased physical activity. The UCC received two visits with a registered dietitian and a monthly newsletter.
Main outcome measures
The main measures were fasting blood glucose, insulin, insulin resistance, body weight, waist circumference, and BMI.
Results
Intent-to-treat analyses of between-group differences in the average of 18- and 24-month measures of outcomes (controlling for baseline values) revealed that the LWL participants experienced greater decreases in fasting glucose (−4.35 mg/dL); insulin (−3.01 μU/ml); insulin resistance (−0.97); body weight (−4.19 kg); waist circumference (−3.23 cm); and BMI (−1.40), all p-values <0.01.
Conclusions
A diabetes prevention program administered through an existing community-based system and delivered by community health workers is effective at inducing significant long-term reductions in metabolic indicators and adiposity.
Trial registration
This study is registered at Clinicaltrials.gov NCT00631345.
Introduction
Latest estimates indicate that almost 26 million people in the U.S. have diabetes (8.3% of the population), and another 79 million adults have predia-betes.1 Although diabetes-related mortality has decreased by 8.3% over the past 10 years, the prevalence of diabetes has increased and is accompanied by greater prevalence of complications (e.g., cardiovascular disease, renal disease, lower extremity dysfunction).2 Thus, despite improved treatment of diabetes, its overall public health burden continues to increase and highlights the critical importance of prevention.3 International clinical trials, including the Diabetes Prevention Program (DPP)4 and the Finnish Diabetes Prevention Study (FDPS),5 have demonstrated that lifestyle weight-loss programs can prevent the incidence of diabetes in individuals with elevated blood glucose by up to 58%. Because these studies involved substantial amounts of resources and specialized personnel, several studies have attempted to translate these programs to more public health–friendly contexts to lower costs and increase access.
Two recent reviews6,7 have examined critically several diabetes prevention translational studies. Although the studies reviewed overlap substantially, the analyses each take individual approaches to examining the literature and reach contrasting conclusions. Whittemore7 reviewed 16 studies that translated the DPP lifestyle weight-loss intervention and categorized each according to the study context: (1) hospital outpatient; (2) primary care; (3) community; and (4) work and church settings. Of the studies reviewed, 81% (n=13) were one-group designs; the length of follow-up ranged from 3 months to 2 years; and weight loss at the longest point of follow-up varied between −1.0 kg and −8.6 kg.
Each study was reviewed critically using the RE-AIM8 framework (reach, effectiveness, adoption, implementation and maintenance). From this review, the authors concluded that (1) the studies varied considerably in terms of the five framework elements; (2) hospital outpatient diabetes education models of care were particularly efficacious at inducing weight loss; and (3) reach and efficacy appear to have an inverse relationship. That is, the settings with the greatest ability to reach diverse samples, such as community and work/church settings, demonstrated the least weight loss and more-restricted settings such as hospital practices demonstrated the greatest weight loss.
Ali and colleagues6 conducted a meta-analysis on 28 studies that tested various translational models for disseminating the methods of the DPP. Of the 28 studies reviewed, four were RCTs, two were cluster-RCTs, 20 were single-group pre–post studies, and two were nonrandomized controlled studies. The authors classified studies based on the type of personnel used to deliver the program: medical and allied health professionals, lay community members, and electronic media–assisted methods. They found that each group of studies demonstrated significant weight loss at 12 months of follow-up: medical and allied health professionals, −4.27 kg; lay community members, −3.15 kg; electronic media–assisted, −4.20 kg; overall mean = −3.99 kg. Based on sensitivity analyses, the authors concluded that programs led by lay community members may have been associated with better weight loss than that achieved by medical and allied health professionals.
A recent analysis of six DPP translational studies that utilized community health workers (CHWs)9 supports the conclusions of Ali and colleagues.8 The authors adopted the definition of CHWs that was developed by the DHHS, Health Services and Resources Administration: lay community members that work in association with local healthcare providers in a variety of settings and share a common culture (i.e., ethnicity, SES, language, life experience) with the individuals they serve.10 Of the six studies reviewed, two were RCTs, one was a cluster RCT, and three were single-group designs. Two studies included follow-up of 12 months and four included follow-up ranging from 3 to 6 months. All studies demonstrated significant weight loss, ranging from −1.5 kg (12 weeks of follow-up) to −7.27 kg (12 months of follow-up).
The manner in which CHWs were utilized varied considerably among studies. In one study, CHWs supported a team of dietitians and exercise specialists.11 Trained YMCA staff delivered a group-based DPP lifestyle weight-loss program in the DEPLOY (delivery of a group-based DPP lifestyle intervention offered at the YMCA) study.12 However, in several studies, the CHWs delivered group-based DPP lifestyle interventions.13–16 Despite the variability in efficacy and the manner in which CHWs are used among the studies reviewed, this approach appears to hold great promise for translating the DPP lifestyle weight-loss intervention.
Taken together, the literature suggests that a number of distinct approaches for translating the DPP lifestyle weight-loss intervention appear to be feasible and efficacious. However, this body of literature is dominated by single-group designs; a large number of the studies reviewed include pilot studies with small samples, and short-term follow-up; and only one study13 reported significant intervention effects on glucose. The Healthy Living Partnerships to Prevent Diabetes (HELP PD)13,17 was an RCT that translated the DPP lifestyle intervention through a partnership between an existing community system and CHWs. The intervention was administered through a local diabetes care center by registered dietitians (RDs) and delivered by CHWs in community settings. At 12 months of follow-up, participants in the lifestyle weight-loss intervention experienced significantly greater reductions in body weight, waist circumference, BMI, glucose, insulin, and insulin resistance as compared to an enhanced usual care comparison group.13
The HELP PD study is also the only DPP translational study to report significant changes in fasting blood glucose (−4.3 mg/dL during the first year, p<0.001).13 Although the results of HELP PD and other DPP translational studies are promising, the long-term effectiveness (beyond 12 months) of these approaches remains to be determined. Therefore, the purpose of the present study is to report the impact of the HELP PD approach during the second year of follow-up on fasting blood glucose, insulin, insulin resistance, body weight, waist circumference, and BMI.
Methods
Study Design
The design and methods of the present study have been reported elsewhere.13,17 Briefly, this RCT sought to translate the DPP lifestyle weight-loss intervention through a local diabetes education program (DEP) and CHWs. Overweight and obese volunteers with elevated fasting blood glucose were assigned randomly to either a group-based, DPP lifestyle weight-loss (LWL) intervention or an enhanced usual care comparison condition (UCC).17 The primary outcome was fasting blood glucose, and other outcomes of interest included insulin, insulin resistance, body weight, BMI, and waist circumference. Outcomes were assessed every 6 months for 24 months. The data were collected during 2007–2011, and analyses were conducted in 2011 and 2012. All study procedures followed a written protocol and all participants provided written consent. The Wake Forest University Health Sciences IRB approved study procedures.
Participants
The recruitment procedures have been reported previously.18 Eligibility criteria were selected to target a sample that was representative of the local community; had an elevated risk of developing type 2 diabetes (i.e., prediabetes) based on fasting blood glucose (95 mg/dL–125 mg/dL) and BMI (25–39); and possessed no contraindications to participation in a weight-loss program or independent physical activity. Main exclusion criteria included clinical history of diabetes, cardiovascular disease occurring within the past 6 months, uncontrolled hypertension, pregnancy, chronic use of medicine known to substantially affect glucose metabolism, other chronic disease likely to limit life span to <2–3 years, and any other factor likely to interfere with participation and willingness to accept randomization (e.g., major psychiatric or cognitive problems).17
The Physical Activity Readiness Questionnaire (PAR-Q) was used to identify participants with contraindications to exercise. The primary recruitment method involved mass mailings to local ZIP codes to target a sample representative of the local population, and there was no racial or gender bias in the selection of participants. Eligible participants were assigned randomly to treatment arms using a permuted block design with varying block size to reduce the time participants had to wait for intervention groups to form.17
Community-Based Implementation
The overarching objective was to test a translation of the DPP model that is implemented in community settings by community organizations and community members. As such, the research team sought to minimize the impact of specialized research personnel and maximize the role of existing community systems and assets (e.g., DEP personnel, CHWs) in the implementation of the intervention.17 To achieve this goal, the Lifestyle Weight-Loss (LWL) intervention was conducted in community-based sites (e.g., parks and recreation centers); implemented and monitored via a local DEP; and led by CHWs. Study investigators and staff were responsible for study administration (e.g., recruitment, data management), but the registered dietitians (RDs) employed by the DEP were responsible for the day-to-day operations of the intervention.13,17
The RDs also recruited, trained, monitored, and supported the CHWs. The CHWs led the group sessions, collected session attendance and self-monitoring data, and primarily were responsible for participant management. The RDs met with an Intervention Committee consisting of study staff and investigators regularly (approximately 2.5 hours per month) to provide updates of intervention implementation and progress. To maximize dissemination potential, a “train the trainer” model was adopted: investigators trained the RDs to train the CHWs. The RDs received 16 hours of training focused on study-specific material and consisted of (1) study protocol; (2) intervention philosophy, goals, and procedures; (3) weight loss (energy balance); (4) physical activity basics; (5) group facilitation; (6) cognitive–behavioral principles; (7) participant monitoring and toolbox methods; (8) role-playing; and (9) data entry.17 The RDs were supported at a level of 0.4 full-time equivalents per year during the 4 years of the program. They also maintained their clinical responsibilities while administering the LWL.17
The CHWs were community members with well-controlled type 2 diabetes (HbA1c <7.0%); a history of healthy eating and physical activity; and evidence of the potential for effective group leadership, such as prior experience and strong interpersonal skills. CHW training consisted of a 36-hour program conducted over the course of 6–9 weeks and involved experiential learning, didactic instruction, peer mentoring, and observation.13 A certification process was developed that consisted of observation, rating, and coaching by the RDs. The CHW training program was developed in conjunction with and delivered by the RDs. CHWs were compensated $100 per week during the first 6 months of their work with an intervention group, when the group met weekly, and $200 per month during months 7–24, when the group met monthly. Ten CHWs were trained in two waves: one group of five was trained prior to recruitment and another group of five began training 4 months into recruitment. Of the ten CHWs that completed training, the mean age was 57.2 years; seven were African-American (three Caucasian); eight were women; seven were employed; eight reported some education beyond high school; and all had completed some form of diabetes self-management education.
Lifestyle Weight-Loss Intervention
The LWL was adapted from the DPPLI4 for use in groups and delivery by nonprofessional staff. The objective was to induce negative energy balance through reductions in daily caloric intake (1200–1800 kcal/day) and increases in moderate-intensity aerobic physical activity (180 minutes/week) in order to produce a weight loss of approximately 0.3 kg per week for the first 6 months of treatment (Phase 1) for a total weight loss of 5%–7%.17 During Phase 2 (Months 7–24), participants were encouraged to continue to meet their weight-loss goals as long as their BMI did not fall below 20, but the primary focus was on weight maintenance.17
Participants met weekly for group sessions during Phase 1 (Months 1–6), and all sessions were coordinated and facilitated by the CHW. In addition, all participants received three personalized consultations with an RD (during Months 1, 3, and 6). The group sessions consist of 8–12 participants and were conducted at community sites (e.g., parks and recreation centers) with arrangements facilitated by study staff. During Phase 2 (Months 7–24), participants received two scheduled contacts with the CHW each month, one group session and one phone contact.
Enhanced Usual Care Comparison Condition
The comparison intervention condition was designed to exceed the usual care provided to similar community members with prediabetes and to enhance retention. The UCC consisted of two individual sessions with an RD nutritionist during the first 3 months that involved discussions of basic aspects of healthy eating and activity to support healthy living and existing community resources that may fit the individual needs of comparison participants. UCC participants also received a monthly newsletter that focused on healthy lifestyle and community resources.13,17
Outcome Measures
Fasting blood glucose, insulin, insulin resistance, body weight, waist circumference, and BMI were assessed at baseline and every 6 months for 24 months by trained study staff (HbA1c was not assessed in this study). All biochemical measurements were performed in a central laboratory by technicians masked to participants’ intervention assignment. Phlebotomy was performed after at least an 8-hour fast in accordance with the American Diabetes Association guidelines.13,19 Glucose was measured using a timed endpoint method supplied by Beckman Coulter for the Synchron LX Analyzer. This method, developed by the CDC, has been accepted as a reference method for glucose determination. Within-run coefficients of variation (CVs) for this method are ≤3.9%, and total CVs are less ≤6.45%.
The insulin assay used was the paramagnetic particle, chemiluminescent immunoassay for the Access Immunoassay Systems from Beckman Coulter. There is <0.3% cross-reactivity with human proinsulin and no detectable cross-reactivity with human C-peptide. Low- and high-level human serum quality-control samples were run during each 24-hour time period. The overall within-assay variability is 3.9%, and the between-assay variability is 5.5%. The assay mean for this laboratory is 44.7 with a CV of 2.56, and 152.7 with a CV of 5.77.17 Insulin resistance was examined using the homeostasis index (HOMA IR= [fasting insulin X fasting glucose]/22.5).20 HOMA IR is a better measure of insulin resistance than is fasting insulin alone and is highly correlated with other more complex and invasive measures of insulin resistance from the frequently sampled intravenous glucose tolerance test and the euglycemic clamp.13,17,20,21
Body weight was assessed with participants wearing street clothes without shoes or outer garments (i.e., jackets, sweaters) using a Cardinal Detecto Digital Scale (758 C Series). Waist circumference was assessed using a Gulick II 150-cm anthropometric tape with the participant in a recumbent position and was taken without clothing directly touching the skin. The tape measure was placed around the torso at the midpoint between the inferior margin of the last rib and the crest of the ilium.22 Body height was assessed by having participants stand erect on the floor with their backs against a vertical stadiometer (Accu-Hite Measure device with level bubble). BMI was calculated using standard formula.
Data Analysis
The authors have previously published results documenting the efficacy of this intervention during the first year of follow-up; hence, the primary focus of these analyses is on the intervention effect during the second year.13 Consequently, the results presented here are based on between-group differences in the averages of the 18- and 24-month outcome measures, adjusted for the baseline measures, and, to be conservative, ignoring the results at Months 6 and 12.
The primary analysis consisted of general linear models for repeated-measures ANCOVA (SAS PROC MIXED) to compare the main effect of the intervention on the average of the 18- and 24-month values measured during the second year of follow-up. For each outcome measure, the value at the baseline value for that outcome was used as a covariate. The primary hypothesis for this report involved comparing the main effect of the LWL intervention versus the UC control on fasting glucose. The test for intervention effect is based on the least-square means of the estimated main effect of intervention (estimate of the average of the 18- and 24-month means). Group and visit interactions were tested and none was significant. An intention-to-treat approach was used and included all postrandomized values according to the group they were assigned.13
A secondary analysis was performed making a reasonable exception to this rule by deleting observations at visit where the subject was taking hypoglycemic medication. This analysis excluded five participants (three in UUC and two in LWL) that took glucose-lowering drugs during this period, and the differences between groups did not change appreciably (the p-values for insulin and HOMA IR changed from 0.006 to 0.011 and 0.012, respectively.) Inferences for comparisons were tested at 5% two-sided level of significance. An unstructured variance model was used for intra-subject longitudinal covariance.
Results
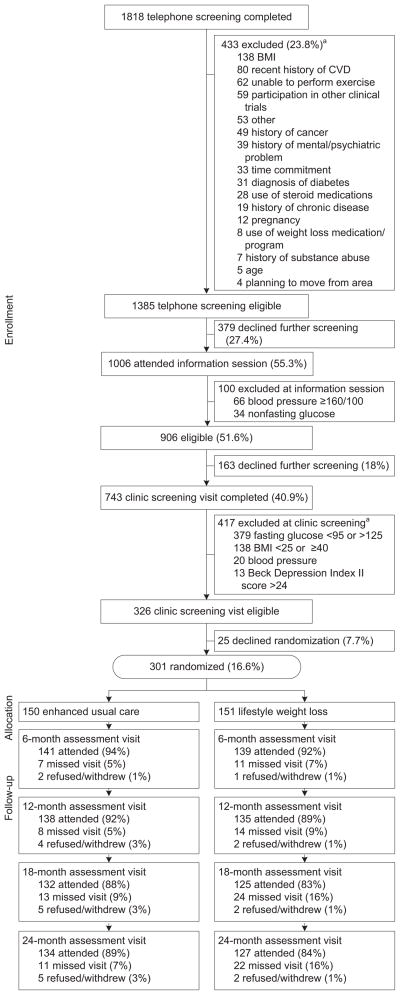
A total of 301 overweight or obese participants with elevated fasting blood glucose were randomized. Figure 1 illustrates the recruiting, screening, randomization, and retention process. The participants who were randomized into the study represented the racial composition of the population of Forsyth County, North Carolina, where the study was conducted. The group was well educated with a mean age of 57.9 (SD=9.5) years. The average BMI was in the obese range (32.7, SD=4.0), and the average fasting blood glucose was elevated (105.5 mg/dL, SD=11.3). No significant differences were detected between the treatment groups at baseline (Table 1).
Figure 1.
Participant flow
aExclusion categories are not mutually exclusive.
Table 1.
Baseline sample characteristics, n (%) or M±SD
| Variable | LWL | UC | Total | p-valuea |
|---|---|---|---|---|
| Number | 151 | 150 | 301 | |
| Gender | 1.00 | |||
| Male | 64 (42.4) | 64 (42.7) | 128 (42.5) | |
| Female | 87 (57.6) | 86 (57.3) | 173 (57.5) | |
| Race | 0.43 | |||
| African-American | 39 (25.8) | 35 (23.3) | 74 (24.6) | |
| White | 111 (73.5) | 111 (74.0) | 222 (73.8) | |
| Other/refused | 1 (0.7) | 4 (2.7) | 5 (1.6) | |
| Age (years) | 57.3±10.1 | 58.5±9.0 | 57.9±9.5 | 0.28 |
| Educational attainment | 0.97 | |||
| High school or less | 29 (19.2) | 32 (21.3) | 61 (20.3) | |
| Associate degree or other | 49 (32.5) | 47 (31.3) | 96 (31.9) | |
| Bachelor’s degree | 37 (24.5) | 37 (24.7) | 74 (24.6) | |
| Beyond bachelor’s degree | 36 (23.8) | 34 (22.7) | 70 (23.3) | |
| Weight (kg) | 94.4±14.7 | 93.0±16.2 | 93.7±15.5 | 0.44 |
| BMI | 32.8±3.9 | 32.6±4.1 | 32.7±4.0 | 0.54 |
| Glucose (mg/dL) | 105.4±12.5 | 105.7±10.0 | 105.5±11.3 | 0.79 |
| Insulin (μU/mL) | 16.7±9.7 | 16.7±10.0 | 16.7±9.8 | 0.95 |
| HOMA IR | 4.4±3.0 | 4.4±2.9 | 4.4±2.9 | 0.99 |
t-tests were used for continuous variables, and Fisher’s exact test was used for categoric variables. HOMA IR, homeostasis index of insulin resistance; LWL, lifestyle weight-loss intervention; UC, enhanced usual care intervention
Follow-up assessments were conducted at 18 months on 125 (83%) of the LWL participants and 132 (88%) of the UCC participants, and 24-month assessments were completed by 127 (84%) of the LWL participants and 134 (89%) of the UCC participants. During Phase 1 (Months 1–6), LWL participants attended 72.2% of intervention sessions; made up 15.5% (by phone); and missed 12.4%. During Phase 2, LWL participants attended 40.4% of intervention sessions, made up 22.9%, and missed 36.7%. Averaged across 24 months, LWL participants attended 58.6% of intervention sessions, made up 18.7%, and missed 22.8%.
Table 2 displays the changes in measures of glucose, insulin resistance, and adiposity across all time points for the full duration of the study and indicates significant long-term intervention effects. During the second year of follow-up and in comparison with the UCC group, the LWL participants experienced significantly greater decreases in fasting glucose (−4.35 mg/dL); insulin (−3.01 μU/ml); insulin resistance (−0.97); body weight (−4.19 kg); waist circumference (−3.23 cm); and BMI (−1.40), with all p-values <0.01. The LWL participants were more likely to experience clinically meaningful weight loss at 12 and 24 months as compared to the UCC participants (Table 3). Table 4 displays the diabetes incidence across the study arms over the course of the trial.
Table 2.
Measures of adiposity and metabolic outcomes of HELP PD over 2 years by treatment group with tests for effect during the second year, M (SE)
| Variable | Group | Baseline | 6-month | 12-month | 18-month | 24-month | Adjusted means over 18 and 24 months of follow-upa | p-value |
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | Control | 93.02 (1.32) | 91.55 (1.38) | 90.93 (1.37) | 90.93 (1.39) | 92.23 (1.43) | 92.80 (0.50) | |
| LWL | 94.38 (1.20) | 87.14 (1.22) | 87.44 (1.28) | 88.33 (1.36) | 88.81 (1.33) | 88.61 (0.51) | ||
| Diff | −4.19 (0.71) | <0.001 | ||||||
| BMI | Control | 32.56 (0.34) | 31.96 (0.34) | 31.95 (0.36) | 31.81 (0.35) | 32.16 (0.36) | 32.42 (0.18) | |
| LWL | 32.85 (0.32) | 30.38 (0.35) | 30.52 (0.36) | 30.76 (0.38) | 30.94 (0.38) | 31.02 (0.18) | ||
| Diff | −1.40 (0.25) | <0.001 | ||||||
| Waistb (cm) | Control | 104.37 (0.87) | 103.37 (0.90) | 103.45 (0.89) | — | 104.05 (0.91) | 104.30 (0.48) | |
| LWL | 104.93 (0.76) | 98.67 (0.84) | 99.22 (0.90) | — | 100.92 (0.93) | 101.06 (0.49) | ||
| Diff | −3.23 (0.69) | <0.001 | ||||||
| Glucose (mg/dL) | Control | 105.71 (0.82) | 106.97 (0.77) | 104.16 (0.99) | 107.42 (0.99) | 107.60 (1.08) | 107.44 (0.79) | |
| LWL | 105.37 (1.02) | 101.69 (0.81) | 101.11 (0.84) | 102.70 (0.98) | 103.28 (0.96) | 103.09 (0.81) | ||
| Diff | −4.35 (1.14) | <0.001 | ||||||
| Insulin (μU/mL) | Control | 16.74 (0.81) | 15.54 (1.07) | 12.81 (0.64) | 14.88 (1.62) | 13.71 (0.78) | 14.38 (0.76) | |
| LWL | 16.67 (0.79) | 10.73 (0.61) | 9.76 (0.46) | 10.15 (0.55) | 12.25 (1.02) | 11.37 (0.77) | ||
| Diff | −3.01 (1.08) | 0.006 | ||||||
| HOMA IR | Control | 4.45 (0.23) | 4.18 (0.30) | 3.33 (0.18) | 4.11 (0.51) | 3.76 (0.24) | 3.96 (0.25) | |
| LWL | 4.44 (0.24) | 2.77 (0.18) | 2.48 (0.13) | 2.63 (0.16) | 3.26 (0.31) | 2.99 (0.25) | ||
| Diff | −0.97 (0.35) | 0.006 | ||||||
| Weight loss (%) | Control | 0.00 | −1.18 (0.29) | −1.33 (0.39) | −0.93 (0.51) | −0.57 (0.55) | −0.77 (0.54) | |
| LWL | 0.00 | −7.52 (0.48) | −7.21 (0.57) | −5.82 (0.62) | −5.39 (0.66) | −5.35 (0.55) | ||
| Diff | −4.59 (0.77) | <0.001 |
Least-square means from a repeated-measures ANCOVA using the baseline value as a covariate; p-values represent the between-group comparison of the average of the 18- and 24-month means
Waist not measured at the 18-month visit
Diff, difference; HELP-PD, Healthy Living Partnerships to Prevent Diabetes; HOMA IR, homeostasis model of insulin resistance; LWL, lifestyle weight-loss intervention; UC, enhanced usual care intervention
Table 3.
Proportion of participants who met various weight-loss criteria at 12 and 24 months of follow-up
| # of participants/total # (%) | p-value | ||
|---|---|---|---|
| UC | LWL | ||
| ≤Baseline weighta | |||
| 12 months | 85/138 (61.6) | 123/135 (91.1) | <0.001 |
| 24 months | 72/133 (54.1) | 99/127 (78.0) | <0.001 |
| ≥5% below baseline weight | |||
| 12 months | 25/138 (18.1) | 79/135 (58.5) | <0.001 |
| 24 months | 20/133 (15.0) | 59/127 (46.5) | <0.001 |
| ≥10% below baseline weight | |||
| 12 months | 2/138 (1.4) | 41/135 (30.4) | <0.001 |
| 24 months | 7/133 (5.3) | 27/127 (21.3) | <0.001 |
Number of participants (percentage) whose weight at 12 and 24 months was less than or equal to their baseline weight
LWL, lifestyle weight-loss intervention; UC, enhanced usual care intervention
Table 4.
Diabetesa incidence across treatment conditions
| Month | Lifestyle weight loss | Usual care | p-valueb |
|---|---|---|---|
| 6 | 2 | 4 | 0.43 |
| 12 | 2 | 6 | 0.19 |
| 18 | 5 | 8 | 0.47 |
| 24 | 4 | 11 | 0.10 |
Fasting glucose >126 mg/dL or using diabetes medications at visit
p-values are based on a Poisson regression model using the number of events as the outcome and testing the difference between randomization groups independently at each visit.
Discussion
The purpose of this study was to examine the impact of the HELP PD intervention on fasting blood glucose, insulin, insulin resistance, and adiposity in the second year of follow-up. Results indicate that the significant reductions in body weight, BMI, waist circumference, fasting blood glucose, insulin, and insulin resistance achieved during the first year of the program largely were maintained in the second year as compared to the enhanced usual care condition. Additionally, at 24 months, the percentage of participants who lost 5% or more of their initial body weight was 46.5% in the LWL group and 15% in the usual care condition. In the first year, body weight and blood glucose were reduced by 7.5% and 4.0 mg/dL, respectively.
Thus, although the LWL effects diminished during the second year (i.e., LWL participants regained some weight back and increased blood glucose), the differences between LWL and UCC remained significant. These results indicate that a community-based lifestyle weight-loss program designed for individuals with prediabetes that is administered through a local DEP and led by CHWs can have powerful, long-term positive effects on numerous variables linked to the development of type 2 diabetes. To our knowledge, this is the first diabetes prevention translational study to document significant reductions in fasting blood glucose and adiposity over 24 months of follow-up.
The results of the present study compare favorably to the DPP,4 other community-based translations of the DPP,6,7,9 and other recently published clinic-based weight-loss studies.23–25 In the DPP, at 2.8 years of follow-up, the lifestyle intervention effects on glucose and body weight were −4 mg/dL and −5.6 kg, respectively. In the present study, the net difference in fasting blood glucose between the groups at 2 years of follow-up was 4.35 mg/dL, and the LWL participants lost 5.23 kg. Comparisons with other DPP translational studies are difficult because no other study of this kind has included more than 12 months of follow-up or reported significant reductions in glucose. However, the average 12-month weight loss achieved in other DPP translational studies was 3.99 kg across all types of settings and 3.15 kg in studies that utilize lay community members to deliver the intervention.6
Although not designed to translate the DPP per se, three large RCTs that tested 24-month behavioral weight-loss programs delivered through primary care settings have been published recently and have documented weight-loss effects of 4.6 kg,23 5.1 kg,25 and −1.2 kg.24 It should be noted that in the Ross24 study, waist circumference was the primary outcome, and they documented a −1.1-cm net effect of the weight-loss intervention at 24 months. In the present study, the net LWL effect was −3.23 cm at 24 months. Thus, the HELP PD model appears to be a powerful approach for delivering a diabetes prevention lifestyle weight-loss program.
As noted above, the net fasting blood glucose effect (i.e., the adjusted estimated difference between the groups) at 24 months in the present study was −4.35 mg/dL. There was a dramatic reduction in glucose in the LWL group over the first year of the trial13 and a slight increase in glucose over the course of the second year (Table 2). However, as one might expect in an untreated sample of patients with prediabetes, there was a gradual increase in glucose over the course of the trial in the UCC group. Thus, although glucose increased slightly over the second year in the LWL participants, the increased glucose levels in the UCC contributed to a large between-group difference. The −4.35 mg/dL glucose effect is about equal to the effects obtained in the DPP that resulted in a 58% reduction in diabetes incidence. Although the present study was not designed to detect differences in diabetes incidence, the glucose effect reported here appears to be large enough to result in significant reductions in diabetes incidence.
Limitations
The results of the present study should be interpreted in the context of several limitations. First, although the study sample was representative of the local population in terms of racial distribution, it was clearly highly educated and included very few Latinos. Second, this study included participants from one county in central North Carolina that included a mid-sized city. It is unknown whether this type of intervention can be implemented effectively in varying geographic locations involving various racial distributions and/or in rural settings.
Third, the HELP PD intervention involved more intervention sessions (24 core sessions) than the original DPP (16 core sessions) and most of the other translational studies. Although it could be argued that the HELP PD intervention was more intense and potentially more costly than other approaches, the additional eight sessions consisted of discussions led by community partners, such as a local grocery store and the YMCA, as well as open discussion sessions designed to facilitate social support and problem solving. These additional sessions may serve to enhance participants’ community engagement and, because of group-based format and involvement of CHWs, it is unlikely that costs significantly increased by offering these additional sessions.26
To our knowledge, HELP PD is the largest RCT to test a translation of the DPP. Additionally, it is the only DPP translational study to document significant reductions in glucose, insulin, insulin resistance, and adiposity to include 24 months of follow-up. Taken together with our first-year results,13 it can be concluded that the HELP PD model is a powerful approach for implementing community-based diabetes prevention programs. In light of the large number of DEPs across the country (there are more than 3000 recognized DEPs in the U.S.) and the partnership with local community members, the HELP model has great potential for large-scale dissemination and implementation and thus to increase access and reduce health disparities. Future research is needed to test the dissemination and implementation of the HELP PD model in diverse geographic locations that involve underserved, at-risk populations (e.g., rural communities).
Acknowledgments
Publication of this supplement was supported by Joslin Diabetes Center and Novo Nordisk.
The authors are grateful for the contributions of (all from the Wake Forest School of Medicine) Erica Rosenberger, MS (study design, data collection); Jorge C. Escandon, MD (study design); Wes Roberson (data collection); Kara A. Foster (recruitment, data collection); Eileen Searson (recruitment); and Terry Tembreull (data collection).
The authors are particularly grateful to the Wake Forest Baptist Medical Center Diabetes Care Center for their invaluable contributions to the study, including Joyce M. Sydell, MEd, RD, LDN; Donna Kernodle, MPH, RD, CDE; and Sonya D.H. Jeffries, MSN, NP, CDE.
This project is supported by Award #R18DK069901 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the NIH.
CFP is an investigator for a trial testing a glucose-lowering medication funded by Merck.
DCG is a consultant for a trial testing a glucose-lowering medication funded by Merck and a DSMB member for a trial testing a glucose-lowering medication funded by Takeda. No other financial disclosures were reported by the authors of this paper.
References
- 1.CDC. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta GA: DHHS; 2011. [Google Scholar]
- 2.Sloan FA, Bethel MA, Ruiz D, Jr, Shea AH, Feinglos MN. The growing burden of diabetes mellitus in the U.S elderly population. 2008;168(2):192–9. doi: 10.1001/archinternmed.2007.35. [DOI] [PubMed] [Google Scholar]
- 3.Vinicor F. Invited commentary: translating diabetes research. Arch Intern Med. 2008;168(2):199. [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 6.Ali MK, Echouffo-Tcheugui JB, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. 2012;31(1):67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 7.Whittemore R. A systematic review of the translational research on the Diabetes Prevention Program. Transl Behav Med. 2011;1(3):480–91. doi: 10.1007/s13142-011-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruggiero L, Castillo A, Quinn L, Hochwert M. Translation of the Diabetes Prevention Program’s lifestyle intervention: role of community health workers. Curr Diab Rep. 2012;12(2):127–37. doi: 10.1007/s11892-012-0254-y. [DOI] [PubMed] [Google Scholar]
- 10.Health Resources and Services Administration, DHHS. Communty Health Worker National Workforce Study. Bureau of Health Professions; 2007. [Google Scholar]
- 11.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care. 2008;31(4):684–9. doi: 10.2337/dc07-1869. [DOI] [PubMed] [Google Scholar]
- 12.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY pilot study. Am J Prev Med. 2008;35(4):357–63. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katula JA, Vitolins MZ, Rosenberger EL, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care. 2011;34(7):1451–7. doi: 10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mau MK, Keawe’aimoku Kaholokula J, West MR, et al. Translating diabetes prevention into native Hawaiian and Pacific Islander communities: the PILI ’Ohana Pilot project. Prog Community Health Partnersh. 2010;4(1):7–16. doi: 10.1353/cpr.0.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruggiero L, Oros S, Choi YK. Community-based translation of the Diabetes Prevention Program’s lifestyle intervention in an underserved Latino population. Diabetes Educ. 2011;37(4):564–72. doi: 10.1177/0145721711411107. [DOI] [PubMed] [Google Scholar]
- 16.West DS, Bursac Z, Cornell CE, et al. Lay health educators translate a weight-loss intervention in senior centers: a randomized controlled trial. Am J Prev Med. 2011;41(4):385–91. doi: 10.1016/j.amepre.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katula JA, Vitolins MZ, Rosenberger EL, et al. Healthy Living Partnerships to Prevent Diabetes (HELP PD): design and methods. Contemp Clin Trials. 2010;31(1):71–81. doi: 10.1016/j.cct.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackwell CS, Foster KA, Isom S, et al. Healthy Living Partnerships to Prevent Diabetes: recruitment and baseline characteristics. Contemp Clin Trials. 2011;32(1):40–9. doi: 10.1016/j.cct.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(S1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Katsuki A, Sumida Y, Gabazza EC, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24(2):362–5. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 22.Williamson DF, Kahn HS, Worthman CM, Burnette JC, Russell CM. Precision of recumbent anthropometry. Am J Hum Biol. 1993;5(2):159–67. doi: 10.1002/ajhb.1310050205. [DOI] [PubMed] [Google Scholar]
- 23.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–68. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross R, Lam M, Blair SN, et al. Trial of prevention and reduction of obesity through active living in clinical settings: a randomized controlled trial. Arch Intern Med. 2012;172(5):414–24. doi: 10.1001/archinternmed.2011.1972. [DOI] [PubMed] [Google Scholar]
- 25.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–79. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]