Abstract
Chikungunya virus (CHIKV), a mosquito-borne alphavirus, recently re-emerged in Africa and spread to islands in the Indian Ocean, the Indian subcontinent, and to South East Asia. Viremic travelers have also imported CHIKV to the Western hemisphere highlighting the importance of CHIKV in public health. In addition to the great burden of arthralgic disease, which can persist for months or years, epidemiologic studies have estimated case-fatality rates of ~0.1%, principally from neurologic disease in older patients. There are no licensed vaccines or effective therapies to prevent or treat human CHIKV infections. We have developed a live CHIKV vaccine (CHIKV/IRES) that is highly attenuated yet immunogenic in mouse models, and is incapable of replicating in mosquito cells. In this study we sought to decipher the role of adaptive immunity elicited by CHIKV/IRES in protection against wild-type CHIKV infection. A single dose of vaccine effectively activated T cells with an expansion peak on day 10 post immunization and elicited memory CD4+ and CD8+ T cells that produced IFN-γ, TNF-α and IL-2 upon restimulation with CHIKV/IRES. Adoptive transfer of CHIKV/IRES-immune CD4+ or CD8+ T cells did not confer protection against wtCHIKV-LR challenge. By contrast, passive immunization with anti-CHIKV/IRES immune serum provided protection, and a correlate of a minimum protective neutralizing antibody titer was established. Overall, our findings demonstrate the immunogenic potential of the CHIKV/IRES vaccine and highlight the important role that neutralizing antibodies play in protection against an acute CHIKV infection.
Keywords: Chikungunya (CHIKV), Interferon (IFN), Humoral immunity, Cellular immunity, protection, A129 mice
1. Introduction
Chikungunya virus (CHIKV) is a mosquito-borne virus that recently reemerged in the Indian Ocean islands, India, and Southeast Asia causing outbreaks affecting millions of people [1–4]. More recently, viremic travelers have imported CHIKV to the Western hemisphere, and autochthonous cases have been reported in Southern Europe [5, 6]. CHIKV infection is clinically indistinguishable from dengue and is characterized by fever, headache, rash, myalgia, and polyarthralgia [7, 8]. Although most signs and symptoms are acute and self-limiting, some patients develop prolonged polyarthralgia a hallmark of CHIKV infection that can persist for months or years [9]. The disease is more severe among newborns, infants and elderly patients with estimated fatality rates of 1:1000 cases in La Reunion and India during the 2005–2006 outbreaks [10].
The RNA genome of CHIKV encodes four non-structural proteins (nsP1 to nsP4) that are required for virus replication and three structural proteins (Capsid, E1 and E2) together with two small cleavage products (E3 and 6K). The E1 glycoprotein mediates pH-dependent fusion with endosomal membranes whereas the E2 glycoprotein interacts with cell surface receptors [11]. The adaptive immune responses to CHIKV have yet to be fully characterized. Recent epidemiological data highlighted the role of antibodies in protection [12–15]. Moreover, antibody-based therapies clearly established the role of humoral immunity in controlling CHIKV replication [12,16], and in mice passively transferred antibodies protect against arthritogenic alphaviruses [17–20]. The role of T cells in CHIKV infection is still largely unknown. In humans, numbers of activated CD4+ and CD8+ T cells were found elevated in peripheral blood cells [21]. The presence of circulating CD8+ T cells was associated with the acute phase of infection, whereas CD4+ T cell responses develop at a later stage of infection [22]. More recently, CD4+ T cells have been implicated in pathology observed in the footpads of infected mice [23].
Currently, there is no licensed CHIKV vaccine or an effective anti-CHIKV therapy although there are several candidate vaccines are under investigation [24–31]. We developed a candidate CHIKV vaccine by employing an attenuation mechanism that also prevents the infection of potential mosquito vectors [18, 32]. This novel CHIKV/IRES vaccine is highly attenuated, immunogenic and efficacious after a single dose against CHIKV-LR [18] or the closely related o`nyong-nyong virus [20] in the A129 mouse model. However, the mechanism(s) by which this vaccine exerts its protective efficacy is largely unknown.
In this study, we sought to examine if CHIKV/IRES elicit a T cell response, determine the role that antibodies and/or cellular immunity play in protection, and establish correlates of protection using the A129 mouse model.
2. Materials and methods
2.1 Mice
129/Sv mice with null mutations in the IFN-α/β receptor (A129) were maintained at the animal isolation unit of Charmany instruction facility (UW-Madison School of Veterinary Medicine). All procedures were carried out in accordance with institutional and NIH guidelines for animal care and use. Despite the lack of a functional IFN response, 129/Sv mice do develop normal humoral and cellular T cell responses [33–35].
2.2 Viruses and vaccines
The CHIKV live-attenuated vaccine CHIKV/IRES was based on a novel attenuation strategy [32] relying on the inactivation of the subgenomic promoter, and the addition of an encephalomyocarditis virus (EMCV) internal ribosome entry sequence (IRES) to drive translation of the structural protein genes. The resulting virus is highly attenuated and incapable of infecting mosquitos. An infectious cDNA clone generated from the wild-type (wt) La Reunion strain (CHIKV-LR) was used as the genetic backbone. A detailed description of the construction and characterization of the CHIKV/IRES vaccine was previously reported [18]. CHIKV 181/25 and CHIKV-LR viruses used for microneutralization assays, and challenge, respectively were obtained from UTMB.
2.3 Virus titration
Confluent Vero cell monolayers were infected in quadruplicate with 100µl of ten-fold diluted virus in DMEM with 2%FBS and 1% P/S. Infected cells were incubated for 3 days at 37°C and 5% CO2 followed by fixation and staining with 10% crystal violet in 10% formalin. The plates were scored by observing cytopathic effects (CPE) under a microscope and the TCID50 titer was calculated by the method of Reed and Muench.
2.4 ELISA for anti-virus antibodies
Whole virus antigen was prepared by formalin inactivation and sucrose gradient purification of CHIKV/IRES. Purified viral antigen in 1× PBS was disrupted using 0.05% w/v Sarkosyl [36] prior to coating at 0.35 µg/ml on 96-well ELISA plates (Nunc Polysorp, Thermo Scientific) using 50µl/well. Plates were blocked with 1% BSA/PBS at room temperature (RT) for 1hr. The blocking buffer was discarded and anti-sera were serially diluted 2-fold in 1% BSA/PBST, and 50µl was added per well. Following incubation at RT for 1h, plates were washed and incubated at RT for 1hr with 50µl of goat anti-mouse IgG H+L conjugated with HRP, (Jackson Immuno Research) at 1:10,000 dilution. IgG isotypes were detected using rat anti-mouse IgG1, rabbit anti-mouse IgG2a, and rabbit anti-mouse IgG2b conjugated with HRP (Life Technologies™) at a dilution of 1:1,000. Plates were then washed six times with PBST and 100µl of TMB substrate (Novex®, Life Technologies™) was added/well. After 10 min incubation in the dark at RT, the reaction was stopped by adding 50µl of 1N HCl. Plates were read on an ELISA plate reader (BioTek® EL×800) at 450 and 630nm. The dilution which produced an OD value of ≥0.2 was considered as the titer.
2.5 Measurement of neutralizing antibodies
Serum samples were heat-inactivated at 56°C for 30 min and then serially diluted 2-fold in DMEM, 2% FBS, 1% P/S and mixed with an equal volume of 2000 TCID50/ml CHIKV 181/25 [25]. The serum/virus mixtures were incubated at 37°C and 5% CO2 for 1.5 hrs. Vero cells monolayers in 96 well plates were then inoculated with 100µl of the serum/virus mixture in triplicate. Anti-CHIKV positive, media only and virus only controls were included in the assay. Plates were incubated at 37°C and 5% CO2 for 3 days and then fixed and stained with crystal violet as above. They were scored by observing CPE per well under a microscope and the titer was defined as the dilution of serum required to neutralize 50% or more of the virus infected wells.
2.6 Analysis of viremia
Viremia analysis was performed in BSL-3. Mouse sera collected on day 3 post-wtCHIKV-LR challenge were diluted ten-fold in DMEM containing 2% FBS and 1% P/S. Vero cells seeded in 96 well tissue culture plates were infected in triplicates with 100µl of each dilution of serum. Cells were incubated for 3 days at 37°C in the presence of 5% CO2, and then fixed and stained with 10% crystal violet in 10% formalin. Scored was performed by observing CPE per well under a microscope. TCID50 titers were calculated by the method of Reed and Muench.
2.7 Flow cytometry
A129 mice immunized subcutaneously (s.c.) with 105 TCID50 of CHIKV/IRES or control mice injected with PBS (3 mice/group) were euthanized 10 weeks post vaccination. Spleens were processed into single splenocyte cell suspensions and red blood cells were lysed with BD Pharm Lyse™ buffer. Cells were washed and suspended in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 I.U./ml penicillin, 100 µg/ml streptomycin and 0.14mM of β-mecaptoethanol. For ex vivo phenotypic analysis, 1×106 splenocytes were surface stained with anti-mouse CD4 FITC (RM4-5), anti-mouse CD8a PerCP (53-6.7), anti-human/mouse CD44 APC (IM7, eBioscience), anti-mouse CD62L PE (MEL-16) and anti-CD69 PE (H1.2F3).
To study intracellular cytokine responses, 1×106 splenocytes were plated to a 96-well flat-bottom plate and stimulated with different concentrations of inactivated or live CHIKV/IRES virus (in 200 µl) for 24h. During the final 5hr of incubation, BD GolgiPlug (Bredfeldin A) was added at a final concentration of 1µg/ml to block protein transport. Cells were stained intracellularly for IFN-γ APC (XMG1.2), IL-2 PE (JES6-5H4) and TNF-α PE (MP6-XT22) after surface staining of CD4 FITC and CD8a PerCP. All antibodies were from BD Bioscience except where it was noted. All samples were acquired on a BD FACSCalibur and analyzed with FlowJo v7.6.5 (Tree Star). The cytokine background from medium-treated groups was subtracted from each sample. The frequency of cytokine-positive T cells was presented as the percentage of gated CD4+ or CD8+ T cells.
2.8 Depletion studies
For depletion studies groups of 8–10 weeks old A129 mice (n=5), were vaccinated s.c. with 105 TCID50 CHIKV/IRES. On days 44 and 47 post priming, immune mice were treated i.p. with 100 µg of anti-CD4 mAb (GK1.5), or 250 µg of anti-CD8a mAb (2.43) or both mAbs (Bio × Cell). Control groups included untreated immune and non-immune animals. Each treatment group was challenged intradermally (footpad) with 100 PFU CHIKV-LR three days later. Mice were monitored for morbidity and mortality for two weeks.
2.9 Adoptive transfer of CD4+ and CD8+ T cells and challenge with CHIKV-LR
Groups of 6–8 weeks old A129 mice (n=8), were vaccinated s.c. with 105 TCID50 CHIKV/IRES. Ten weeks post-priming, spleens were harvested and pooled. CD4+ and CD8+ T cells were negatively isolated using the Miltenyi T cell isolation kit II according to the manufacturer’s instructions. The purity of isolated CD4+ and CD8+ T cells was 90.6% and 88.4%, respectively as assessed by flow cytometry after cell surface staining with anti-mouse CD4 FITC (RM4-5) and anti-mouse CD8a PerCP (53-6.7). Isolated cells (2.0 ×106/mouse) in 100µl volume were adoptively transferred via the retro-orbital sinus under light anesthesia into groups of five naïve A129 mice (6–8 weeks old). Mice were challenged 24hr later as described above. Serum samples collected on day 3 post challenge were tested for viremia. Mice were monitored for morbidity and mortality for two weeks.
2.10 Passive transfer of anti-CHIKV/IRES immune serum and challenge with CHIKV-LR
Groups of A129 mice (5 mice/group) aged 12–14 weeks old were injected intraperitoneally (i.p.) with 200 µl of either undiluted or 1:5, 1:10, 1:20 or 1:40 diluted serum pooled from CHIKV/IRES immunized mice. Dilutions of sera for passive transfer were done using 1× PBS. A control group of five A129 mice was injected with 200 µl of normal A129 mouse serum. Mice were bled 24 hr following passive transfer, to determine the titer of circulating anti-CHIKV neutralizing antibodies and then challenged and monitored as described in 2.8. Serum samples collected on day 3 post-challenge were tested for viremia.
2.11 Statistics
All data were analyzed with GraphPad Prism5 software. P values are reported in the legends of figures.
3. Results
3.1 Humoral and cellular immune responses to CHIKV/IRES
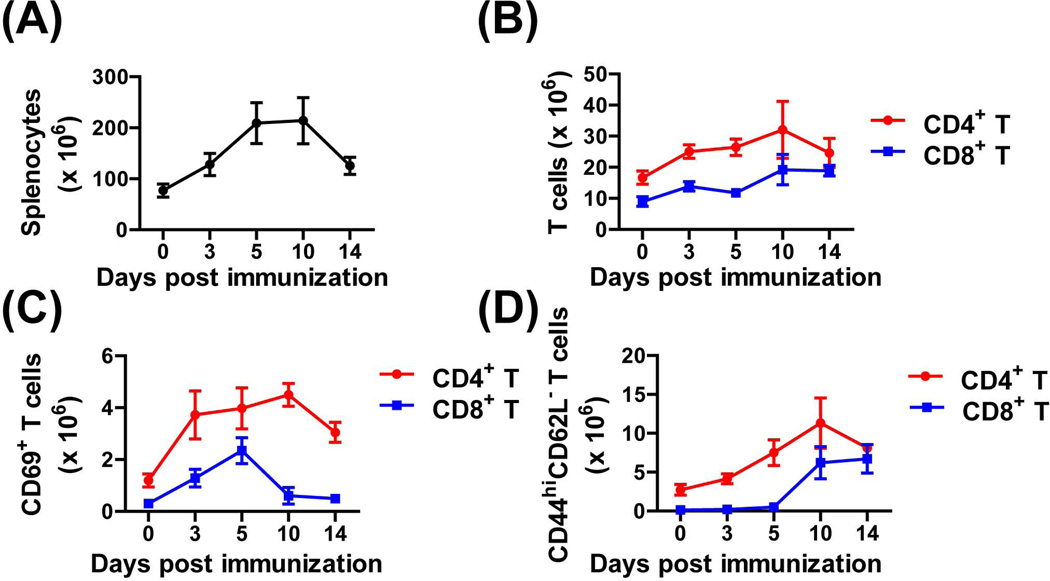
Following CHIKV/IRES immunization we examined the kinetics of T cell activation at different time points. As shown in Figure 1A, spleen cellularity increased after vaccination on day 3, peaked on day10, and gradually returned to normal levels by day14. Absolute numbers of CD4+ and CD8+ T cells in the spleen were also increased and peaked on day10 post vaccination (Fig. 1B). The expansion of CD4+ and CD8+ T cells was accompanied by the upregulation of the early T cell activation marker CD69+ (Fig. 1C). While the percentage of CD4+CD69+ T cells peaked on day10, the CD8+CD69+ peaked earlier on day 5 and returned to basal level by day14 (Fig. 1C). Mature activated CD4+ and CD8+ T cells that were CD44hiCD62L- gradually increased and peaked on day10 post-vaccination for both T cell subsets (Fig. 1d). Moreover, their levels remained high even 10-weeks post-vaccination (Fig. 2A and B).
Fig.1.
CD4+ and CD8+ T cell activation and expansion kinetics following immunization with CHIKV/IRES. A129 mice were vaccinated s.c. with 105 TCID50 of CHIKV/IRES. On day 3, 5, 10, and 14 post vaccination, spleens were collected and single splenocytes suspension were stained ex vivo for CD4 and CD8a, CD69, CD44 and CD62L. The total spleen cellularity (A), absolute CD4+ and CD8+ T cells (B), early activated CD69+ T cells (C) as well as mature activated CD44hiCD62L- T cells numbers (D) are shown. Data were presented as mean ± SD (3 mice per group).
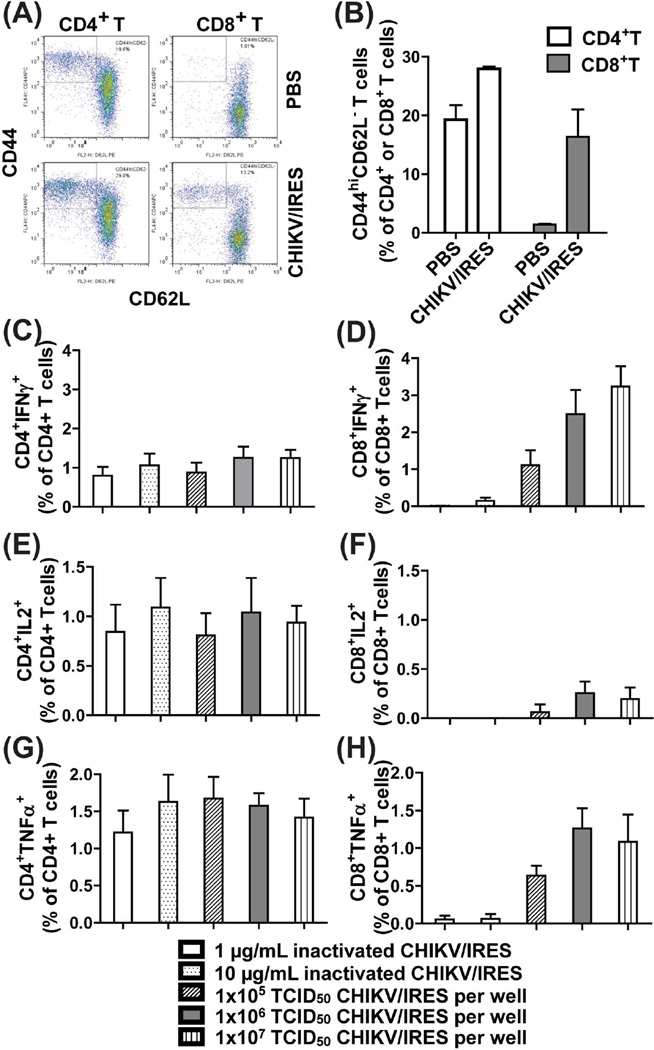
Fig.2.
CHIKV/IRES induced T cell responses. (A–B) CHIK/IRES induced sustained T cell activation (CD44hiCD62-) of CD4+ and CD8+ T cells in A129 mice 10-weeks post vaccination. Cells were stained as described in Figure1. (A) Representative flow cytometry dot plots of activated T cells. (B) The relative activated CD44hiCD62L- T cells were gated among CD4+ or CD8+ T cells. p=0.004 for CD4+ T cells and p=0.005 for CD8+ T cells, two-tailed unpaired T-test was used to compare PBS and CHIKV/IRES vaccinated groups. (C–H) CHIKV/IRES elicited CHIKV-specific CD4+ and CD8+ T cell responses. Ten-weeks post CHIKV/IRES vaccination, splenocytes were restimulated in vitro with inactivated (0.1 and 10 µg/ml) or live (1×105 to 1×107 TCID50/culture) CHIKV/IRES for 24 hr. Cells were stained and analyzed by flow cytometry for intracellular IFN-γ (C–D), IL-2 (E–F) and TNF-α (G–H). T cells from PBS immunized animals did not produce any cytokine responses under the same condition (the net values after subtracting medium background were < or =zero). The cytokine background from medium-treated groups was subtracted from each sample. Data were presented as mean ± SD (3 mice per group).
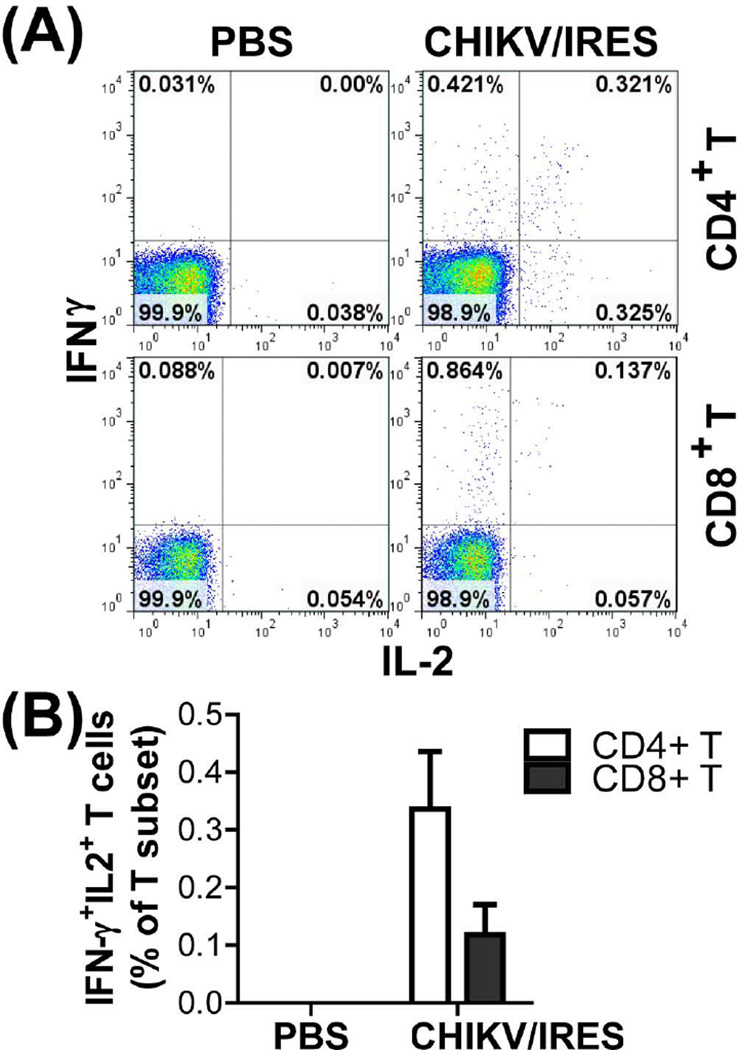
CHIKV/IRES-specific T cell responses were measured using intracellular cytokine staining assay in which T cells that produce IFN-γ, IL-2, and TNF-α in response to in vitro restimulation with either live or inactivated CHIKV/IRES. As shown in Fig. 2C–H, CHIKV/IRES immune CD8+ T cells showed dose-dependent IFN-γ responses upon in vitro restimulation with live CHIKV/IRES virus but not with inactivated virus. By contrast, CHIKV/IRES immune CD4+ T cells had a much lower IFN-γ response but higher TNF-α and IL-2 production. T cell responses elicited by CHIKV/IRES were multifunctional with double positive IFNγ+IL2+ CD4+ and CD8+ T cells (Fig. 3). CD4+ or CD8+ T cells from PBS control mice did not show any cytokine producing positive cells (data not shown).
Fig.3.
CHIKV/IRES elicited CHIKV-specific multifunctional IFNγ+IL2+ T cells. Ten-weeks post CHIKV/IRES or sham PBS vaccination, splenocytes were restimulated in vitro with live 1×106 TCID50/culture of CHIKV/IRES or medium for 24 hr as that in Figure 2. (A) Reprehensive flow cytometry dot plots of IFNγ+IL2+ double-positive cells among gated CD4+ or CD8+ T cells. (B) The relative percentage of IFNγ+IL2+ T cells among gated CD4+ or CD8+ T cells. Data were presented as mean ± SD (3 mice per group). P=0.0044 for CD4+ T cells and P=0.017 for CD8+ T cells based on two-tailed unpaired t-test when PBS and CHIV/IRES vaccinated groups were compared.
A single dose of CHIKV/IRES vaccine administered s.c. elicited a strong anti-CHIKV antibody response four weeks post vaccination with the IgG1, IgG2a, and IgG2b isotypes detected in the serum (Fig. 4A). Neutralizing antibody responses reached a mean titer of 1152 (four weeks post-vaccination), and remained high up to eight weeks post- vaccination (Fig. 4B).
Fig.4.
Humoral responses induced by CHIKV/IRES. (A) ELISA assays of IgG isotypes of anti-CHIKV/IRES serum. Average ODs from five individual serum samples collected on day 27 are presented. The vaccine induced dominant anti-CHIKV IgG2a isotype followed with IgG2b than IgG1 indicating the involvement of Th1 response. NMS-Normal mouse serum. (B) Anti-CHIKV neutralizing antibody titers in A129 mice are after immunization with a single dose of 105 TCID50 of CHIKV/IRES. Serum collected from individual mice on day 27 and day 55 post-vaccination was subjected to microneutralization test based on the detection of cytopathic effect (n=5 mice per group). The data were presented as geometric mean ± SD. Normal A129 mouse serum neutralizing antibody titers were < 5 (data not shown).
3.2 Role of humoral and cellular immunity to CHIKV/IRES in protection against wt CHIKV-LR challenge
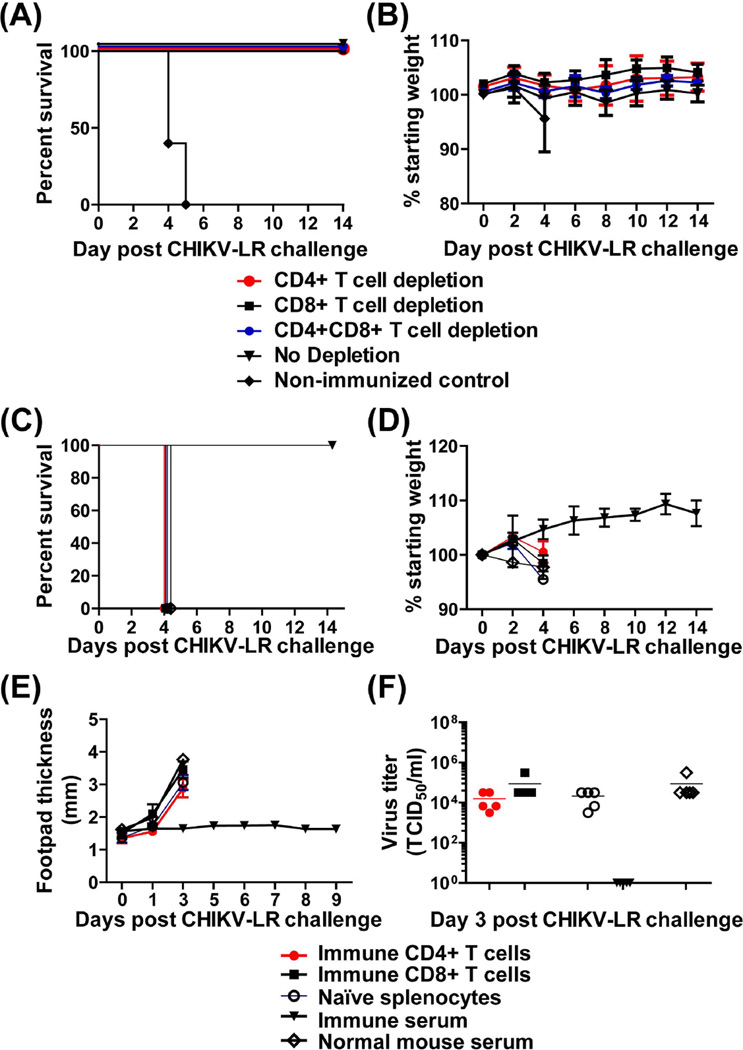
To determine the role of CHIKV/IRES-specific T cells in protection we depleted the CD4+ and/or CD8+ T cells prior to challenge with wtCHIKV-LR. As shown in Figures 5A and B, depleted immune animals remained healthy until the end of the monitoring period as did the control immune undepleted animals. In contrast, non-immune mice succumbed to infection. In addition, we conducted adoptive transfer studies of immune T cells. Figures 5C–F show that adoptively immunized mice or control animals when challenged with CHIKV-LR developed clinical signs of disease (hunched posture, ruffled appearance, footpad swelling) and had detectable viremia. By day 4, all mice became moribund (unable to move, lethargic) and were euthanized (Fig.5C–F). In contrast, positive control mice that received immune serum prior to challenge were fully protected (Fig. 5C–F).
Fig.5.
Role of T cells in protection against wtCHIKV-LR challenge. Mortality (A) and morbidity (B) of CHIKV/IRES immune A129 mice (n=5) following T cell depletion. Six weeks post CHIKV/IRES immunization, A129 mice were depleted of CD4+ or CD8+ T cells or both T cell subsets. Immune depleted and non-depleted animals, including control non immune animals were then challenged with wtCHIV-LR as described in materials and methods. T cell depleted groups showed no difference in survival when compared to the non-depleted group. All mice survived (p=0.0023) as compared to the non-immune control group. (C–F) Mortality and morbidity of A129 mice following adoptive transfer of CHIKV/IRES immune T cells. Ten-weeks post-CHIKV/IRES vaccination immune CD4+ and CD8+ T cells were isolated and 2×106 purified cells were adoptively transferred to groups of A129 naïve mice (n=5). Control mice received naïve splenocytes, or passively immunized with CHIKV/IRES immune serum or non-immune serum (n=5). 24h later, all mice were challenged intradermally (footpad) with 100 PFU of CHIKV-LR. Mice were monitored for 2 weeks for survival (C), weight loss (D) and foot pad swelling (E). Error bars represent standard deviation (SD). (F) Mice were bled on day 3 post challenge and serum samples were tested for viremia by TCID50.
3.3 Minimum neutralizing antibody titers required for protection
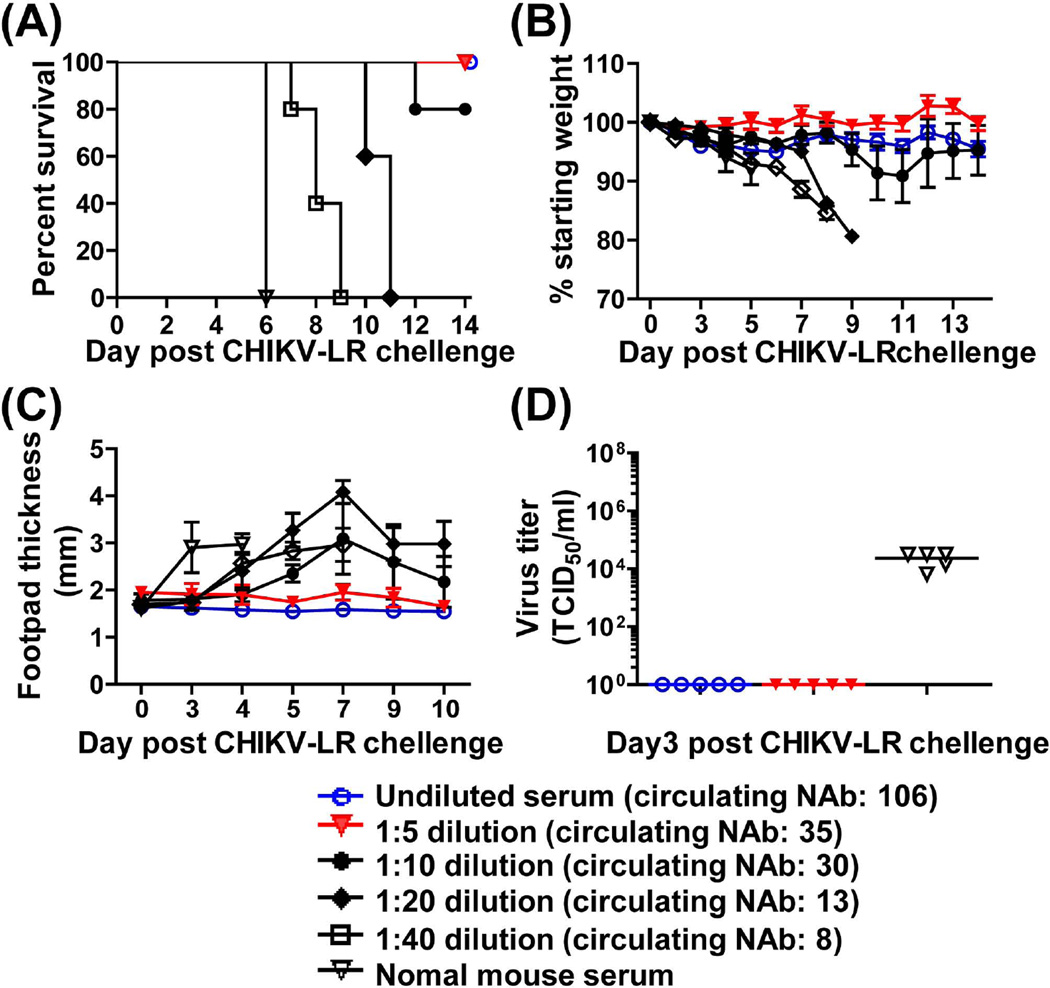
Since protection by CHIKV/IRES appears to be predominantly mediated by neutralizing antibodies we sought to determine the minimum protective neutralizing antibody. Dilutions of pooled anti-CHIIKV/IRES immune serum (collected from various bleeds post primary immunization; neutralizing titer of 640) were passively transferred into naïve A129 mice that were then challenged with wtCHIKV-LR, 24hrs later. As shown in Figure 6A, a minimum neutralizing antibody titer of 35 in recipient mice was sufficient to confer complete protection with no clinical signs of disease as compared to the control animals that succumbed to infection (Fig. 6B and C). Sera from fully protected animals had also no detectable viremia. In contrast, sera of mice passively immunized with normal mouse serum had high levels of viremia 3 days post-challenge (Fig. 6D).
Fig.6.
The morbidity and mortality and day3 viremia of mice after passive transfer of serial diluted CHIKV/IRES immune serum. A129 mice (n=5) were administrated i.p. with 200 µl of undiluted, 1:5, 1:10, 1:20, 1:40 diluted serum pooled from CHIKV/IRES immunized mice, or normal A129 mouse serum as the negative control. Mice were challenged I.D. (footpad) with 100 PFU of CHIKV-LR 24 hr later, and monitored for 2 weeks for survival (A), weight (B) and foot pad swelling (C). Numbers in parenthesis indicate the neutralization titers of circulating antibodies (NAb) following passive transfer of diluted serum. (D) Mice were bled on day 3 post challenge and serum samples were tested for viremia. When survival curves of mice receiving immune serum were compared to the control group that received the non-immune serum (A) they were found to be significantly different (p=0.0027) based on the Long-rank test. In addition, the Long-rank test for trend was significant (p<0.0001) indicating that survival was dose-dependent.
4. Discussion
Our findings demonstrate that the CHIKV/IRES vaccine elicit humoral and cellular immune responses, and during the acute phase of CHIKV infection humoral immunity is the major mediator of protection.
CHIKV infection is an acute illness that generally is not considered life-threatening. However, severe forms with neurological and hepatological complications could lead to fatalities [7]. Despite the fact that the immune responses to CHIKV are not well documented, recent findings suggest that following infection in humans, the innate immunity plays a critical role in controlling virus replication [22, 37, 38]. This is followed by the activation of adaptive immunity [22]. In the A129 mouse model, in the absence of an IFN-α/β response, IFN-γ is able to partially control replication of an attenuated CHIKV [17], suggesting that although the IFN-γ response is subordinate to IFN-α/β in terms of antiviral activity it contributes to the control of viral replication and spread. Consistent with the proposed model of human immune response to CHIKV, we demonstrated here that immunization of A129 mice with CHIKV/IRES results in the activation of both CD4+ and CD8+ T cells (CD69+ and CD44hiCD62L-) that are maintained at high levels for at least10-weeks post immunization. Further analysis of the T cell responses highlight that both CD4+ and CD8+ T cell subsets exhibit a proinflammatory cytokine profile producing IFN-γ, TNF-α and IL-2 upon restimulation with CHIKV/IRES. In addition, both T cell subsets appear to be multifunctional as judged by the double IFN-γ+IL-2+ cytokine positive cells. These findings are in agreement with recent observations demonstrating the induction of CHIKV-specific CD4+ and CD8+ T cells producing IFN-γ following CHIKV infection of C57BL/6 mice [23].
Although CHIKV-induced T cells have been isolated from human patients [21, 22] their direct role in controlling infection is not well known. We show here that in the absence of a functional IFN-α/β response, T cells may not play a significant role in protection against acute CHIKV infection. This inference was supported; i) by depletion studies that had no negative impact in protection of immune mice, and ii) by adoptive transfer studies that showed no detectable protective efficacy by either T cell subset. These findings are in line with recent observations that neither CD4+ nor CD8+ T cells are needed to control CHIKV viremia in C57BL/6 mice [23]. Interestingly, CD4+ T cells were recently shown to play a pathogenic role contributing to the joint swelling during the early CHIKV infection stage [23]. In general, activated CD8+ T cells are implicated in controlling virus infected cells as was shown with persistently infected macrophages with Ross River virus [39], whereas CD4+ T cells are predominantly associated with the development of humoral immunity.
Our studies have also shown that CHIKV/IRES elicit strong neutralizing antibody response consisting all IgG isotypes detected in the serum. Passive transfer of anti-CHIKV/IRES immune serum with a minimum circulating titer of 35 was required for full protection against wtCHIKV-LR challenge. Several epidemiological studies have suggested the protective role of antibodies against CHIKV [12–15], and recent therapeutic approaches based on passively transferred neutralizing antibodies highlighted their protective effect [12, 16]. The protection afforded by antibodies could be attributed to their capacity to directly neutralize CHIKV and/or to induce other protective immune responses such as antibody-dependent, and complement– mediated cellular cytotoxicity. In general, our findings are in agreement with data demonstrating protection by passively transferred antibodies as shown in the case of the antigenically related RRV and ONNV [19, 20], and suggest that low levels of antibody responses might be sufficient in mediating effective protection.
In conclusion, our findings demonstrate the immunogenic potential of CHIKV/IRES vaccine, and highlight the important role that neutralizing antibodies play in protecting against acute CHIKV infection.
Highlights.
A single dose of CHIKV/IRES vaccine elicits neutralizing antibody and virus-specific T cell responses
CHIKV/IRES immune CD4+ or CD8+ T cells do not protect A129 mice against CHIKV LR challenge
A correlate of a neutralizing antibody titer and protection has been established.
Acknowledgments
We gratefully thank Erin Plisch for helping with the Flow cytometry. This research was supported by NIH grant 1 R01 AI093491-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 2.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Bréhin AC, Cubito N, Desprès P, Kunst F, Rey FA, Zeller H, Brisse S. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laras K, Sukri NC, Larasati RP, Bangs MJ, Kosim R, Djauzi, et al. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg. 2005;99:128–141. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Mavalankar D, Shastri P, Raman P. Chikungunya epidemic in India: a major public-health disaster. Lancet Infect Dis. 2007;7:306–307. doi: 10.1016/S1473-3099(07)70091-9. [DOI] [PubMed] [Google Scholar]
- 5.Grandadam M, Caro V, Plumet S, Thiberge JM, Souarès Y, Failloux AB, et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17:910–913. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poletti P, Messeri G, Ajelli M, Vallorani R, Rizzo C, Merler S. Transmission potential of chikungunya virus and control measures: the case of Italy. PLoS One. 2011;6:e18860. doi: 10.1371/journal.pone.0018860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pialoux G, Gaüzère BA, Jauréguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 8.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012;379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 9.Dupuis-Maguiraga L, Noret M, Brun S, Le Grand R, Gras G, Roques P. Chikungunya disease: infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia. PLoS Negl Trop Dis. 2012;6:e1446. doi: 10.1371/journal.pntd.0001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8:491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- 11.Voss JE, Vaney MC, Duquerroy S, Vonrhein C, Girard-Blanc C, Crublet E, Thompson A, Bricogne G, Rey FA. Glycoprotein organization of chikungunya virus particles revealed by X-ray crystallography. Nature. 2010;468:709–712. doi: 10.1038/nature09555. [DOI] [PubMed] [Google Scholar]
- 12.Couderc T, Khandoudi N, Grandadam M, Visse C, Gangneux N, Bagot S, et al. Prophylaxis and therapy for chikungunya virus infection. J Infect Dis. 2009;200:516–523. doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kam YW, Lee WW, Simarmata D, Harjanto S, Teng TS, Tolou H, et al. Longitudinal analysis of the human antibody response to chikungunya virus infection: implications for serodiagnosis and vaccine development. J Virol. 2012;86:13005–13015. doi: 10.1128/JVI.01780-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, et al. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J Infect Dis. 2012;205:1147–1154. doi: 10.1093/infdis/jis033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kam YW, Lum FM, Teo TH, Lee WW, Simarmata D, Harjanto S, et al. Early neutralizing IgG response to chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol Med. 2012;4:330–343. doi: 10.1002/emmm.201200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fric J, Bertin-Maghit S, Wang CI, Nardin A, Warter L. Use of human monoclonal antibodies to treat chikungunya virus infection. J Infect Dis. 2013;207:319–322. doi: 10.1093/infdis/jis674. [DOI] [PubMed] [Google Scholar]
- 17.Partidos CD, Weger J, Brewoo J, Seymour R, Borland EM, Ledermann JP, et al. Probing the attenuation and protective efficacy of a candidate chikungunya virus vaccine in mice with compromised interferon (IFN) signaling. Vaccine. 2011;29:3067–3073. doi: 10.1016/j.vaccine.2011.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plante K, Wang E, Partidos CD, Weger J, Gorchakov R, Tsetsarkin K, et al. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 2011;7:e1002142. doi: 10.1371/journal.ppat.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holzer GW, Coulibaly S, Aichinger G, Savidis-Dacho H, Mayrhofer J, Brunner S, et al. Evaluation of an inactivated Ross River virus vaccine in active and passive mouse immunization models and establishment of a correlate of protection. Vaccine. 2011;29:4132–4141. doi: 10.1016/j.vaccine.2011.03.089. [DOI] [PubMed] [Google Scholar]
- 20.Partidos CD, Paykel J, Weger J, Borland EM, Powers AM, Seymour R, et al. Cross-protective immunity against o'nyong-nyong virus afforded by a novel recombinant chikungunya vaccine. Vaccine. 2012;30:4638–4643. doi: 10.1016/j.vaccine.2012.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184:5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 22.Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi-Mbiguino A, Leroy EM. The acute phase of chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. 2011;204:115–123. doi: 10.1093/infdis/jiq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teo TH, Lum FM, Claser C, Lulla V, Lulla A, Merits A, et al. A pathogenic role for CD4+ T cells during chikungunya virus infection in mice. J Immunol. 2013;190:259–269. doi: 10.4049/jimmunol.1202177. [DOI] [PubMed] [Google Scholar]
- 24.Wang E, Volkova E, Adams AP, Forrester N, Xiao SY, Frolov I, Weaver SC. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26:5030–5039. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Jr, Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;3:157–162. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- 26.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed Chikungunya vaccine. J Immunol. 1971;107:643–647. [PubMed] [Google Scholar]
- 27.Tiwari M, Parida M, Santhosh SR, Khan M, Dash PK, Rao PV. Assessment of immunogenic potential of Vero adapted formalin inactivated vaccine derived from novel ECSA genotype of Chikungunya virus. Vaccine. 2009;27:2513–2522. doi: 10.1016/j.vaccine.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 28.Muthumani K, Lankaraman KM, Laddy DJ, Sundaram SG, Chung CW, Sako E, Wu L, Khan A, Sardesai N, Kim JJ, Vijayachari P, Weiner DB. Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus. Vaccine. 2008;26:5128–5134. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akahata W, Yang Z-Y, Andersen H, Sun S, Holdaway HA, Kong W-P, Lewis MG, Higgs S, Rossmann MG, Rao S, Nabel GJ. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Suhrbier A, Penn-Nicholson A, Woraratanadharm J, Gardner J, Luo M, Le TT, Anraku I, Sakalian M, Einfeld D, Dong JY. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine. 2011;29:2803–2809. doi: 10.1016/j.vaccine.2011.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chattopadhyay A, Wang E, Seymour R, Weaver SC, Rose JK. A chimeric vesiculo/alphavirus is an effective alphavirus vaccine. J Virol. 2013;87:395–402. doi: 10.1128/JVI.01860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkova E, Frolova E, Darwin J, Forrester NL, Weaver SC, Frolov I. IRES-dependent replication of Venezuelan equine encephalitis virus makes it highly attenuated and incapable of replicating in mosquito cells. Virology. 2008;377:160–169. doi: 10.1016/j.virol.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, et al. A protective role for dengue virus-specific CD8+ T cells. J Immunol. 2009;182:4865–4873. doi: 10.4049/jimmunol.0801974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yauch LE, Prestwood TR, May MM, Morar MM, Zellweger RM, Peters B, et al. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J Immunol. 2010;185:5405–5416. doi: 10.4049/jimmunol.1001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zompi S, Santich BH, Beatty PR, Harris E. Protection from secondary dengue virus infection in a mouse model reveals the role of serotype cross-reactive B and T cells. J Immunol. 2012;188:404–416. doi: 10.4049/jimmunol.1102124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das SC, Hatta M, Wilker PR, Myc A, Hamouda T, Neumann G, et al. Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine. Vaccine. 2012;30:6871–6877. doi: 10.1016/j.vaccine.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schilte C, Couderc T, Chretien F, Sourisseau M, Gangneux N, Guivel-Benhassine F, et al. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J Exp Med. 2010;207:429–442. doi: 10.1084/jem.20090851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Her Z, Malleret B, Chan M, Ong EK, Wong SC, Kwek DJ, et al. Active infection of human blood monocytes by chikungunya virus triggers an innate immune response. J Immunol. 2010;184:5903–5913. doi: 10.4049/jimmunol.0904181. [DOI] [PubMed] [Google Scholar]
- 39.Linn ML, Mateo L, Gardner J, Suhrbier A. Alphavirus-specific cytotoxic T lymphocytes recognize a cross-reactive epitope from the capsid protein and can eliminate virus from persistently infected macrophages. J Virol. 1998;72:5146–5153. doi: 10.1128/jvi.72.6.5146-5153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]