Abstract
Objective
To provide an analysis of multiple predictors of cognitive and behavioral traits for children with fetal alcohol spectrum disorders (FASD).
Method
Multivariate correlation techniques were employed with maternal and child data from epidemiologic studies in a community in South Africa. Data on 561 first grade children with fetal alcohol syndrome (FAS), partial FAS (PFAS), and not FASD and their mothers were analyzed by grouping 19 maternal variables into categories (physical, demographic, childbearing, and drinking) and employed in structural equation models (SEM) to assess correlates of child intelligence (verbal and non-verbal) and behavior.
Results
A first SEM utilizing only seven maternal alcohol use variables to predict cognitive/behavioral traits was statistically significant (B = 3.10, p < .05), but explained only 17.3% of the variance. The second model incorporated multiple maternal variables and was statistically significant explaining 55.3% of the variance. Significantly correlated with low intelligence and problem behavior were demographic (B = 3.83, p < .05) (low maternal education, low socioeconomic status (SES), and rural residence) and maternal physical characteristics (B = 2.70, p < .05) (short stature, small head circumference, and low weight). Childbearing history and alcohol use composites were not statistically significant in the final complex model, and were overpowered by SES and maternal physical traits.
Conclusions
While other analytic techniques have amply demonstrated the negative effects of maternal drinking on intelligence and behavior, this highly-controlled analysis of multiple maternal influences reveals that maternal demographics and physical traits make a significant enabling or disabling contribution to child functioning in FASD.
Keywords: fetal alcohol spectrum disorders (FASD), fetal alcohol syndrome (FAS), partial fetal alcohol syndrome (PFAS), verbal intelligence, non-verbal intelligence, problem behaviors in children, maternal risk factors
Introduction
Maternal Risk Factors for FASD
Alcohol is a teratogen, and the quantity, frequency, and timing (QFT) of prenatal alcohol use by a mother produces fetal alcohol spectrum disorders (FASD).1-3 The pattern of drinking that is most harmful to the fetus is heavy episodic (binge) drinking.4-9 So it is important that studies of human maternal risk factors for FASD include multiple measures of drinking that define the specific pattern of consumption. But obtaining accurate drinking information from maternal reports has proven to be a substantial challenge.10-24 In essence, the more often a woman drinks in the prenatal period, the greater amounts she drinks (especially over short periods of time), and if drinking continues throughout gestation, the more likely a child is to have an FASD, particularly a severe form such as fetal alcohol syndrome (FAS) and partial fetal alcohol syndrome (PFAS).2,25-29
Maternal risk is not as simple as the alcohol alone. Multiple maternal co-factors of risk for FASD have been identified.3,17,20,30,31 Maternal risk has been found to vary in individual women based on childbearing variables such as: maternal age, gravidity, and parity.8,9,32-35 Older women who frequently drink larger quantities of alcohol in an episodic fashion, who have been pregnant more (gravidity), and have had more children (parity), are more likely to bear a child with an FASD. FASD occurrence also varies by demographic variables such as socioeconomic status (SES).3,8,9,36-39 Although cases of FASD have been documented to occur in all strata, children with FASD are most likely to be born to women of the lower social strata.7-9,31,38,40 Finally, recent population-based studies have pointed to maternal physical variables as important risk or protective factors for FASD: mother's height, weight, and a summary measure, body mass index (BMI), are influential in determining outcome in the child. Lighter, shorter women, with a low BMI are overrepresented among mothers who have children with FASD. Larger women appear to be less likely to give birth to a child with an FASD,8,9,41,42 as alcohol metabolism is affected by body size and maternal nutrition likely plays a role in qualitative outcome.2,41,43-48
Multiple Correlation Studies of Maternal Risk for FASD
Maternal risk factor studies have seldom benefitted from large samples of accurately-diagnosed children with FASD or from population-based maternal risk data matched to the child data. Therefore, few multiple correlation studies have been carried out on maternal risk for FASD. A study of 25 mothers of children with FAS and 50 controls43 reported that mothers of children with FAS were characterized as: higher in maternal age, predominantly Black, higher gravidity and parity, having higher scores on the Michigan Alcohol Screening Test (MAST), more drinking days during pregnancy, greater amounts of alcohol consumed on drinking days, a binge drinking pattern, and as drinking mostly beer. Using discriminant function analysis, four variables accounted for the majority of all variance in fetal damage: more drinking days, a high MAST score, high parity, and Black race. Then regression analysis indicated that in the presence of these four factors, only one of which concerned alcohol, there was an 85% chance that a child would have FAS, and in their absence a 2% chance of having FAS.43
Recently a model assessing the multiple influences on the dysmorphology of children with FAS and PFAS used sequential regression to conclude that maternal drinking measures (e.g. quantity and frequency of drinking) were powerful predictors of a child's physical anomalies (R2 = .30). Drinking was followed in significance by maternal measures of SES (R2 = .24), maternal physical characteristics (height, weight, and head circumference) (R2 = .15), and childbearing variables (gravidity, parity, and age at delivery) (R2 = .06).49 A structural equation model (SEM) of these four categories predicted child dysmorphology, explaining 62% of total variance. In this model drinking behavior was by far the strongest predictor of dysmorphology (B = 2.26, p < .05), even when all other maternal risk variables were included. Maternal SES variables were the only other statistically significant category (B = 0.87, p = .05), but maternal co-factors exclusive of drinking all contributed to associations with FASD dysmorphology (physical outcome).49
Studies Specific to the Intelligence and Behavioral Traits of Children with FASD
While the above findings focused on a child's physical traits, cognitive and behavioral functioning is also affected by prenatal alcohol exposure. Children exposed to high levels of alcohol prenatally have diminished verbal IQ skills, frequently characterized as difficulty taking in new verbal information and holding the information in memory for later use.50-56 For example, heavily-exposed children perform more poorly than normal controls on both letter and category fluency tasks.57-59 In addition, instruments such as the Test of Reception of Grammar (TROG),60 which measure a participant's ability to match a picture with a spoken word or sentence, can be used to differentiate between children with FAS and controls in a variety of cultures.58,61 Verbal ability is a particularly sensitive discriminator between children with FAS and controls.62
Non-verbal, or fluid, intelligence refers to the agility of the brain to analyze a problem and construct a solution. Alcohol-exposed children generally have less mental agility and complex problem-solving ability,58,61 and therefore do not score as high as controls on tests of non-verbal intelligence.63,51,58,64 Children whose mothers consumed alcohol during pregnancy have more difficulty with fluid intelligence tests involving mental agility and executive functioning (EF) than with crystallized intelligence tests, which examine accumulated knowledge and skills.52,65 The effects of alcohol exposure on fluid intelligence of children also appear to be cross-cultural, as shown in a study of Italian children with FASD utilizing the Raven Colored Progressive Matrices (CPM).58,61
Prenatal alcohol exposure also affects a child's behavior.66,67 Alcohol-exposed persons often display more problem behaviors than controls, particularly in social situations where difficulty in understanding social consequences of actions may result in inappropriate interactions with others.68 The Personal Behavior Checklist-36 (PBCL-36)69 and the Children's Executive Functioning Inventory (CEFI) have been used to assess behavior problems associated with prenatal alcohol exposure. Also, children with FASD frequently lack the ability to regulate their emotional responses, and, for example, may speak or act before considering the social acceptability of their actions.66,70
While maternal alcohol consumption adversely impacts cognitive functioning and behavior of a child with FASD,63,66,67,69,71-75 additional maternal factors may amplify or suppress the prenatal effects of alcohol on behavior and functioning. For example, low parental education and higher numbers of children at home are associated with lowered child IQ,76 and children born to older mothers who consumed alcohol during pregnancy display lower, full scale IQ scores.77 Because previous research indicates that patterns of prenatal alcohol use alone do not fully explain the neurobehavioral traits of exposed children79,80 this study examines how multiple maternal variables interact to influence intellectual functioning and behavior in children with FAS and PFAS.
Methods
The Model And Tests Used as Dependent Variables
Causal modeling techniques are used to better understand influences on intelligence and behavior among children with FASD. Using a structural equation model (SEM) approach, we attempt to determine which maternal variables are most influential on a child's cognitive functioning and behavior at age 7 years. Three tests were used as the dependent variable in the SEM: the Raven Colored Progressive Matrices, the Test for the Reception of Grammar (TROG), and the Personal Behavior Checklist (PBCL-36).
The Raven measures non-verbal or fluid intelligence, and has been shown to be more sensitive to the assessment of EF than tests of crystallized intelligence.52 The Raven requires the participant to solve a series of problems by selecting a specific piece that most appropriately completes the missing portion of a given pattern. Patterns are shown to the child in sequence, with each pattern being more difficult than the one previous.78
A test of verbal intelligence, the TROG, is also used. It measures ability to understand grammatical constructs by asking58 the participant to select from a series of four pictures, the one that most closely matches the word or sentence spoken by the examiner.60 The TROG was selected to derive child's receptive language abilities and easure understanding of grammatical constructs in a multiple choice format. This test is culturally appropriate and sensitive for the South African student population, because it requires minimal verbal effort for the child, and literacy is an issue in the South African context of this study. Pairing this receptive language test with the Raven, pproximate measures of verbal and nonverbal cognition were derived. All testing was completed by three Afrikaans-speaking psychometrists who, to ensure fidelity, were trained by one of the authors (CMA) of this study. The PBCL-3669 was used to measure behavior. A questionnaire of 36 items completed by the child's teacher, it reliably measures behavioral characteristics associated with FASD: academic performance, social skills, bodily functions, communication, mannerisms, emotions, and motor skills. A higher score indicates a greater number of problem behaviors. The children's teachers were blinded and had no indication provided of whether the child was diagnosed with an FASD or not.
Sample: Children with FASD and Normal Comparison Children
This study utilizes data on the characteristics of children with FAS, PFAS, and comparison subjects without an FASD attending the same schools in a town in the Western Cape Province of South Africa (SA). The data originate from three waves of population-based, in-school, FASD research in SA. All protocols and procedures were approved by the University of New Mexico Human Subjects Research Review Committee, the Research Ethics Committee of the University of Cape Town, and a single site assurance committee at the local municipality.
All first grade children attending public schools with active consent to participate (slightly less than 3,000 or 91% of enrolled students), were screened first for height, weight, and head circumference. All of the smaller children (≤ 10th centile on height and weight and/or head circumference) were advanced further in the diagnostic process along with randomly-selected controls from the same classrooms. Dysmorphologists (blinded from the child's history) examined all children for signs of FASD or other anomalies. Because all children in the sample reported here were advanced through the study together and received the same screening, diagnostic services, and testing, all necessary data were collected to determine a proper diagnosis: FAS or PFAS.
Measurements and observations of physical characteristics were recorded on a quantified dysmorphology checklist, where a high score indicates more features of FASD.28, 51,81 Following the dysmorphology exam, children suspected of having FASD and randomly-selected controls were administered the dependent variable tests by psychometrists blinded to the reason for entry into the study,57,61 and each participant's classroom teacher completed the PBCL-36. Biological mothers of all children (potential FASD cases and controls) were interviewed about maternal risks by Afrikaans-speaking, blinded interviewers. None of the mothers of children with FASD who were alive and contacted, refused to be interviewed, and few of the mothers of normal controls refused. Studies in this SA population have evidenced mothers to be quite forthright with sensitive information about health and FASD risk factors including details of alcohol use before, during, and after pregnancy.7-9 When the mothers were dead or could not be located (< 6%), information was collected from knowledgeable collateral sources.
The Maternal Questionnaire
All mothers were administered identical questionnaires developed specifically for this population7,8 to reconstruct maternal traits before, during, and after gestation of the index child. Recent research indicates that retrospective assessment of prenatal alcohol consumption is more accurate than data collected during gestation in prenatal clinics.20,24,82 Alcohol questions are embedded in a context of general health status and dietary recall to ease sensitivities for accurate reporting.83 The context, quantity, and frequency of the mother's current drinking were explored via a 1-week, day-by-day log which became benchmarks for timeline follow-back questions84 regarding gestational drinking. Drinks were measured in standard ethanol units that equal 0.5 ounces of absolute alcohol: 340 ml can/bottle of beer (5% ethanol), 120 ml of wine (11% ethanol), 95 ml of wine (13.5% ethanol), or 44 ml of distilled spirits (43% ethanol). Respondents viewed pictures of standard containers of local brands (of beer, wine, etc.) to calibrate the exact amounts consumed.85,86
Mothers were also measured for their height, weight, and head circumference at each interview. The Body Mass Index (BMI) was calculated via the standard metric formula:87 weight in kilograms ÷ (height in meters)2.
Final Diagnosis Made by Case Conference
Finally, a formal case conference was held for each child where clinicians from each of the study domains reviewed findings on all tests/exams for all children. Final diagnoses were made using revised Institute of Medicine (IOM) criteria.27,28 Children with FAS have a distinct pattern of facial anomalies, evidence of prenatal and/or postnatal growth retardation, evidence of deficient brain growth and/or cognitive and behavioral disorders, and evidence of prenatal maternal alcohol consumption. Children with PFAS must have similar dysmorphology as children with FAS, either growth retardation or evidence of deficient brain growth (head circumference ≤10th centile or a complex pattern of cognitive and/or behavioral abnormalities), and direct or indirect conformation of maternal alcohol consumption during pregnancy.27,28 Both FAS and PFAS are diagnoses made by the above criterion and also by exclusion of other anomalies with similar phenotypes.28 The data set contains information on 561 children. Overall, 185 children were identified in the sample with a diagnosis within the FASD continuum: 134 children (72.4%) with FAS and 51 (27.6%) with PFAS. Also, all 376 comparison children in the final sample were confirmed to not have an FASD or another recognizable anomaly. Some (25%) mothers of normal controls reported consuming alcohol during the index pregnancy. As with all maternal trait data, all alcohol use data were utilized in the multiple correlation models in this study which normalizes the distribution of drinking somewhat over the sample.
Data Entry, Preliminary Analysis and Analytical Scheme
Data were entered via EPI INFO and transformed to SPSS and other formats for specific statistical applications and analytic techniques via programs as described below. Preliminary correlation analyses first examined the prediction of child neuropsychological traits from individual variables which fall onto four categories of maternal characteristics: physical, demographic, childbearing, and drinking. Two structural equation models (SEM) examined which maternal variables predict children's neurobehavioral characteristics. Model 1 utilized only maternal drinking variables (Figure 1), and Model Two added other maternal traits as possible predictors (Figure 2).
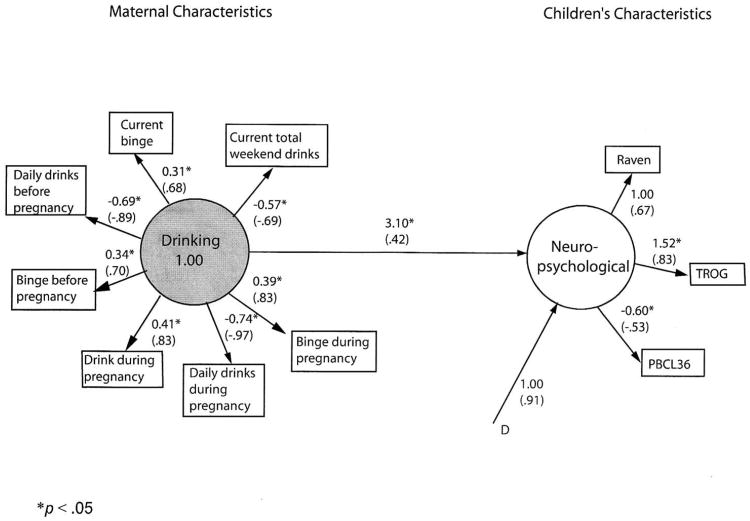
Figure 1. Final model of children's neuropsychological functioning predicted from maternal drinking patterns.
Unstandardized and standardized (in parentheses) parameter estimates. Correlated residuals are in Table 2. Residuals for measured variables are not shown. D* indicates disturbance (error) for the dependent latent variable. All residual variances for measured variables (E*) also are to be estimated (not shown).
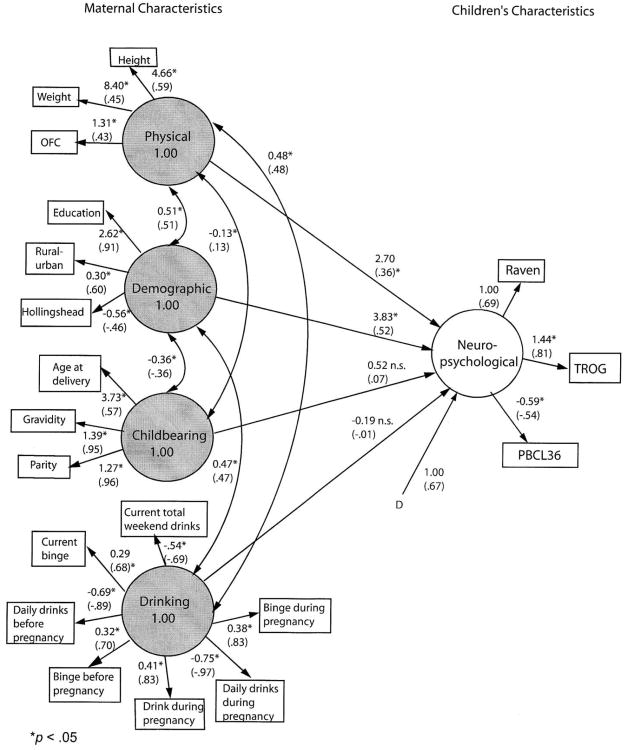
Figure 2. Final model of children's characteristics predicted from maternal characteristics.
Unstandardized and standardized (in parentheses) parameter estimates. Residuals for measured variables are not shown. Asterisks are parameters to be estimated. D* indicates disturbance (error) for the dependent latent variable. All residual variances for measured variables (E*) also are to be estimated (not shown).
Data Preprocessing and Assumptions
Three of the variables measuring drinking quantity were trichotomized to reduce unacceptable skewness and kurtosis associated with low levels of drinking for the group of mothers whose children were not diagnosed with FAS. Current weekend drinking (number of drinks consumed Friday, Saturday, and Sunday) was coded as follows: 0 = “no drinks”, 1 = “1-6 drinks”, 2 = “7 or more drinks”. Variables representing quantity of drinking on a typical day before and during pregnancy were coded as follows: 0 = “no drinks”, 1 = “1-4 drinks”, 2 = “5 or more drinks”.
Varying amounts of data were missing on the measures, from about 5% for Hollingshead index to 36% missing for mother's height. Logarithmic transformations were applied to several of the variables within the SAS PROC MI (multiple imputation) procedure to comply with multivariate normality assumptions of the multiple imputation process. These variables then were back-transformed to create five complete (imputed) data sets, each with N = 561. Impossible values (e.g., negative number of problem behaviors) were adjusted to fall within reasonable range. Relative efficiency was greater than .89 for all variables, indicating relatively little uncertainty regarding missing values. Although deviating from normality, these imputed data sets were considered appropriate for robust estimation in structural equation modeling. Analyses of the five imputed data sets produced negligible differences among them. Therefore only the results from the first imputation are reported. Seventeen cases were identified through Mahalanobis distance as multivariate outliers with p < .001. Analyses with and without the outliers were not substantively different, so the 17 cases were retained in reported analyses. EQS structural equation modeling indicated that these data deviated significantly from normality, Mardia's Normalized Coefficient = 36.31, p < .001. Therefore maximum likelihood estimation was used in EQS modeling with Satorra-Bentler chi-square model tests and with adjustment of standard errors to the extent of nonnormality.88
Results
Key demographic variables are presented in Table 1. The sample is predominately low socioeconomic (SES) status with the average maternal education of formal education, and 70% classified as unskilled laborers, mostly in the vineyards, orchards, and farms. Gravidity and parity are relatively high at 3.1 pregnancies and 2.9 children per mother. The average alcohol consumption values (Table 1) are moderate because of the merging of data from heavy drinkers through non-drinkers, and the large standard deviations indicate substantive variation in the sample. Description of specific quantity, frequency, and timing of maternal drinking by diagnosis in this sample is published elsewhere.8,9 Heavy episodic (binge) drinking on the weekends is the common pattern among those who drink, as evidenced by one-third having binged three months prior to pregnancy and over 85% of all alcohol consumed on Friday, Saturday, and Sunday. Almost half of all women drank at least some during the pregnancy, but the mothers of children with FASD did not quit once they found out they were pregnant. 8,9 The sample is almost equally divided by rural and urban residents.
Table 1. Frequencies and Means of Variables Included in Structural Equation Models (n = 561).
| Variable | |
|---|---|
|
| |
| Mother's Educational Level, Mean (SD) | 7.0 (3.3) |
|
| |
| Occupations (%) (Hollingshead Occupational Codes) | |
| Higher executives, major professionals, owners of large businesses | 0.0 |
| Business managers, medium businesses, lesser professionals, school teachers, nurses | 1.1 |
| Administrative personnel, small businesses, minor professionals, school teachers without degrees | 4.7 |
| Clerical and sales, technician, little businesses | 3.2 |
| Skilled manual (including crafts workers and artists) | 1.1 |
| Semiskilled (factory worker, or farm work who operates machinery) | 8.8 |
| Unskilled (farm worker, tender of vines, unspecified and unemployed) | 70.1 |
| Homemaker | 8.4 |
| Student, disabled, or no occupation | 2.6 |
|
| |
| Gravidity, Mean (SD) | 3.1 (1.4) |
|
| |
| Parity, Mean (SD) | 2.9 (1.3) |
|
| |
| Current Use of Alcohol, Total Drinks Consumed on Weekends (Fri-Sun) Mean (SD) | 3.4 (7.3) |
|
| |
| Total Drinks Consumed on Weekend, % | 85.9 |
|
| |
| Current Use of Alcohol, Binging (3+) in week preceding interview, % | 22.8 |
|
| |
| Use of Alcohol in 3 months before pregnancy, quantity of drinks consumed per typical drinking day, Mean (SD) | 2.4 (3.8) |
|
| |
| Binging (3+) in 3 months before pregnancy, % | 30.0 |
|
| |
| Age during pregnancy, Mean (SD) | 26.3 (6.3) |
|
| |
| Residence during pregnancy, % | |
| Rural | 43.4 |
| Urban | 56.6 |
|
| |
| Drank during pregnancy, % | 49.1 |
|
| |
| Use of Alcohol during pregnancy, quantity of drinks consumed per typical drinking day, Mean (SD) | 2.2 (3.9) |
|
| |
| Binging (3+) during pregnancy, % | 30.7 |
|
| |
| Child's Age (months), Mean (SD) | 86.5 (9.9) |
|
| |
| Child's verbal IQ, Mean (SD) | 81.0 (13.9) |
|
| |
| Child's performance IQ, Mean (SD) | 83.6 (10.9) |
|
| |
| Child's PBCL Score, Mean (SD) | 8.8 (8.3) |
Table 2 provides the Pearson product-moment correlations among all measures. In Table 2, the mother's physical characteristics show fairly robust correlations with testing outcomes, r = .30 and r = .29 between child's performance on the TROG and the mother's height and head circumference, respectively. For the mother's demographic characteristics, correlations of r = .40 and r = .41 are produced between child's performance on the Raven and TROG, respectively, and the mother's education. Inter-correlations appear to be strongest for drinking measures during pregnancy and neuropsychological test performance and behavior, r = .28 and r = -.26 between the child's scoring on the TROG and the PBCL-36 (respectively) and the drinking during pregnancy variable. Likewise, daily drinking during pregnancy shows inter-correlations of r = -.31 and r = .33 with TROG and PBCL-36 scores.
Table 2.
Correlations among variables in structural equation models (N = 563).
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mother's physical | ||||||||||||||||||
| 1. Height | ||||||||||||||||||
| 2. Weight | .31 | |||||||||||||||||
| 3. Head circumference | .26 | .13 | ||||||||||||||||
| Demography | ||||||||||||||||||
| 4. Education | .28 | .24 | .12 | |||||||||||||||
| 5. Rural-urban | .20 | .18 | .16 | .49 | ||||||||||||||
| 6. Hollingshead | -.13 | -.05 | .04 | -.38 | -.28 | |||||||||||||
| Childbearing | ||||||||||||||||||
| 7. Age at delivery | -.00 | .10 | .00 | -.28 | .02 | -.05 | ||||||||||||
| 8. Gravidity | -.19 | -.05 | -.05 | -.38 | -.16 | .12 | .54 | |||||||||||
| 9. Parity | -.16 | -.03 | -.03 | -.38 | -.15 | .31 | .55 | .90 | ||||||||||
| Drinking | ||||||||||||||||||
| 10. Current total weekend | -.22 | -.26 | -.39 | -.30 | -.36 | .10 | -.01 | .16 | .12 | |||||||||
| 11. Current binge | .21 | .26 | .38 | .28 | .34 | -.14 | .07 | -.11 | -.19 | -.88 | ||||||||
| 12. Daily drinks pre-pregnancy | -.24 | -.24 | -.22 | -.30 | -.34 | .20 | .01 | .13 | .10 | .62 | -.59 | |||||||
| 13. Binge pre-pregnancy | .01 | .12 | .22 | .32 | .33 | -.18 | .01 | -.05 | -.03 | -.47 | .51 | -.66 | ||||||
| 14. Drink during pregnancy | .19 | .27 | .20 | .33 | .37 | .24 | .02 | -.14 | -.13 | -.55 | .54 | -.74 | .56 | |||||
| 15. Daily drinks during pregnancy | -.20 | -.26 | -.26 | -.36 | -.38 | .23 | .04 | .17 | .14 | .66 | -.65 | .86 | -.67 | -.82 | ||||
| 16. Binge during pregnancy | .20 | .26 | .21 | .39 | .40 | -.20 | -.01 | -.15 | -.11 | -.66 | .67 | -.71 | .72 | .66 | -.81 | |||
| Child neuropsychological | ||||||||||||||||||
| 17. Raven matrices | .25 | .17 | .13 | .40 | .30 | -.29 | -.02 | -.24 | -.20 | -.23 | .21 | -.22 | .14 | .18 | -.25 | .25 | ||
| 18. TROG | .30 | .18 | .29 | .41 | .30 | -.29 | -.01 | -.17 | -.13 | -.28 | .28 | -.26 | .21 | .28 | -.31 | .32 | .57 | |
| 19. PCBL 36 | -.22 | -.07 | -.24 | -.31 | -.30 | .23 | -.06 | .09 | .07 | .26 | -.25 | .28 | -.27 | -.26 | .33 | -.32 | -.33 | -.44 |
Model 1: Predicting Neuropsychological Characteristics from Drinking Patterns
The hypothesized structural equation model was similar in substantive content to the final model in Figure 1, with circles representing latent variables (factors) and rectangles representing measured variables. Lines with single arrows represent hypothesized direction of prediction (e.g., from maternal drinking characteristics to children's neuropsychological characteristics).
Patterns of drinking behavior before and during the index pregnancy as well as at the time of interview were expected to predict children's neuropsychological characteristics: non-verbal intelligence, verbal intelligence, and problem behavior.
The test of the hypothesized model indicated significant differences between hypothesized and observed covariance matrices, Satorra-Bentler Π2 (34, N = 563) = 515.80, p < .001. The robust comparative fit index (CFI) of .80 reflects an unacceptable fit, as well.
The model was significantly improved by adding two pairs of correlated residuals suggested by the Lagrange multiplier test, Satorra-Bentler Π2difference (2, N = 563) = 209.00, p < .001. Paths for correlated residuals were added between total weekend drinks and current bingeing, r = -.77, p < .05 and between bingeing before and during pregnancy, r = .35, p < .05.
Even this modified model produced significant differences between the estimated and observed covariance matrix, Satorra-Bentler Π 2 (32, N = 563) = 111.18, p < .001. However, the fit was quite good, robust CFI = .98, RMSEA = .07. A bivariate correlation was performed between common unstandardized parameter estimates from the hypothesized and final models, to support the addition of post hoc modifications. The r(8) > .99, p < .001 indicates a virtually perfect relationship between the hypothesized and final models.
Figure 1 shows unstandardized and standardized coefficients for the final model number 1. All of the measures loaded significantly on their respective factors. The added correlations between residuals, although significantly enhancing model fit, do not add substantively to interpretation. They simply indicate that the strengths of some of the drinking relationships are not fully captured by the model.
As expected, in the simple model 1, drinking behavior predicts these children's neuropsychological characteristics (B = 3.10, p < .05). More problematic drinking behavior produces poorer child performance (e.g., more problem behaviors, lower intelligence). Despite the good model fit, however, only 17.3% of the variance in child's characteristics is accounted for by maternal drinking.
Model 2: Adding Non-Drinking Variables to Prediction of Neuropsychological Characteristics
Other maternal characteristics were added to account for more variance in children's functioning. Figure 3 shows the four maternal factors (physical, demographic, childbearing characteristics, and drinking behavior before and during the index pregnancy as well as at the time of interview) expected to predict the childrens' neuropsychological characteristics. Correlations are hypothesized between maternal physical and demographic characteristics, between maternal physical and childbearing characteristics, and between childbearing and demographic characteristics. Drinking behavior was hypothesized to correlate with maternal physical and demographic characteristics.
The test of the hypothesized model indicated significant differences between hypothesized and observed covariance matrices, Satorra-Bentler Π2(143, N = 563) = 1048.57, p < .001. The robust comparative fit index (CFI) of .86 reflects a poor fit, as well.
The model was significantly improved by adding two pairs of correlated residuals suggested by the Lagrange multiplier test, Satorra-Bentler Π2difference(2, N = 563) = 212.17, p < .001. The correlated residuals were the same as in Model 1. Even this modified model produced significant differences between the estimated and observed covariance matrix, Satorra-Bentler Π2(141, N = 563) = 562.19, p < .001, but the fit was improved considerably, robust CFI = .93, RMSEA = .07.
A bivariate correlation was then performed between common, unstandardized parameter estimates from the hypothesized and final models, to support the addition of post hoc modifications. The r(25) = .99, p < .001 indicates a virtually perfect relationship between the hypothesized and final models.
Figure 2 presents unstandardized and standardized coefficients for the final model. All individual variables loaded significantly on their respective factors. The added correlations between residuals, although significantly enhancing model fit, do not add substantively to interpretation. These simply indicate that the strengths of some drinking relationships are not fully captured by the model.
All measured variables have significant loadings for their respective factors, but of the four composite factors, only two are statistically significant predictors. The strongest correlation with a child's neuropsychological functioning is demography (B = 3.83, p < .05). Greater child functioning is associated with more maternal education, urban lifestyle, and higher SES. Maternal physical characteristics are also correlated significantly with child neuropsychological function (B = 2.70, p < .05). Mothers with higher functioning children are apt to be heavier, taller, and have larger head circumference. Once these factors are taken into account, however, neither childbearing characteristics nor drinking patterns significantly predict child's neuropsychological functioning, p > .05. That is, the significant prediction evident from drinking pattern in isolation is removed when demography and maternal physical and childbearing characteristics are considered. However, in summary of this second model, more than half (R2 = 55.3%) of the variance in children's neuropsychological functioning was accounted for by the combination of all four of the maternal characteristics.
A third model was tested in which the drinking construct contained only variables reflecting drinking during pregnancy. This model did not differ substantially from Model 2.
Discussion
In this study both a simple, alcohol-specific model of causation and a complex statistical model of multiple maternal traits influencing cognition and behavior among children with FASD have been presented; the strong performance of the complex, multi-variable model underscores the prime importance of the postnatal environment on the development of children with FASD and lends credence to the promise of early intervention. The highest functioning children with fewer behavioral problems were born to women who are higher SES (higher education, urban dwellings, and higher income) with more robust physical characteristics which are generally indicative of better maternal health status (heavier, taller, and larger head circumference). Although other types of statistical analyses of material risk typically cite childbearing variables as influential on FASD risk, the combined variables of maternal age at delivery, gravidity, and parity were not significantly correlated with child neurobehavior at seven years of age. Furthermore even though the simple, alcohol only, SEM model proved significantly influential on child outcomes and explained 17.3% of the variance in child neurobehaviors, alcohol use was not significantly correlated with the cognitive skills and behavior of these children. Maternal risk for neurobehavioral problems caused by FASD is a complex phenomenon that is highly influenced by co-factors other than alcohol exposure alone. The final, complex model explains 55% of the variance in cognition and behavior of children with FASD and normal controls.
These findings are important for understanding the major developmental role played by: 1.) postnatal or early childhood influences and 2.) the characteristics of mothers on children with FASD. In the final model, maternal drinking variables are overpowered by SES and maternal physical variables in their effect on neurobehavior. This does not mean that prenatal alcohol consumption was not significantly negative for neurobehavior, for alcohol is an agent which adversely impacts brain development and sets the stage for disability. But, while the heavy prenatal alcohol exposure predisposed children to relative impairment in dysmorphology and neurobehavior, maternal education, higher SES, urban residence, and good general health of the mother may provide a protective influence in both the prenatal and postnatal period that translates to higher intelligence and better behavior by age seven. The significant variables may reflect a general prenatal advantage of less severe physical brain impairment derived from adequate nutrition of the mothers89-92 and a postnatal advantage from mothers who are able to provide a more nurturing and stimulating environment for their children's neurodevelopment.2,45,93 Pre and postnatal advantages have mitigated the negative teratogenic effects of alcohol exposure. Such findings have broad implications. Better outcomes have been documented for prenatal-alcohol exposed babies in populations where these same maternal characteristics exist to a greater degree: Europe and the United States.34,79,80,92 These better outcomes may reflect the importance of the significant variables identified here: a mother with robust health status and the influence of higher SES environments. Conversely, this study may also highlight the negative impact of prenatal stress and postnatal disadvantage as contributors to increased neurological vulnerability of low SES children who are alcohol exposed.94,95
Differences in Models of Maternal Risk for Physical and Neurobehavioral Traits
A previous publication from this same data set examined maternal risk factors for child physical characteristics, specifically alcohol-related dysmorphology. Dysmorphological traits were most significantly influenced by the quantity, frequency, and timing of alcohol consumption49 than by any of the other independent variables used in this study. Physical characteristics of FASD were most correlated with maternal drinking variables which overpowered all others. Maternal demographics and physical characteristics were also significant categories predicting dysmorphology, but alcohol use dominated all other variables. The overall physical model, using these same four independent categories, explained 62% of the variance in child dysmorphology.49
Therefore, higher levels of maternal prenatal drinking clearly predict child dysmorphology,49 but the multivariate model of neurobehavioral determinants in study revealed that maternal SES and physical health may be more influential on intellectual functioning and behavior by age seven than prenatal alcohol use alone. That is good news that is consistent with child development literature. A positive, healthy, and stimulating postnatal environment with a well-educated mother is significantly linked to improvements in child functioning, even when prenatal alcohol exposure has occurred. Better understanding of the specific ways that these traits are translated to the children is important for effective pre-and post-natal intervention.
Limitations of the Statistical Model and Interpretations of a Complex Reality
This statistical model is exactly that, a model. While many possible, theory-backed influences on child behavior are included and statistically controlled, the model may not be completely accurate in the final picture that is provided. Other research methods and statistical analysis techniques have shown repeatedly, that prenatal exposure to alcohol is a powerful force in limiting both a child's physical and neurobehavioral capabilities.34,50,52-57,61-63 But this model from a low SES, primarily weekend drinking population of unique genetic traits de-emphasizes or masks the influence of prenatal alcohol damage on neurobehavior as measured by seven years of age. Slightly more advantaged social and developmental circumstances have likely mitigated some of the teratogenic damage exerted by alcohol prenatally.96 So even though this model underestimates the negative effects of prenatal alcohol exposure, it promotes a more complete understanding of maternal traits which protect or remediate exposed children. Therefore the promise of early intervention via intellectual and behavioral stimulation for FASD children is demonstrated here in a disadvantaged population.97-100 It seems likely the same variables are positively influential in higher SES populations.79,80 Furthermore, if a mother's education, SES, and more robust physical status correlates with better outcomes here, then relative nutritional advantages also must be considered.89-93,101Research in this population, USA, and Italy has shown that mothers of children with FASD have lower average BMI than mothers of children without an FASD.8,9,42,49,104 Do these data indicate that nutrition is an important factor in both the prenatal and postnatal period? First, the better nutrition of the mother both in her early life and the prenatal period, as in animal models, mitigate the teratogenic effects of alcohol on the developing brain.44,101,102 Furthermore, it is reasonable to assume that better functioning children have been raised in homes for up to seven years where better nutrition has aided brain growth and development.44,103 Early intervention with both cognitive/behavioral stimulation and nutritional supplementation are implicated for adequate brain development which is not a controversial or unsubstantiated approach to child development.105-109
Conclusion
Alcohol is a teratogen that causes major harm to fetuses which may last a lifetime. But substantial individual variation is evident in both the physical and cognitive/behavioral outcomes of children. As presented here, the neurobehavioral traits of children vary significantly by the mother's demographic (SES) and physical status, while prenatal alcohol exposure is not a statistically significant factor. This provides objective data for optimism about potential development for children with FASD and supports the efficacy of early intervention. If correct, substantial remediation is possible for some of the cognitive and behavioral deficits of children with FASD. Further research into the complex relationships between alcohol use and maternal health, nutrition, social environment, biological, and genetic influences on child development is warranted.
Acknowledgments
This research was funded in part by grants RO1AA09440, RO1/UO1AA11685, and RO1 AA15134 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the NIH National Center on Minority Health and Health Disparities (NCMHD). We thank the mothers and children who provided the information for this study. The interviewers for this study have been exceptional: Julie Croxford, Leslie Brooke, Anna-Susan Marais, Magdalena September, Loretta Hendricks, and Leana Marais. The psychometrists who completed the testing were also exceptional. Andrea Hay (deceased) Ansie Ketching. Gwyneth Moya and Chandra Stellavato, University of New Mexico student employees, also deserve thanks for their assistance in data entry. Finally, Phyllis Trujillo Lewis and Cheryl Ritson assisted with important tasks of initial manuscript preparation and submission.
Footnotes
The authors have no conflicts of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.May PA. A multiple-level, comprehensive approach to the prevention of FAS and other alcohol-related birth defects. Int J Addictions. 1995;30:1549–1602. doi: 10.3109/10826089509104417. [DOI] [PubMed] [Google Scholar]
- 2.Abel EL. Fetal Alcohol Abuse Syndrome. New York: Plenum Press; 1998. [Google Scholar]
- 3.May PA, Gossage JP. Maternal risk factors for Fetal Alcohol Spectrum Disorders: not as simple as it might seem. Alcohol Res Health. 2011;34:15–26. [PMC free article] [PubMed] [Google Scholar]
- 4.West JR, Goodlett CR. Teratogenic effects of alcohol on brain development. Ann Med. 1990;22:319–325. doi: 10.3109/07853899009147914. [DOI] [PubMed] [Google Scholar]
- 5.Thomas JD, Wasserman EA, West JR, et al. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Dev Psychobiol. 1996;29:433–452. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- 7.Viljoen D, Croxford J, Gossage JP, et al. Characteristics of mothers of children with fetal alcohol syndrome in the Western Cape Province of South Africa: a case control study. J Stud Alcohol. 2002;63:6–17. [PubMed] [Google Scholar]
- 8.May PA, Gossage JP, Brooke LE, Snell, et al. Maternal risk factors for fetal alcohol syndrome in the Western Cape Province of South Africa: a population-based study. Am J Public Health. 2005;(95):1190–1199. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May PA, Gossage JP, Marais AS, et al. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res. 2008;32:738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 10.Waterson EJ, Murray-Lyon IM. Drinking and smoking patterns amongst women attending an antenatal clinic — II during pregnancy. Alcohol Alcohol. 1989;24:163–173. doi: 10.1093/oxfordjournals.alcalc.a044880. [DOI] [PubMed] [Google Scholar]
- 11.Robles N, Day NL. Recall of alcohol consumption during pregnancy. J Stud Alcohol. 1990;51:403–407. doi: 10.15288/jsa.1990.51.403. [DOI] [PubMed] [Google Scholar]
- 12.Serdula M, Williamson DF, Kendrick JS, et al. Trends in alcohol consumption by pregnant women: 1985 through 1988. JAMA. 1991;265:876–879. [PubMed] [Google Scholar]
- 13.Jacobson SW, Jacobson JL, Sokol RJ, et al. Maternal recall of alcohol, cocaine, and marijuana use during pregnancy. Neurotoxicol Teratol. 1991;13:535–540. doi: 10.1016/0892-0362(91)90062-2. [DOI] [PubMed] [Google Scholar]
- 14.Primatesta P, DelCorno G, Bonazzi MC, et al. Alcohol and pregnancy: An international comparison. J Pub Health Med. 1993;15:69–76. doi: 10.1093/oxfordjournals.pubmed.a042822. [DOI] [PubMed] [Google Scholar]
- 15.Bad Heart Bull LB, Kvigne VL, Leonardson GL, et al. Validation of a self-administered questionnaire for prenatal alcohol use in Northern Plains Indian women. Am J Preven Med. 1999;16:240–3. [PubMed] [Google Scholar]
- 16.Floyd RL, Decouflé P, Hungerford DW. Alcohol use prior to pregnancy recognition. Am J Prev Med. 1999;17(2):101–107. doi: 10.1016/s0749-3797(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 17.Hankin JR. Fetal alcohol syndrome prevention research. Alcohol Res Health. 2002;26(1):58–65. [PMC free article] [PubMed] [Google Scholar]
- 18.Whaley SE, O'Connor MJ. Increasing the report of alcohol use among low-income pregnant women. Am J Health Promo. 2003;17:369–372. doi: 10.4278/0890-1171-17.6.369. [DOI] [PubMed] [Google Scholar]
- 19.Malet L, de Chazeron I, Llorca PM, et al. Alcohol consumption during pregnancy: A urge to increase prevention and screening. Eur J Epidemiol. 2006;21:787–788. doi: 10.1007/s10654-006-9056-3. [DOI] [PubMed] [Google Scholar]
- 20.Alvik A, Haldorsen T, Groholt B, et al. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcohol Clin Exp Res. 2006a;30:510–515. doi: 10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 21.Alvik A, Haldorsen T, Grohol B, Lindemann, et al. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcohol Clin Exp Res. 2006;30:510–515. doi: 10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 22.Alvik A, Heyerdahl S, Haldorsen T, et al. Alcohol use before and during pregnancy: A population-based study. Acta Pediatr. 2006;85:1292–1298. doi: 10.1080/00016340600589958. [DOI] [PubMed] [Google Scholar]
- 23.Kristjanson AF, Wilsnack SC, Zvartau E, et al. Alcohol use in pregnant and nonpregnant Russian women. Alcohol Clin Exp Res. 2007;31:299–307. doi: 10.1111/j.1530-0277.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- 24.Hannigan JH, Chiodo LM, Sokol RJ, et al. A 14-Year Retrospective Maternal Report of Alcohol Consumption in Pregnancy Predicts Pregnancy and Teen Outcomes. Alcohol. 2010;44L:583–594. doi: 10.1016/j.alcohol.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce DR, West JR. Blood alcohol concentration: A critical factor for producing fetal alcohol syndrome. Alcohol. 1986;3:269–272. doi: 10.1016/0741-8329(86)90036-4. [DOI] [PubMed] [Google Scholar]
- 26.Ernhart CB, Sokol RJ, Martier S, et al. Alcohol teratogenicity in the human: a detailed assessment of specificity, critical period, and threshold. Am J Obstet Gynecol. 1987;156:33–39. doi: 10.1016/0002-9378(87)90199-2. [DOI] [PubMed] [Google Scholar]
- 27.Stratton KR, Howe CJ, Battaglia FC. Institute of Medicine (Division of Biobehavioral Sciences and Mental Disorders, Committee to Study Fetal Alcohol Syndrome and National Institute on Alcohol Abuse and Alcoholism. Fetal alcohol syndrome diagnosis, epidemiology, prevention, and treatment. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 28.Hoyme HE, May PA, Kalberg WO, et al. A Practical Clinical Approach to Diagnosis of Fetal Alcohol Spectrum Disorders: clarification of the 1996 Institute of Medicine Criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn HA, Chermack S. Prenatal alcohol use: The role of lifetime problems with alcohol, drugs, depression, and violence. J Stud Alcohol Drugs. 2008;69:500–509. doi: 10.15288/jsad.2008.69.500. [DOI] [PubMed] [Google Scholar]
- 30.Pierog S, Chandavsu O, Wexler I. The Fetal Alcohol syndrome: some maternal characteristics. Int J Gyn Obstet. 1979;16:412–415. doi: 10.1002/j.1879-3479.1979.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 31.Abel EL, Hannigan JH. Maternal risk factors in Fetal Alcohol Syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1995;17:445–465. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- 32.May PA, Hymbaugh KJ, Aase JM, et al. Epidemiology of Fetal Alcohol Syndrome among American Indians of the Southwest. Soc Biol. 1983;30:374–385. doi: 10.1080/19485565.1983.9988551. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson JL, Jacobson SW, Sokol RJ. Increased vulnerability to alcohol-related birth defects in the offspring of mothers over 30. Alcohol Clin Exp Res. 1996;20:359–363. doi: 10.1111/j.1530-0277.1996.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 34.May PA, Fiorentino D, Gossage JP, et al. The epidemiology of FASD in a province in Italy: prevalence and characteristics of children in a random sample of schools. Alcohol Clin Exp Res. 2006;30:1562–1575. doi: 10.1111/j.1530-0277.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson JL, Jacobson SW, Sokol RJ, et al. Relation of maternal age and pattern of pregnancy drinking to functionally significant cognitive deficit in infancy. Alcohol Clin Exp Res. 1998;22:345–351. doi: 10.1111/j.1530-0277.1998.tb03659.x. [DOI] [PubMed] [Google Scholar]
- 36.Baily S. Women with alcohol problems: a psycho-social perspective. Drug Alcohol Rev. 1990;9:125–131. doi: 10.1080/09595239000185181. [DOI] [PubMed] [Google Scholar]
- 37.Darrow SL, Russell M, Cooper ML, et al. Sociodemographic correlates of alcohol consumption among African-American and white women. Women Health. 1992;18:35–50. doi: 10.1300/J013v18n04_03. [DOI] [PubMed] [Google Scholar]
- 38.Abel EL. An update on the incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol. 1995;17:43. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- 39.Crome IB, Glass Y. The DOP system: A manifestation of social exclusion. A personal commentary on “consumption amongst South African workers”. Drug Alcohol Depend. 2000;59:207–208. doi: 10.1016/s0376-8716(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 40.Bingol N, Schuster C, Fuchs M, et al. The influence of socioeconomic factors on the occurrence of fetal alcohol syndrome. Adv Alcohol Subst Abuse. 1987;6:105–118. doi: 10.1300/J251v06n04_08. [DOI] [PubMed] [Google Scholar]
- 41.Khaole NC, Ramchandani VA, Viljoen DL, et al. A pilot study of alcohol exposure and pharmacokinetics in women with or without children with fetal alcohol syndrome. Alcohol Alcohol. 2004;39:503–508. doi: 10.1093/alcalc/agh089. [DOI] [PubMed] [Google Scholar]
- 42.May PA, Hamrick KJ, Brooke LE, et al. The possible contribution of maternal nutrition to fetal alcohol syndrome among offspring of Coloured women in the Western Cape Province, South Africa. Alcohol Clin Exp Res. 2004;28:125A. [Google Scholar]
- 43.Sokol RJ, Ager J, Martier S, et al. Significant determinants of susceptibility to alcohol teratogenicity. Ann NY Acad Sci. 1986;477:87–102. doi: 10.1111/j.1749-6632.1986.tb40323.x. [DOI] [PubMed] [Google Scholar]
- 44.Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1995 Jul-Aug;17(4):445–62. doi: 10.1016/0892-0362(95)98055-6. Review. Erratum in: Neurotoxicol Teratol. 1995;Nov-Dec;17(6):689. [DOI] [PubMed] [Google Scholar]
- 46.Badger TM, Hidestrand M, Shankar K, et al. The effects of pregnancy on ethanol clearance. Life Sci. 2005;77:2111–2126. doi: 10.1016/j.lfs.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 47.Shankar K, Hidestrand M, Liu X, et al. Physiologic and genomic analyses of nutrition-ehtanol interactions during gestation: Implications for fetal ethanol toxicity. Exp Biol Med. 2006;231:1379–1397. doi: 10.1177/153537020623100812. [DOI] [PubMed] [Google Scholar]
- 48.Shankar K, Ronis MJ, Badger TM. Effects of pregnancy and nutritional status on alcohol metabolism. Alcohol Res Health. 2007;30:55–59. [PMC free article] [PubMed] [Google Scholar]
- 49.May PA, Tabachnick B, Gossage J, et al. Maternal risk factors predicting child physical characteristics and dysmorphology in fetal alcohol syndrome and partial fetal alcohol syndrome. Drug Alcohol Depend. 2011;119:18–27. doi: 10.1016/j.drugalcdep.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattson SN, Riley EP, Delis DC, et al. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- 51.May PA, Gossage JP, Marais AS, et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kodituwakku PW, Handmaker NS, Cutler SK, et al. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1995;19:1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 53.Mattson SN, Goodman AM, Caine C, et al. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- 54.Willford JA, Richardson GA, Leech SL, et al. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Comparative Study. Alcohol Clin Exp Res. 2004;28(3):497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- 55.Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:875–882. [PubMed] [Google Scholar]
- 56.Streissguth AP, Bookstein FL, Sampson PD, et al. Neurobehavioral effects of prenatal alcohol: Part III. PLS analyses of neuropsychologic tests. Neurotoxicol Teratol. 1989;11:493–507. doi: 10.1016/0892-0362(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 57.Mattson SN, Riley EP. Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. J Int Neuropsychol Soc. 1999;5:462–471. doi: 10.1017/s1355617799555082. [DOI] [PubMed] [Google Scholar]
- 58.Kodituwakku PW, Adnams CM, Hay A, et al. Letter and category fluency in children with fetal alcohol syndrome from a community in South Africa. J Stud Alcohol. 2006;67:502–509. doi: 10.15288/jsa.2006.67.502. [DOI] [PubMed] [Google Scholar]
- 59.Korkman M, Liikanen A, Fellman V. Neuropsychological consequences of very low birth weight and asphyxia at term: follow-up until school-age. J Clin Exp Neuropsychol. 1996;18:220–233. doi: 10.1080/01688639608408277. [DOI] [PubMed] [Google Scholar]
- 60.Bishop DVM. Test for the reception of grammar. London: Medical Research Council; 1989. [Google Scholar]
- 61.Aragon AS, Coriale G, Fiorentino D, et al. Neuropsychological Characteristics of Italian Children With Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2008;32:1909–1918. doi: 10.1111/j.1530-0277.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adnams CM, Kodituwakku P, Hay A, et al. Patterns of cognitive-motor development in children with fetal alcohol syndrome from a community in South Africa. Alcohol Clin Exp Res. 2001;25:557–562. [PubMed] [Google Scholar]
- 63.Kodituwakku PW, Kalberg W, May PA. The effects of prenatal alcohol exposure on executive functioning. Alcohol Res Health. 2001;25:92–198. [PMC free article] [PubMed] [Google Scholar]
- 64.Urban M, Chersich MF, Fourie LA, et al. Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: prevalence and risk factors. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2008;98:877–882. [PubMed] [Google Scholar]
- 65.Kodituwakku PW, May PA, Clericuzio CL, et al. Emotion-related learning in individuals prenatally exposed to alcohol: an investigation of the relation between set shifting, extinction of responses, and behavior. Neuropsychologia. 2001;39:699–708. doi: 10.1016/s0028-3932(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 66.Kalberg WO, Buckley D. Educational planning for children with fetal alcohol syndrome. Ann Ist Super Sanita. 2006;42:58–66. [PubMed] [Google Scholar]
- 67.Kalberg WO, Buckley D. FASD: What types of intervention and rehabilitation are useful? Neurosci Biobehav Rev. 2007;31:278–285. doi: 10.1016/j.neubiorev.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 68.Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotox teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Streissguth AP, Bookstein FL, Barr HM, et al. A fetal alcohol behavior scale. Alcohol Clin Exp Res. 1998;22:325–333. doi: 10.1111/j.1530-0277.1998.tb03656.x. [DOI] [PubMed] [Google Scholar]
- 70.Streissguth AP. Fetal alcohol syndrome a guide for families and communities. Baltimore: Paul H. Brookes Pub; 1997. [Google Scholar]
- 71.Streissguth AP, Clarren SK, Jones KL. Natural history of the Fetal Alcohol Syndrome, A ten-year follow-up of eleven patients. Lancet. 1985;2:85–92. doi: 10.1016/s0140-6736(85)90189-8. [DOI] [PubMed] [Google Scholar]
- 72.Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- 73.Steinhausen HC, Spohr HL. Long-term Outcome of Children with Fetal alcohol Syndrome: Psychopathology, Behavior, and Intelligence. Alcohol Clin Exp Res. 1998;22:334–338. doi: 10.1111/j.1530-0277.1998.tb03657.x. [DOI] [PubMed] [Google Scholar]
- 74.Coles CD, Platzman KA, Raskind-Hood CL, et al. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- 75.Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcohol Clin Exp Res. 1990;14:650–655. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 76.Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 ½ years. Alcohol Clin Exp Res. 1990 Oct;14(5):662–9. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 77.Jacobson SW, Jacobson JL, Sokol RJ, et al. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- 78.Raven JC, Court JH, Raven J. Manual for Raven's Progressive Matrices and Vocabulary Scales section 1, general overview and section 2, Coloured Progressive Matrices. London: H.K. Lewis and Co. Ltd.; 1947. [Google Scholar]
- 79.Skogerbø Å, Kesmodel U, Wimberley T, et al. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on executive function in 5-year-old children. BJOG. 2012;119:1201–1210. doi: 10.1111/j.1471-0528.2012.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Underbjerg M, Kesmodel U, Landrø N, et al. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on selective and sustained attention in 5-year-old children. BJOG. 2012;119:1211–1221. doi: 10.1111/j.1471-0528.2012.03396.x. [DOI] [PubMed] [Google Scholar]
- 81.Viljoen DL, Gossage JP, Adnams C, et al. Fetal Alcohol Syndrome epidemiology in a South African community: a second study of a very high prevalence area. J Stud Alcohol. 2005;66:593–604. doi: 10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Czarnecki DM, Russell M, Cooper ML, et al. Five-year reliability of self-reported alcohol consumption. J Stud Alcohol. 1990;51:68–76. doi: 10.15288/jsa.1990.51.68. [DOI] [PubMed] [Google Scholar]
- 83.King AC. Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. AJPH. 1994;84:294–296. doi: 10.2105/ajph.84.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sobell LC, Sobell MB, Leo GI, et al. A. Reliability of a timeline method: assessing normal drinker's reports of recent drinking and a comparative evaluation across several populations. Brit J Addiction. 1998;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 85.Kaskutas LA, Graves K. Pre-pregnancy drinking: how drink size affects risk assessment. Addiction. 2001;96:1199–1209. doi: 10.1046/j.1360-0443.2001.968119912.x. [DOI] [PubMed] [Google Scholar]
- 86.Kaskutas LA, Kerr WC. Accuracy of photographs to capture respondent-defined drink size. J Stud Alc Drugs. 2008;69:605–610. doi: 10.15288/jsad.2008.69.605. [DOI] [PubMed] [Google Scholar]
- 87.Molarius A, Seidell JC, Sans S, et al. Educational level, relative body weight, and changes in their association over 10 years: an international perspective from the WHO MONICA Project. AJPH. 2000;90:1260–1268. doi: 10.2105/ajph.90.8.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ullman JB. Structural equation modeling. In: Tabachnick BG, Fidell LS, editors. Using multivariate statistics. Boston: Allyn and Bacon; 2007. pp. 770–772. [Google Scholar]
- 89.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–50. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeisel SH. What choline metabolism can tell us about the underlying mechanisms of fetal alcohol spectrum disorders. Mol Neurobiol. 2011 Oct;44(2):185–91. doi: 10.1007/s12035-011-8165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeisel SH, Niculescu MD. Perinatal choline influences brain structure and function. Nutr Rev. 2006 Apr;64(4):197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lewis SJ, Zuccolo L, DaveySmith G, et al. Fetal alcohol exposure in IQ at age 8: evidence from a population-based birth-cohort study. PLoS One. 2012;7(11):e49407. doi: 10.1371/journal.pone.0049407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009 Nov;67(11):615–23. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schneider ML, Moore CF, Kraemer GW, et al. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendicrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- 95.Hellemans KGC, Sliwowska JH, Verma P, et al. Prenatal alcohol exposure: Fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davies L, Dunn M, Chersich M, et al. Developmental delay of infants and young children with and without fetal alcohol spectrum disorder in the Northern Cape Province, South Africa. Afr J of Psychiatry. 2011;14:298–305. doi: 10.4314/ajpsy.v14i4.7. [DOI] [PubMed] [Google Scholar]
- 97.Coles CD, Platzman KA, Lynch ME, et al. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res. 2002;26:263–271. [PubMed] [Google Scholar]
- 98.O'Connor MJ, Paley B. The relationship of prenatal alcohol exposure and the postnatal environment to child depressive symptoms. J Pediatr Psychol. 2006;31:50–64. doi: 10.1093/jpepsy/jsj021. [DOI] [PubMed] [Google Scholar]
- 99.Paley B, O'Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15:258–267. doi: 10.1002/ddrr.67. [DOI] [PubMed] [Google Scholar]
- 100.Adnams CM, Sorour P, Kalberg WO, et al. Language and literacy outcomes from a pilot intervention study for children with fetal alcohol spectrum disorders in South Africa. Alcohol. 2007;41:403–414. doi: 10.1016/j.alcohol.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keen CL, Uriu-Adams JY, Skalny A, et al. The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. BioFactors. 2010;36:125–135. doi: 10.1002/biof.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Research Support, N.I.H., Extramural. Neurotoxicol Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas JD, Biane JS, O'Bryan KA, et al. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- 104.May PA, Fiorentino D, Coriale G, et al. Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: new estimated rates are higher than previous estimates. Int J Environ Res Public Health. 8(6):2331–51. doi: 10.3390/ijerph8062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Allen LH, Peerson JM, Olney DK. Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient-deficient children and adults. J Nutr. 2009;139:1022–1030. doi: 10.3945/jn.107.086199. [DOI] [PubMed] [Google Scholar]
- 106.Barros AJD, Matijasevich A, Santos IS, et al. Child development in a birth cohort: Effect of child stimulation is stronger in less educated mothers. Int J Epidemiol. 2010;29:285–294. doi: 10.1093/ije/dyp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Engle PL, Black MM, Behrman J, et al. Child development in developing countries 3: Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369:229–242. doi: 10.1016/S0140-6736(07)60112-3. [DOI] [PubMed] [Google Scholar]
- 108.Gardner J, Powell C, Baker-Henningham H, et al. Zinc supplementation and psychosocial stimulation: Effects on the development of undernourished Jamaican children. Am J Clin Nutr. 2005;82:399–405. doi: 10.1093/ajcn.82.2.399. [DOI] [PubMed] [Google Scholar]
- 109.Lozoff B, Smith J, Clark K, Perales C, Rivera F, Castillo M. Home intervention improves cognitive and social-emotional scores in iron-deficient anemic infants. Pediatrics. 2010;126:884–894. doi: 10.1542/peds.2009-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]