Abstract
Purpose
Patients with Common Variable Immunodeficiency (CVID) are subject to the development of a liver disease syndrome known as nodular regenerative hyperplasia (NRH). The purpose of this study was to define the characteristics and course of this complication of CVID.
Methods
CVID patients were evaluated by retrospective and prospective clinical course review. Liver biopsy specimens were evaluated for evidence of NRH and studied via RT-PCR for cytokine analysis.
Results
NRH in our CVID patient population occurred in approximately 5% of the 261 patients in our total CVID study group, initially presenting in most cases with an elevated alkaline phosphatase level. While in some patients the disease remained static, in a larger proportion a more severe disease developed characterized by portal hypertension, the latter leading to hypersplenism with neutropenia and thrombocytopenia and, in some cases, to ascites. In addition, a substantial proportion of patients either developed or presented initially with an autoimmune hepatitis-like (AIH-like) liver disease that resulted in severe liver dysfunction and, in most cases to death due to infections. The liver histologic findings in these AIH-like patients were characterized by underlying NRH pattern with superimposed interface hepatitis, lymphocytic infiltration and fibrosis. Immunologic studies of biopsies of NRH patients demonstrated the presence of infiltrating T cells producing IFN-γ, suggesting that the NRH is due to an autoimmune process.
Conclusion
Overall, these studies provide evidence that NRH may not be benign but, can be a severe and potentially fatal disease complication of CVID that merits close monitoring and intervention.
Keywords: CVID, NRH, Autoimmune, Liver, Hypertension, Cytokine
Introduction
Common variable immunodeficiency (CVID) is the most clinically significant of the genetically-related immunodeficiencies because of its relatively high prevalence rate of 1 in 25,000 individuals and its potential to result in recurrent infection [1]. The main immunological feature of CVID is impaired B cell function and hypogammaglobulinemia, but many patients have demonstrable T cell or even antigen-presenting cell abnormalities as well [2–6]. In untreated patients these abnormalities result in recurrent sinopulmonary bacterial infections, including acute pneumonias and chronic bronchiectasis leading ultimately to chronic pulmonary insufficiency. However, with the advent of immunoglobulin replacement therapy (either IV or subcutaneous), this problem has been minimized and the majority of treated patients are relatively free of pulmonary infection, although increased risk of the latter continues to persist [7].
From the time CVID was first described it was recognized that some patients exhibited autoimmune manifestations and in recent years these have become more prominent, possibly because patients are now free of chronic infection and are living longer. These take the form of immune cytopenias and gastrointestinal malabsorption syndromes [1–3,8–10]. In addition, a subset of CVID patients can develop a form of liver disease known as nodular regenerative hyperplasia (NRH), which may also have an autoimmune basis [11,12].
NRH is an abnormality observed in a variety of hepatic diseases and is thought to result, at least in part, from an intra-hepatic vasculopathy common to these diseases. The vasculopathy is characterized by alterations in microvascular perfusion that leads on the one hand to hepatocyte injury and, on the other to hepatocyte regeneration. The latter results in the formation of characteristic nodules, which compress surrounding hepatic parenchyma as well as the portal and central veins and thus have the potential to cause portal hypertension, esophageal varices and splenomegaly [13,14]. NRH is similar to cirrhosis in that both exhibit macroscopic multi-nodularity; however, NRH lacks the perinodular fibrosis and intrahepatic vascular shunts occurring in cirrhosis.
In this study we report on 14 patients with CVID and NRH who are part of a large cohort of CVID patients currently being followed at the National Institutes of Health. Clinical and pathologic studies revealed that CVID patients with NRH in this CVID study group frequently exhibit a disease course characterized by severe portal hypertension and splenic abnormalities leading to neutropenia and thrombocytopenia. In addition, in some cases the presence of NRH is accompanied by the presence of autoimmune hepatitis-like pathologic changes that are associated with severe, life-threatening illness.
Methods
Patients
The study included 14 patients with CVID who were part of an IRB-approved natural history study sponsored by the National Institute of Allergy and Infectious Diseases entitled: “Studies of Immune Regulation in Patients with Common Variable Immunodeficiency and Related Humoral Immunodeficiency Syndromes.” This study evaluated all clinical and laboratory data obtained from these 14 patients as well as all other CVID patients that were part of this protocol. Patients with CVID were enrolled regardless of severity of disease. Resultant data were recorded and reviewed during periods of continuous follow up ranging from 1 to 22 years.
RT-PCR and Real-Time PCR
Total RNA was extracted from liver biopsy tissue and cDNA was synthesized by reverse transcription using commercially available kits (Applied Biosystems) according to manufacturer’s instructions. Cytokine RT-PCR was performed with TaqMan gene expression assays as well as gene-specific primer and probe settings; data shown are respective gene expression relative to GAPDH via 2−Δct method [15]. Equal loading was confirmed by simultaneous HPRT amplification.
Extraction of RNA from Liver tissue slides
In some cases DNA was extracted from formalin-fixed, paraffin-embedded liver tissue slides by a guanidine thiocyanate/CsCl isolation method as previously described [16,17]
Immunohistochemical Studies
Available tissue was stained with a panel of four labeled antibodies enabling identification of various cell types. Four micron-thick sections of unstained tissue initially fixed in 10% buffered formalin and embedded in paraffin were de-paraffinized by passage through various grades of alcohol and then washed in water. Antigen “retrieval” was then performed by incubating the tissue in citrate buffer (pH 6.0) at boiling temperature for 20 minutes inside a microwave pressure cooker. For detection of CD20 (with Clone L26, Dako#M0755 (CD20cy), 1:1000 dilution) and CD3 (with Clone F7.2.38, Dako#M7254 (CD3e), 1:100 dilution), tissues were stained on a Dako Autostainer with 20 minutes of incubation and then developed using the Dako EnVision+ HRP detection system. For detection of CD4 (with Clone 1F6, Novocastra NCL-CD4-1F6, 1:40 dilution) and CD8 (with Clone 144B, Dako#M7103, 1:50 dilution), tissues were stained on a Ventana Benchmark XT with 20 minutes of incubation and then developed using the Ultraview DAB detection system.
Results
The general characteristics of the cohort of 14 patients with CVID and NRH followed at the NIH are shown in Table 1. The cohort was comprised of 10 males and 4 females having an average age of 42 yrs. and an average age of CVID onset of 29.5 yrs. In each case the diagnosis of CVID was based the European Society for Immunodeficiences/Pan-American group criteria [18]. Accordingly, all patients at the time of diagnosis demonstrated a marked decrease of IgG of at least 2 standard deviations below the normal mean and a similar decrease of IgM and/or IgA. The CVID patients with NRH were identified in each case by liver biopsy performed after evidence of liver disease had appeared (see below); this sub-group of CVID patients constituted 5.0% of all CVID patients seen at NIH over the time range of observation.
Table 1.
Demographics of CVID patients with NRH
| Patient | Gender | Age (Yr) | Age of CVID onset (Yrs) | Concomitant Autoimmune Disease (if present) |
|---|---|---|---|---|
| 1 | M | 39 | 30 | ITP arthropathy |
| 2 | M | 59 | 51 | Malabsorptive enteropathy |
| 3 | F | 48 | 26 | Hemolytic anemia, malabsorptive enteropathy |
| 4 | M | 36 | 23 | ITP |
| 5 | F | 35 | 17 | Vasculitis |
| 6 | M | 47 | 21 | Psoriasis |
| 7 | M | 60 | 45 | |
| 8 | M | 63 | 50 | |
| 9 | M | 45 | 20 | Scleroderma-like skin disease |
| 10 | M | 36 | 35 | |
| 11 | M | 40 | 27 | malabsorptive enteropathy |
| 12 | M | 8 | 5 | malabsorptive enteropathy |
| 13 | F | 42 | 35 | ITP |
| 14 | F | 33 | 29 | Hemolytic anemia |
The mean IgG level in the subject group did not differ from the mean level in patients with CVID without liver abnormalities (data not shown). The patients’ mean IgG trough level after stable IVIG supplementation was 899 +/− 103 mg/dl, again not different than patients with CVID without liver abnormalities. As noted in Table 1, CVID patients with liver abnormalities sometimes exhibited any of a variety of autoimmune-like manifestations, including ITP, autoimmune hemolytic anemia, gastrointestinal enteropathy, vasculitis, arthropathy, psoriasis, and scleroderma-like skin disease.
Patient Laboratory Values
Analysis of laboratory values of the CVID patients with NRH followed at (or referred to) the NIH over a period of 1 to 22 years, revealed that the first abnormal liver values to appear were elevations in alkaline phosphatase and ALT/AST levels. An increased alkaline phosphatase level, the liver parameter exhibiting the most prominent change, were first observed in this group at a mean of 7.8 +/− 2.8 years after the time of initial CVID diagnosis (range 2 to 19 yrs.) and, as shown in Table 2, consisted in most patients of a mean increase of up to 2 fold above the upper limit of normal baseline alkaline phosphatase (range 74–712 U/L vs. normal baseline 37–116 U/L). While in some patients the values plateaued at these levels, in others (patients 2,4,6,10,12) they underwent a further increase so that at the time of liver biopsy they had reached a mean level that was 3-fold above the upper limit of normal baseline levels (mean 383 +/− 119 U/L; range 72–1860 U/L) before stabilizing. The mean time to liver biopsy after diagnosis of CVID was 10.3 +/− 1.8 years.
Table 2.
Laboratory Indices of CVID NRH Patients
| Patient | Alkaline * phosphatase (37–116 U/L) | SGOT (6–41 U/L) | SGPT (9–34 U/L) | Total Bilirubin (0.1–1.0 mg/dL) | Albumin (3.7–4.7 g/dL) | Prothrombin Time (11.6– 15.2 sec) | WBC ** (4.2–9.0 k/uL | Platelet count (154–134 k/uL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 200/(166) | 28/(26) | 80/(54) | 0.7/(5.9) | 4.0/(2.2) | 16.3/(19.3) | 2.2/(0.9) | 63/(43) |
| 2 | 566/(610) | 93/(103) | 108/(113) | 1.1/(1.3) | 2.9/(2.4) | 12.5/(12.6) | 2.4/(2.9) | 144/(131) |
| 3 | 268/(248) | 58/(67) | 81/(105) | 0.6/(0.6) | 3.1/(2.7) | 15.3/(16.6) | 2.9/(0.9) | 77/(26) |
| 4 | 712/(1860) | 39/(30) | 54/(30) | 1.2/(1.1) | 3.7/(2.8) | 14.2/(14.5) | 10.9/(12) | 106/(84) |
| 5 | 119 | 84 | 71 | 0.9 | 3.8 | 12.1 | 2.5 | 61 |
| 6 | 391/(497) | 34/(34) | 75/(83) | 1.0/(0.9) | 3.9/(2.7) | 12.5/(13.1) | 3.4/(2.7) | 97/(68) |
| 7 | 209 | 72 | 72 | 0.3 | 3.0 | 13.5 | 2.5 | 85 |
| 8 | 226/(217) | 101(163) | 87/(111) | 1.0/(0.9) | 3.7/(3.6) | 12.4/(12.9) | 14.9/(10.2) | 329/(251) |
| 9 | 140 | 73 | 68 | 0.4 | 3.7 | 13.1 | 3.8 | 115 |
| 10 | 455/(644) | 114/(436) | 83/(113) | 0.3/(1.8) | 3.7/(1.8) | 12.9/(16.9) | 5.0/(3.7) | 106/(21) |
| 11 | 105/(148) | 45/(58) | 39/(43) | 0.6/(0.5) | 3.7/(3.2) | 17.2/(18.1) | 1.5/(1.0) | 58/(67) |
| 12 | 239/(295) | 114/(122) | 106/(142) | 0.9/(1.0) | 2.3/(2.2) | 16.7/(17.5) | 11.7/(8.2) | 210(187) |
| 13 | 74/(72) | 27/(23) | 20/(26) | 0.5/(0.6) | 4.1/(3.7) | 13.9/(14.2) | 3.5/(3.3) | 142/(133) |
| 14 | 151/(135) | 41/(142) | 22/(118) | 1.1/(3.1) | 3.9/(4.3) | 13.4/(16.7) | 4.5/(3.2) | 268/(102) |
Alkaline phosphatase values at the time an increase was first noted and in parenthesis values at the time of liver
WBC and platelet values at the time of liver biopsy and in parenthesis values at a later time point
As also shown in Table 2, increases in ALT/AST levels occurred over the same time period as that of the increases in alkaline phosphatase levels but were much milder, consisting of a 2–3 fold increase over baseline values. In some contrast, elevations of total bilirubin and changes in albumin levels and prothrombin time did not occur until approximately 1.5 – 2.5 years after the onset of the rise in alkaline phosphatase levels. Parenthetically, these data indicate that in NRH occurring in CVID patients, abnormalities of liver synthetic function do eventually occur and thus NRH in CVID may differ from NRH in other disease states where synthetic liver function abnormalities have not been reported [14].
As also shown in Table 2, the CVID patients with NRH frequently exhibited decreases in WBC and platelet counts that made their appearance about 1.5 years after elevations in liver-associated enzymes were observed. Thus, at the time of initial liver biopsy, the mean WBC count was 5.1 k/uL with a range of 1.5 to 14.9 and mean platelet count was 132,900 with a range of 58,000 to 329,000. It should be noted, however, that at that time liver biopsy two of the patients were receiving G-CSF in order to support their WBC counts and the patient with the highest WBC count had a prior history of CMV infection. Importantly, WBC and platelet counts in some patients continued to decline (patients 1,3,5,6,10,11 and 14) and this led to an increase incidence of serious infectious episodes that consisted of pneumonias and bacteremias. The most common bacterial isolates were Streptococcus pneumonia, Staphylococcal aureus, Hemophilis influenza and Klebsiella pneumoniae.
With the onset of liver function abnormalities, the patients with NRH were subjected to serologic studies to determine the presence of hepatitis-associated antibodies and antigens as well as autoimmune antibodies. Most importantly, HBV and HCV PCR assays revealed that the patients were uniformly negative for the presence of hepatitis B antigens in the liver or negative for hepatitis B or hepatitis C in the peripheral blood. In addition, as expected for patients with CVID and defects in Ig synthesis, no patient displayed antibodies for other liver-associated autoimmune antibodies (i.e., ANA, anti-smooth muscle, anti-liver-kidney microsomal, cryoglobulin, or Sjögren’s-syndrome associated SS-A and SS-B antibodies).
In addition to searches for the presence of viruses specifically associated with hepatitis, all patients were tested and found negative for the presence of EBV antigen as well as routine bacterial and fungal pathogens. One patient with a moderate degree of NRH had a prior history of cervical/axillary adenopathy that tested positive for CMV antigen; however, subsequent examination of his blood for several years after treatment and prior to the onset of the NRH was repeatedly negative for CMV by PCR despite the persistence of the adenopathy.
Liver Biopsy Findings
All CVID patients with NRH underwent liver biopsy to define the nature of their liver injury. In 10 out of the 14 patients the biopsy was performed by trans-jugular insertion in lieu of standard liver percutaneous needle biopsy because the latter was precluded by the presence of platelet count abnormalities. Such trans-jugular biopsies were usually accompanied by measurement of hepatic venous pressure gradient and, in some instances, pulmonary artery pressure as well.
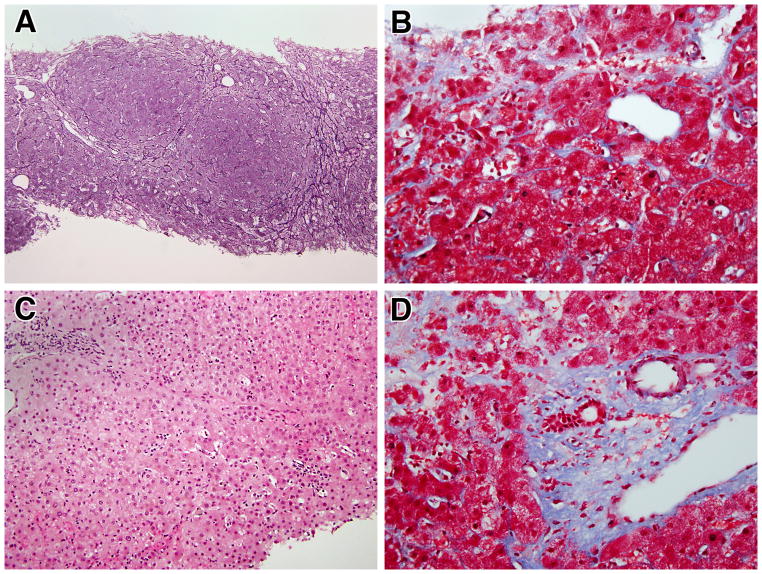
As shown in Figure 1A, all liver biopsies of the CVID patients with NRH revealed the presence of nodular regeneration (best observed in reticulin stained-tissue) that was typically evident as nodular areas of enlarged hepatocytes organized into two-cell thick plates alternating with compressed liver cell plates. This was accompanied in three patients (patients 2, 4, 7) by peri-sinusoidal fibrosis in the compressed zones (Figure 1B). Although usually rare in NRH, these three biopsies also exhibited spotty lobular inflammatory foci, which varied from rare to numerous in the different cases (Figure 1C). These inflammatory foci consisted predominantly of lymphocytes, although rare microgranulomas were also noted in two patients. In six NRH patients, mild to moderate focal portal inflammatory infiltrates were observed in addition to lobular infiltrates noted above, leading to a diagnosis of interface hepatitis (patients 3, 5, 8, 9, 11, 13). However, this was not associated with piecemeal necrosis except in one patient in which extensive portal fibrosis that led to bridging of portal and central areas was also observed (Figure 1D). A milder level of portal fibrosis than the one just mentioned was observed in three other patients in this group. All iron and copper staining for evidence of cholestasis were negative except for one patient; which revealed minimal staining.
Figure 1.
Typical histologic findings in patients with nodular regenerative hyperplasia: A) Reticulin staining demonstrating the characteristic nodularity of NRH (100x); B) Masson trichrome staining demonstrating a mild degree of perisinusoidal fibrosis (400x); C) H&E staining demonstrating sparse lobular inflammation (in most cases) (200x): D) Masson trichrome staining demonstrating mild degree of periportal fibrosis and portal inflammation. (400x).
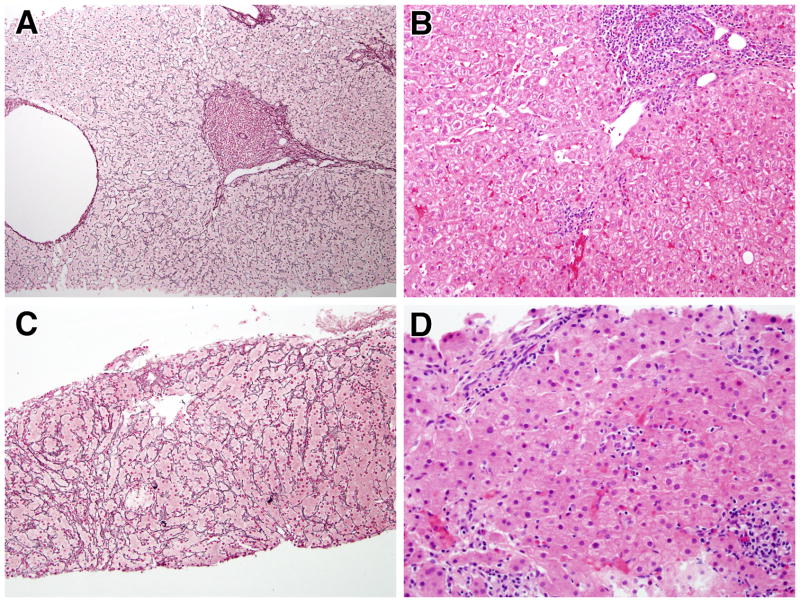
In a final group of four patients, those who were considered to have an autoimmune hepatitis (AIH)-like liver disease, the pathologic features of NRH noted above were associated with: 1) a level of portal inflammatory infiltration sufficient to justify a diagnosis of interface hepatitis and associated bridging necrosis (Figure 2A) (patients 1, 6, 12, 14); and 2) prominent bridging periportal and perisinusoidal fibrosis, seemingly an exaggeration of the milder degrees of perisinusoidal fibrosis seen in NRH rather than the thick bands of fibrosis seen in chronic viral hepatitis or that of typical AIH (Figure 2B). In addition, the liver pathology in these patients was marked by the presence of pseudoxanthomatous changes in the hepatocytes at the edges of the portal areas; this, plus positive copper staining in most of the patients (three patients), suggested the presence of a chronic cholestatic component to the hepatitis (Figure 2C) although no duct lesions were seen and the number of ducts appeared to be normal. Finally, as shown in Figures 3A–D and reviewed in Supplemental Table 1, the AIH-like liver disease in CVID was pathologically distinct from “classical” AIH not associated with CVID: in the AIH-like disease tissue the inflammatory infiltrates, interface hepatitis and piecemeal necrosis were superimposed on a nodular hepatic parenchymal pattern indicative of NRH whereas in “classical” AIH (studied at NIH or reported in the literature) there was no evidence of an underlying nodular hepatic parenchymal pattern; this difference strongly suggests that the AIH-like disease in CVID is a unique form of AIH that is related to NRH.
Figure 2.
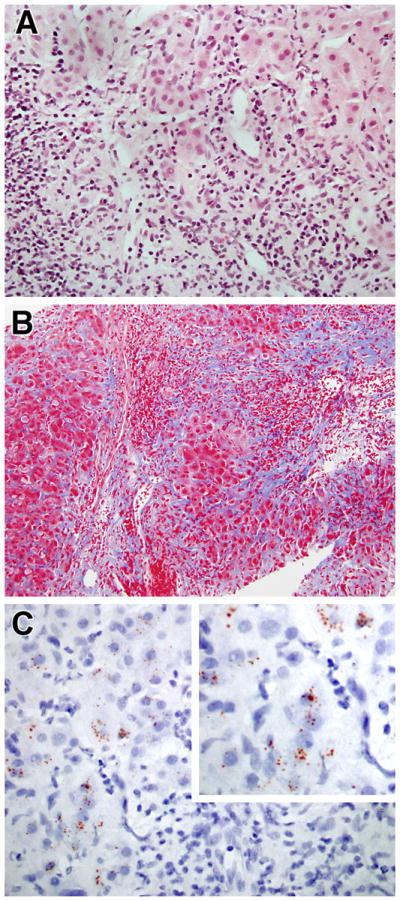
Progression of hepatic findings in CVID: A) Severe hepatitis with extensive interface hepatitis and focal bridging necrosis (H&E, 400x); B) Extensive fibrosis bridging portal areas (Masson trichrome, 200x); C) Rhodamine stain demonstrating red granules inside hepatocytes indicate of copper deposition (600x).
Figure 3.
Typical histologic findings in patients with “classical” autoimmune hepatitis (AIH) vs. NRH and AIH-like liver disease: A) Reticulin staining in classical AIH demonstrating lack of nodularity characteristic of NRH (100x); B) H&E staining in classical AIH showing severe hepatitis with interface hepatitis and focal bridging necrosis (400x); C) Reticulin staining in NRH-AIH demonstrating the characteristic nodularity of NRH (100x); D) H&E staining in NRH-AIH demonstrating severe hepatitis with extensive interface hepatitis and focal bridging necrosis (400x).
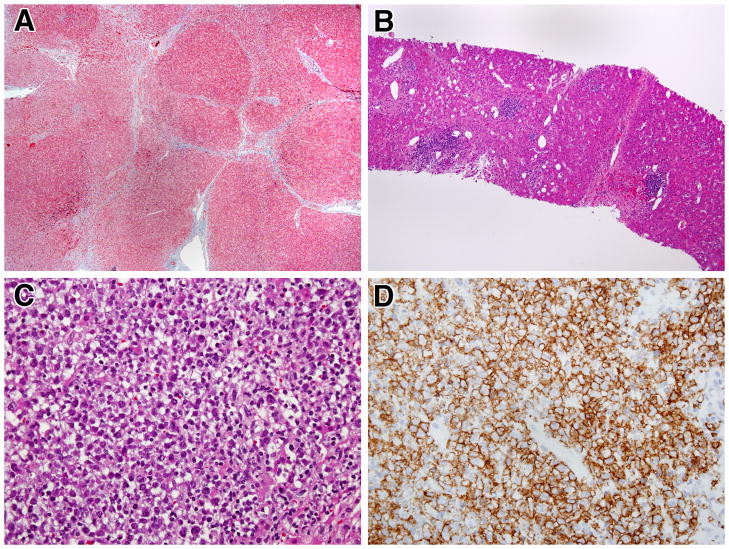
In another of the NRH patients with CVID (patient 10, not included in the patients described above) evidence was obtained via 7 serial liver biopsies over a span of 6 years that mild NRH could evolve into a severe AIH-like liver disease. The early biopsies from this patient exhibited NRH with the mild degree of inflammation and perisinusoidal fibrosis described above in other patients with NRH. In subsequent biopsies, however, the hepatic architecture was significantly distorted by extensive bridging fibrosis and focal regenerative nodular formation (Figure 4A). In addition, these later biopsies showed inflammation similar to the four cases with marked interface hepatitis on their initial biopsies. This included lymphoid aggregates (free of plasma cells) in the portal areas as well as around the central veins (Figure 4B) and, in addition, changes consistent with chronic cholestasis in the periportal and periseptal hepatocytes. In the final biopsy these findings were accompanied by a level of fibrosis and nodularity more usually seen in cirrhosis as well as the presence of a liver mass resulting from a B cell lymphoma (Figure 4C) positive for CD20, Bcl-6 and MUM-1 (Figure 4D). Hepatocellular carcinoma but not B cell lymphoma has been observed previously in the context of NRH [19] although the latter type of tumor is a known complication of CVID [2,3].
Figure 4.
Progressive liver disease and development of malignant lymphoma in a CVID patient with NRH: A) Masson trichrome staining showing incomplete cirrhosis with regenerative nodules partially and completely surrounded by fibrosis. (40x); B) H&E staining demonstrating lymphoid aggregates in portal areas and near central veins (100x); C) H&E staining showing late development of a large intra-hepatic lymphomatous mass (400x); D) Immunostain for CD20 demonstrating that the lymphomatous mass is largely composed of B cells (anti-L26, 400x)
Portal and Pulmonary Pressure Measurements, CT/MRI Scans and Endoscopy
Ten of the NRH patients underwent trans-jugular liver biopsy and in nine of these patients this was accompanied by measurement of portal pressures. As shown in supplemental Table 2, these nine patients had elevated hepatic venous pressure gradients (HVPG) consistent with portal hypertension (range 7 to 17 mmHg; upper limit of normal: 5mm Hg). In one patient (patient 1) with NRH/AIH features a pulmonary arterial systolic pressure of 62 mm/Hg and a pulmonary artery mean pressure of 43 was obtained, indicative of the presence of pulmonary hypertension.
As shown in Figure 5, results of CT/MRI scans obtained at the time of pressure measurements revealed portal vein dilatation and collateral vessel formation in seven patients, findings consistent with the above increased portal pressure values. As also shown in Figure 5, the presence of portal hypertension was associated with the presence of splenomegaly in 11 of 14 patients; in addition, hepatomegaly, a finding not normally associated with NRH, was noted in four of these 11 patients. All patients underwent upper endoscopy to determine the presence of esophageal varices and, indeed, seven patients with portal hypertension exhibited evidence of grade 1–2 varices. One patient (patient 10), underwent a splenectomy due to lymphomatous infiltrates; however, this did not alter his laboratory parameters or clinical course.
Figure 5.
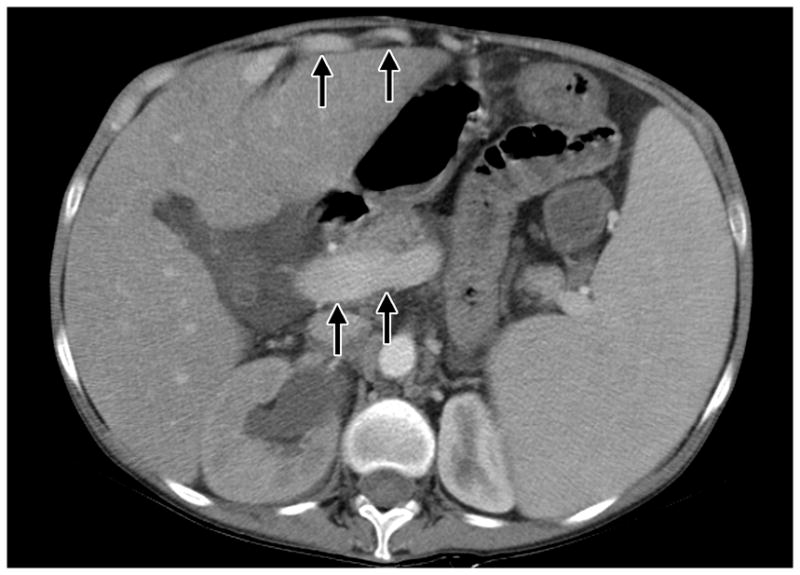
CT SCAN with contrast in a patient with CVID and NRH. Marked hepatosplenomegaly associated with evidence of dilated portal vein (indicated by arrows) and enhancing umbilical varices anterior to the liver (indicated by arrows) is present.
Clinical Course and Treatment
The patients with CVID and NRH can be divided into three clinical groups: Category I patients with non-progressive NRH; Category II patients with NRH that progressed to portal hypertension and splenomegaly; and Category III patients with an NRH/AIH-like syndrome. The clinical course of patients in category I (three of the 14 patients under study), was marked by the onset of liver function abnormalities that either remained static or increased slowly to levels not causing clinical liver disease; these patients did not require specific therapy. On the other hand, the clinical course of patients in category II (six of the 14 patients under study), was associated by slowly developing but progressive disease in which initial elevations in alkaline phosphatase levels was followed after by frank liver disease associated with the presence of disturbances in portal blood flow and hematologic changes (WBC and platelet count); these patients developed significant liver disease that ultimately required treatment. Finally, the clinical course of the patients in category III with both NRH and AIH-like disease evolved even more rapidly than those in category II in that the first appearance of liver abnormalities was followed within a short period of time (1–2 years) by liver disease characterized by significant hepatic dysfunction (changes in PT/PTT and synthetic protein formation), portal hypertension ascites and jaundice.
The most notable feature of the clinical course of NRH patients in category II was the development of significant portal hypertension associated with low WBC and platelet levels, the latter attributable to the development of splenomegaly (hypersplenism) (see Table 2 and supplemental Table 2). As previously mentioned, these patients were subject to increased infections despite IVIG therapy presumably due to the presence of neutropenia. Four of these patients were treated with G-CSF to ameliorate the neutropenia (as well as accompanying low platelet levels) which led to partial reversal of these abnormalities as well as lower susceptibility to infecton. Two other patients with portal hypertension developed severe and persistent ascites (patients 3 and 11) as well as neutropenia. In these cases surgical treatment consisting of either TIPS placement or a spleno-renal shunt procedure was necessary to control ascites as well as to obtain increased WBC and platelet counts and cessation of serious infection. Finally, a third patient (patient 5) has been identified as a possible candidate for a shunt placement because of the development of portal hypertension associated with significant varices (esophageal, umbilical, splenic hilum), and changes in white blood cell and platelet counts.
In the patients in category 3 with AIH-like disease (patients 1, 6, 10, 12 and 14) the effects of severe hepatitis on hepatic excretory and synthetic function was superimposed on the effects of portal hypertension and hypersplenism and the severe hepatitis was the main focus of therapy. Consequently, two of these patients (patients 1 and 6) were administered a modified standard immunosuppressive regimen (prednisone and immuran) for AIH despite the increased risk for infection of such therapy in a patient with CVID. In addition, patient one was administered sildenafil for treatment of pulmonary hypertension. This led to a decrease in portal pressure in both patients (and in patient one, to decreased pulmonary pressure as well); however, it had only a mild effect on the cytopenias and these patients died of progressive liver disease and associated systemic infection. Similarly, one patient (patient 14) died of a systemic bacteremia prior to the start of any therapy. The lack of success obtained in treating patients with AIH-like disease with immunosuppressives suggests that these patients may have arrived at a stage of liver disease that was refractory to treatment by the time such treatment was begun. This is exemplified by one of the five patients in this disease category, a young patient (patient 12) who has had liver disease that has been successfully controlled with steroids and an anti-metabolite (6-mercaptopurine) treatment. Whether earlier identification and treatment offers any hope of controlling this severe form of NRH/AIH and correcting this fatal outcome remains to be seen.
Immunologic studies of NRH
To obtain insight into the cause of NRH patient liver tissue was subjected to immune cell-specific immunohistochemical and cytokine-specific RT-PCR studies.
Histochemical tissue staining and RT-PCR analysis of cytokine production
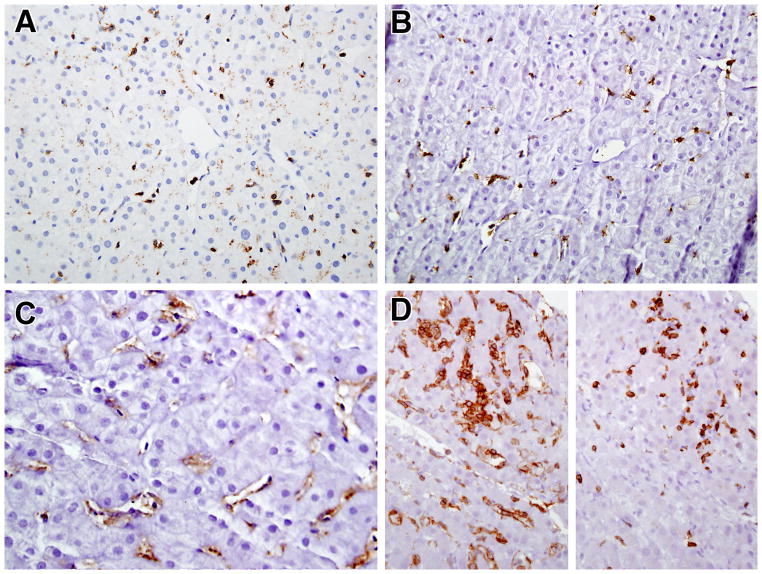
Basic immunohistochemical phenotyping was performed on tissue sections using labeled antibodies against CD20, CD3, CD4 and CD8. In all patients very few B cells were identified, even in the patients with severe hepatitis, despite the presence of a normal number of peripheral blood CD19 and CD20 B cells (data not shown), and the few cells that were identified were mainly found in the portal areas. Similar findings concerning T cell lymphocyte infiltration were seen in most of the mild to moderate CVID NRH biopsies. In these cases, the vast majority of the cells in the infiltrate were CD3+ T cells that co-expressed CD8 and were found in both the parenchyma and portal areas (Figures 6A and 6B). Lightly-stained CD4+ cells indicative of activated macrophages (Kupffer cells) were also present, predominately in the parenchyma areas (Figure 6C). An exception to the above findings occurred in the one patient noted to have a B cell lymphoma who exhibited a predominance of CD4+ T cell infiltrate admixed with a smaller number of CD8+ cells and numerous CD4+ macrophages (see Figure 6D).
Figure 6.
Immunophenotype of lymphocyte infiltrates in CVID and NRH (All 400x). A and B) Most of the lymphocytes in the liver parenchyma are CD3+ (A) and CD8+ (B); C) Anti-CD4 staining of liver tissue with mild/moderate NRH with anti-CD4 reveals lightly stained Kupffer cells; D) Increased numbers of CD4+ lymphocytes (left) as compared to CD8+ lymphocytes (right) in the liver of the patient with progressive liver disease and lymphoma.
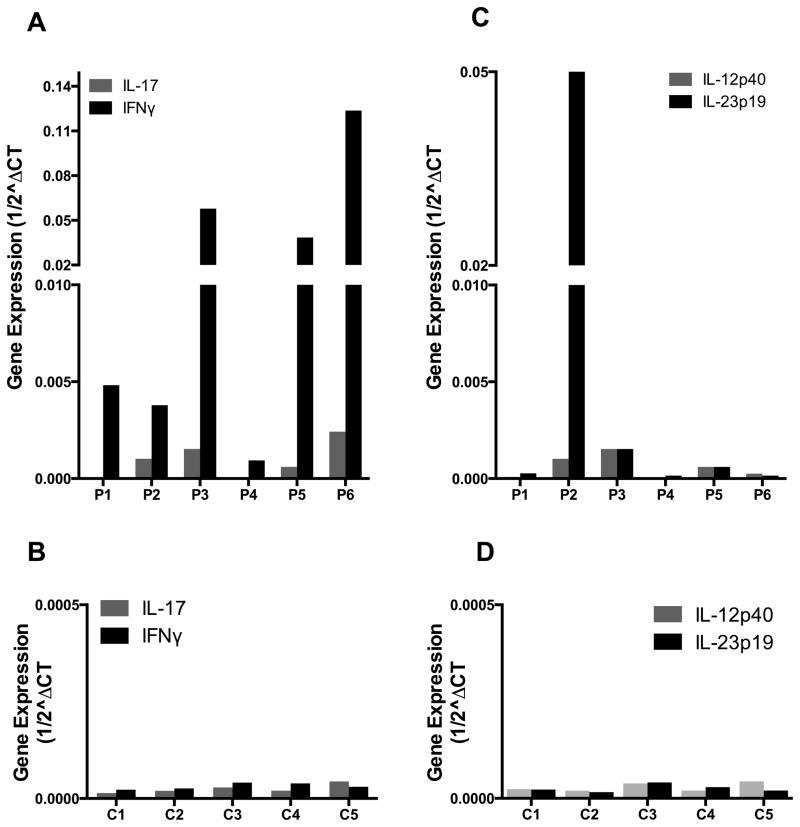
RT-PCR analysis for quantitation of cytokine production in liver tissue was conducted in liver biopsy samples obtained from 6 of the patients undergoing trans-jugular liver biopsies as well as 5 normal control individuals who had consented to performance of tissue biopsies. As shown in Figure 7A and 7B, five of the six patients with NRH exhibited greatly increased production of IFN-γ mRNA compared to controls which in some cases were increased 100-fold. As might be expected, these increases were most prominent in the patients with the most severe NRH (patient 1,3,10, 13, 14). In contrast, only minimal increases in IL-17 mRNA were observed in the CVID patients as compared to the controls. As shown in Figure 7C and 7D, in most patients these cytokine abnormalities were not associated with significant increases in IL-12p40 or IL-23p19 mRNA production. The exception, to this rule was again the one patient who developed severe NRH and B cell lymphoma who did manifest a marked increase in IL-23 p19 (as well as IFN-γ) in liver tissue obtained at the time of initial NRH diagnosis (when his liver disease was still relatively mild).
Figure 7.
CVID patients with NRH exhibit increased production of IFN-γ mRNA compared to control individuals. (A,B) Real-time RT-PCR analysis for IFN-γ and IL-17 of mRNA extracted from liver biopsy specimens obtained from 6 CVID patients with NRH and 5 normal control individuals respectively. (C,D) Real-time RT-PCR analysis for IL-23p19 and IL-12p40 of mRNA from liver biopsy specimens obtained from 6 CVID patients with NRH and 5 normal control individuals respectively.
Discussion
In prior studies liver pathology has been observed with hypogammaglobulinemia although predominately associated with granulomatous infiltrative disease or viral etiology [2, 20–22]. In some contrast to these studies in which few if any cases of NRH were found in patients with CVID, in a recent study of CVID and other B cell immunodeficiencies conducted by Malamut et. al. [11], 51 patients with chronic liver abnormalities were identified and 50% of the patients studied had documented portal hypertension, a manifestation of NRH; this, plus liver biopsy studies led the authors to conclude that NRH lesion was the most common liver abnormality in CVID [11]. However, while this study established that NRH is more common in CVID than previously thought, it did not provide a clear picture of incidence since the size of the total pool of immunodeficiency patients from which the patients with liver disease were drawn was not defined. Perhaps a clearer picture of the incidence of NRH in CVID comes from a recent study by Ward et. al. [12] as well as the present study wherein the incidence of NRH was found to be 12% of 108 CVID patients and 5% of 261 patients respectively. However, these percentages could be higher than that seen at other centers because they are both derived from studies of patient cohorts seen in referral centers that may treat more severely ill patients.
The major finding in the present study is that while a minority of the CVID patients with NRH in this study group (the three patients gathered in Category I above) had a static disease that did not result in clinically significant liver abnormalities, the majority of the patients (the eleven patients gathered in Categories II and III) develop a liver disease that posed a major health risk to the patient such as severe portal hypertension and its consequences or the latter associated with an autoimmune hepatitis-like disease. This more serious manifestation of NRH in CVID is perhaps predicted by the prior study by Malamut et al. [11] wherein, as noted above, portal hypertension was documented in 50% of patients; however, in this prior study the course of the NRH patients and therefore the clinical significance of this CVID complication was not described. A contrasting pattern of NRH, however, was obtained in another study of NRH in patients with CVID was reported by Ward et al. [12]. In this case, while portal hypertension identified by clinical criteria was observed in some patients the course of NRH was characterized as benign. The reason the findings in this study differ so dramatically from those of the present study is unclear. However, it is possible that in the Ward et al. study, patients were not followed for a sufficient length of time to identify more severe NRH complications.
As alluded to above, one cause of morbidity in patients with CVID with NRH could be traced to the development of a liver disease marked by the presence of severe portal hypertension that resulted in splenomegaly and, in a sub-group of patients, in severe ascites as well. In these patients (designated here as Category II patients) the splenomegaly was associated with the development of hypersplenism and the occurrence of neutropenia and thrombocytopenia, the latter directly attributable to the portal vascular obstruction by the fact that distal portal shunting in at least two of the patients led to improved neutrophil and platelet levels. These cytopenias were a particularly serious complication of NRH in CVID patients because it further undermined the CVID patient’s ability to deal with infection. It was treated by administration of G-CSF in some cases or, in several of the patients manifesting severe ascites, with measures to relieve the underlying portal hypertension, a TIPS placement or a spleno-renal shunt procedure. It was not felt to be amenable to treatment by simple splenectomy because it is likely that the spleen in NRH is acting as a vascular sink that relieves pressure in the portal system.
Another cause of morbidity in CVID patients with NRH (occurring in patients designated here as Category III patients) was due to a liver disease in which the portal hypertension noted in Category II patients was present, but was in this case the latter was over-shadowed by an inflammatory liver disease best described as an autoimmune hepatitis (AIH)-like disease. It seems likely that this liver abnormality represents a severe stage of NRH since examination of liver biopsy tissue that had AIH-like inflammatory features were superimposed on an underlying nodular hepatic parenchymal pattern indicative of NRH. It thus differed from “classical” AIH where no underlying nodular hepatic parenchymal was present. Additional evidence that NRH can evolve into a dominating AIH-like pattern over time was obtained in one of our five Category III patients in whom serial biopsies revealed gradual development of an AIH picture in a liver originally exhibiting uncomplicated NRH; the other patients came to our attention with the full-blown AIH picture already present but did have documented antecedent history of mild liver disease changes suggestive of NRH. Thus, while NRH in CVID may gradually evolve into an AIH-like disease, the latter is more likely to present de novo as a severe liver disease.
Category III NRH patients with AIH-like liver disease may require early and aggressive treatment, the latter frequently involving agents (i.e., steroids and imuran (or other anti-metabolites)) that put these already immunodeficient patients at further risk for severe infection. Indeed, the two patients with AIH-like disease studied here were administered such treatment both died of progressive liver compromise and an associated infectious episode while a third patient died of a septic episode prior to any treatment; only a pediatric patient treated early in the course of his liver disease is currently alive and stable.
Previous studies of NRH in a variety of conditions not involving CVID suggest that the underlying pathology can result from either a vasculopathy that secondarily affects hepatic architecture or a hepatocyte abnormality that secondarily results in vascular ischemia [23]. Relevant data that supports this comes from a previous study of NRH in a group of patients with a variety of autoimmune, neoplastic disease and idiopathic NRH (not including patients with CVID). In this study, it was found that in about a third of patients with NRH, liver sinusoids adjacent to areas of atrophic hepatocytes contained CD8+ T cells [24]. In addition, there was evidence that these T cells were cytotoxic T cells in that the cells were granzyme B+ and were found in area of apoptotic endothelial cells lining the sinusoids. Finally, analysis of liver T cell TCR clonality revealed liver-specific clonality or oligoclonality suggesting that the sinusoidal T cells were composed of cells specifically targeting sinusoidal endothelial cells. That a similar process is at play in NRH associated with CVID comes from a study of CVID and NRH already cited by Malamut et al. in which it was shown that the livers of the great majority of CVID patients with NRH did in fact exhibit a predominantly CD8+ T cell infiltrate in the sinusoids coinciding with areas of sinusoid dilatation [11]. In addition, portal vein endotheliitis was noted in about 50% of patients [11]. The present study offers further data relating to immunologic factors in the NRH in CVID in that we found that most of the patients studied exhibited a parenchyma CD8+ T cell infiltrate. This cellular infiltrate was the likely source of an accompanying increase in liver IFN-γ production, the magnitude of which correlating roughly with disease severity. Taken together, these studies suggest that NRH occurring in CVID is initiated by a destructive autoimmune process usually mediated by CD8+ cells that leads first to hepatocyte loss and then to a regenerative process that results in vascular abnormalities and portal hypertension.
Conclusion
NRH occurring in CVID is a not infrequent complication of this immunodeficiency, which in all likelihood has an autoimmune basis. The striking new finding to arise from this study of patients with NRH is that the latter can be a severe liver disease leading either to portal hypertension and its various sequelae or to portal hypertension complicated by an inflammation with autoimmune hepatitis-like liver pathology. Thus, NRH is a potential cause of severe liver function abnormalities and disease increased risk of infection.
Supplementary Material
Acknowledgments
Sources of funding: Funding is from the intramural program of the NIAID, NCI, National Institutes of Health. None of the authors has any financial relationships with industries that have an interest in the subject matter or materials discussed in the manuscript
References
- 1.Oksenhendler E, Gérard L, Fieschi C, Malphettes M, Moillot G, Jaussaud R, Viallard JF, Gardmbas M, Gallicier L, Schleinitz N, Suarez F, Soulas-Sprauel P, Hachulla E, Jaccard A, Gardeur A, Théodorou I, Rabian C, Debre P DEFI Study Group. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008;46:1547–54. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S, Cunningham-Rundles C. Autoimmunity in common variable immunodeficiency. Curr Allergy Asthma Rep. 2009;9:347–52. doi: 10.1007/s11882-009-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119:1650–57. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham-Rundles C, Radigan L, Knight AK, Bauer L, Nakazawa A. TLR9 activation is defective in common variable immune deficiency. J Immunol. 2006;176:1978–87. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]
- 5.Salzer U, Grimbacher B. Common variable immunodeficiency: the power of co-stimulation. Seminars in Immunology. 2006;18:337–46. doi: 10.1016/j.smim.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Giovanetti A, Pierdominici M, Mazzetta F, Marziali M, Renzi C, Mileo AM, De Felice M, Mora B, Esposito A, Carello R, Pizzutti A, Paggi M, Paganelli R, Malorni W, Aiuti F. Unravelling the complexity of T cell abnormalities in common variable immunodefiency. J Immunol. 2007;178:3932–43. doi: 10.4049/jimmunol.178.6.3932. [DOI] [PubMed] [Google Scholar]
- 7.Busse PJ, Farzan S, Cunningham-Rundles C. Pulmonary complications of common variable immunodeficiency. Ann Allergy Asthma Immunol. 2007;98:8–11. doi: 10.1016/S1081-1206(10)60853-8. [DOI] [PubMed] [Google Scholar]
- 8.Knight A, Cunningham Rundles C. Inflammatory and autoimmune complications of common variable immune deficiency. Autoimmunity Reviews. 2006;5:156–9. doi: 10.1016/j.autrev.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Mannon PJ, Fuss IJ, Friend J, Hornung R, Yang Z, Yi C, Quezado M, Brown M, Strober W. Excess IL-12 but not IL-23 accompanies the inflammatory bowel disease associated with common variable immunodeficiency. Gastroenterology. 2006;13:748–56. doi: 10.1053/j.gastro.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Malamut G, Verkarre V, Suarez F, Villard JF, Lascaux AS, Cosnes J, Bouhnik Y, Lambotte O, Bechade D, Ziol M, Lavergne A, Hermine O, Cerf-Bensussan N, Cellier C. The enteropathy associated with common variable immunodeficiency: The delineated frontiers with Celiac disease. Amer J Gastroenteropathy. 2010;105:2262–75. doi: 10.1038/ajg.2010.214. [DOI] [PubMed] [Google Scholar]
- 11.Malamut G, Ziol M, Suarez F, Beaugrand M, Viallard JF, Lascaus AS, Verkarre V, Bechade D, Poynard T, Hermine O, Cellier C. Nodular regenerative hyperplasia: the main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. Journal of Hepatology. 2008;48:74–82. doi: 10.1016/j.jhep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Ward C, Lucas M, Piris J, Collier J, Chapel H. Abnormal liver function in common variable immunodeficiency disorders due to nodular regenerative hyperplasia. Clin Exp Immunol. 2008;153:331–7. doi: 10.1111/j.1365-2249.2008.03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanless IR. Micronuodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787–97. doi: 10.1002/hep.1840110512. [DOI] [PubMed] [Google Scholar]
- 14.Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology. 2006;44:7–14. doi: 10.1002/hep.21258. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H) 17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 16.Lopatin U, Yao X, Williams RK, Blessing JJ, Dale JK, Wong D, Teruya-Feldstein J, Fritz S, Morrow MR, Fuss I, Sneller MC, Raffeld M, Fleisher TA, Puck JM, Strober W, Jaffe ES, Straus SE. Increases in circulating and lymphoid tissue interleukin-10 in autoimmune lymphoproliferative syndrome are associated with disease expression. Blood. 2001;97:3161–70. doi: 10.1182/blood.v97.10.3161. [DOI] [PubMed] [Google Scholar]
- 17.Quintanilla-Martinez L, Kumar S, Fend F, Reyes E, Teruya-Feldstein J, Kingma DW, Sorbara L, Raffeld M, Straus SE, Jaffe ES. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443–51. [PubMed] [Google Scholar]
- 18.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American group for immunodeficiency) and ESID (European society for immunodeficiencies) Clin Immunol. 1999;93:190–97. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 19.Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma and nodular regenerative hyperplasia: possible pathogenic relationship. Am J Gastroenterol. 1996;91:879–84. [PubMed] [Google Scholar]
- 20.Ravindran J, Gillis D, Rowland R, Heddle R. Common variable immunodeficiency associated with nodular regenerative hyperplasia of the liver. Aust NZ J Med. 1995;25:741. doi: 10.1111/j.1445-5994.1995.tb02867.x. [DOI] [PubMed] [Google Scholar]
- 21.Ardeniz O, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clin Immunol. 2009;133:198–207. doi: 10.1016/j.clim.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razvi S, Schneider L, Jonas MM, Cunningham-Rundles C. Outcome of intravenous immunoglobulin-transmitted hepatitis C virus infection in primary immunodeficiency. Clin Immunol. 2001;101:284–88. doi: 10.1006/clim.2001.5132. [DOI] [PubMed] [Google Scholar]
- 23.Shimamatsu K, Wanless IR. Role of ischemia in causing apoptosis, atrophy, and nodular hyperplasia in human liver. Hepatology. 1997;26:343–50. doi: 10.1002/hep.510260214. [DOI] [PubMed] [Google Scholar]
- 24.Ziol M, Poirel H, Koutchou GN, Boyer O, Mohand D, Mouthon L, Tepper M, Guillet JG, Guettier C, Raphael M, Geaugrand M. Intrasinusoidal cytotoxic CD8+ T cells in nodular regenerative hyperplasia of the liver. Hum Pathol. 2004;35:1241–51. doi: 10.1016/j.humpath.2004.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.