Abstract
In the present work, the concentrations of Aβ11-x and Aβ17-x peptides (x=40 or 42), which result from the combined cleavages of β-amyloid precursor protein (AβPP) by β'/α or α/γ-secretases, respectively, were assessed in cerebrospinal fluid (CSF) samples from patients with Alzheimer's disease (AD) or mild cognitive impairment (MCI). Specific multiplexed assays were set up using new anti-40 and anti-42 monoclonal antibodies (mAbs) for the capture of these N-truncated Aβ peptides and anti-11 or anti-17 mAbs for their detection. The specificity, sensitivity and reproducibility of such assays were assessed using synthetic peptides and human cell models. Aβ11-x and Aβ17-x were then measured in CSF samples from patients with AD (n=23), MCI (n=23) and controls with normal cognition (n=21). Aβ11-x levels were significantly lower in patients with MCI than in controls. Compared with the combined quantification of Aβ1-42, total Tau (T-Tau) and phosphorylated Tau (P-Tau; AlzBio3, Innogenetics), the association of Aβ11-40, Aβ17-40 and T-Tau improved the discrimination between MCI and controls. Furthermore, when patients with MCI were classified into two subgroups (MCI ⩽1.5 or ⩾2 based on their CDR-SB (Cognitive Dementia Rating–Sum of Boxes) score), the CSF Aβ17-40/Aβ11-40 ratio was significantly higher in patients with CDR-SB ⩽1.5 than in controls, whereas neither Aβ1-42, T-Tau nor P-Tau allowed the detection of this subpopulation. These results need to be confirmed in a larger clinical prospective cohort.
Keywords: amyloid peptide, biomarkers, prodromal, secretase, truncated
Introduction
Alzheimer's disease (AD) is the most common form of dementia and is characterized by loss of memory and progressive cognitive impairment. The major histopathological hallmarks of AD are extracellular senile plaques, which mainly consist of β-amyloid peptides (Aβ),1 and intracellular neurofibrillary tangles, which are mostly composed of hyperphosphorylated microtubule-associated Tau protein.2, 3 Accumulation of Aβ peptide aggregates could lead to hippocampal synaptic dysfunction,4 thereby explaining the AD memory deficits. Episodic memory loss is generally considered as the core requirement for the diagnosis of mild cognitive impairment (MCI).5, 6 Early and reliable AD diagnosis at the stage of MCI would improve AD prognosis and provide the means to examine the putative efficacy of newly designed drugs as disease modifiers. Today, the combined measurement of total Tau (T-Tau), phosphorylated Tau (P-Tau) and Aβ1-42 in cerebrospinal fluid (CSF) allows the best biochemical characterization of the patients' clinical status, even from a prognostic point of view.7, 8, 9, 10, 11, 12 However, despite their good diagnostic performance, we clearly need complementary biomarkers to differentiate between AD and non-AD disorders,13, 14, 15 particularly at early stages (MCI).
In normal conditions, the β-amyloid precursor protein (AβPP) mainly undergoes a nonamyloidogenic cleavage by α-secretase activity that precludes Aβ generation.16 Conversely, in the amyloidogenic pathway, AβPP is sequentially cleaved by the β-secretase BACE1 and by the γ-secretase proteolytic complex to produce various Aβ peptides, including the full-length (fl) species Aβ1-40 and Aβ1-42.16, 17 Besides flAβ peptides, many N- and C-terminally truncated variants have also been identified and isolated from cell supernatants, animal models and brain extracts from patients with AD,18, 19, 20, 21, 22 and they could have escaped immunodetection in the CSF because of technical limitations. This is not anecdotal as within this plethoric Aβ-linked peptidome, several Aβ truncated variants could have physiopathological and diagnostic relevance. For instance, N-truncated peptides at residue 11 of flAβ (Aβ11-x) results from BACE1-mediated β'-cleavage23 and might be seen as an indicator of β-secretase-associated AβPP processing that could happen in pathological conditions.24 Aβ17-x variants result from α-secretase activity and could also be revelatory of a pathological situation, because α-secretase activity is apparently downregulated in AD.25, 26, 27, 28
Here, to evaluate Aβ11-x and Aβ17-x levels in complex fluids, including human CSF, we describe new specific multiplexed assays based on the capture of the different Aβ peptides by new specific anti-C-terminal (Cter) monoclonal antibodies (mAbs; 6H7 anti-40 antibody and 12E8 anti-42 antibody) and their detection by very specific anti-N-terminal mAbs (7H1 anti-11 antibody and 8H5 anti-17 antibody) that were previously obtained and characterized.24 We then assessed the ability of these assays to monitor CSF Aβ11-x and Aβ17-x levels at very early AD stages and show that, unlike the currently used assays, the Aβ17-40/Aβ11-40 ratio allows discriminating between patients with very early MCI and controls. Although the number of patients was limited, our study indicates that additional N-truncated Aβ-related fragments could be used as biomarkers of AD pathology onset.
Materials and methods
Peptide synthesis
The immunogenic peptide C-KKKGS-Aβ33-42 used for the production of the anti-42 antibody included the 10 C-terminal amino acids of human Aβ starting at residue #33 (33GLMVGGVVIA42, referred to as Aβ33–42). The immunogenic peptide C-KKKGS-PADRE-Aβ31-40 used for anti-40 antibody production comprised the 10 C-terminal amino acids of human Aβ starting at residue #31 (31IIGLMVGGVV40, referred to as Aβ31–40). The PADRE sequence (pan HLA-DR epitope; sequence: aK(X)VAAWTLKAAa, where X=L-cyclohexylalanine and a=D-amino acid) can bind to C57BL/6 mouse MHC-II molecules (H-2b haplotype) and elicit the T helper type 2 response.29, 30 Additional details on their synthesis, purification and integrity analysis are described in Supplementary Information. Both peptides were coupled via their N-terminal cysteine residue to maleimide-activated mcBSA (#77607 Pierce Conjugation Kit, Rockford, IL, USA) for immunization.
Generation of antibody-producing hybridoma clones
Experimental protocols requiring the use of mice were reviewed by the Institutional Animal Ethics Committee (Sysdiag HT-Mab facility, Montpellier, France). The detailed description of the immunization protocol, hybridoma production with the Sp2/0Ag14 myeloma cell line and mAb purification are in Supplementary Information. Clone selection (specific reactivity toward the relevant biotinylated peptide and absence of reactivity against the other biotinylated peptide) was done by sandwich enzyme-linked immunosorbent assay based on the capture of N-terminally biotinylated Aβ1-40 or Aβ1-42 (AnaSpec, Fremont, CA, USA) on streptavidin-coated plaques and their detection by hybridoma supernatants and goat anti-Fc antibodies (Sigma, St Louis, MO, USA). Specific anti-40 (6H7 clone) and anti-42 (12E8 clone) antibodies were selected, amplified and purified on protein A Sepharose columns (GE Healthcare, Piscataway, NJ, USA).
X-MAP assays
All Aβ peptides were purchased from AnaSpec as lyophilized powder, solubilized in dimethyl sulphoxide (2 mg ml−1) and conserved at −20 °C. Standard aliquots (2 μg ml−1 in dimethyl sulphoxide) were prepared and stored at −20 °C. For test reproducibility, a new aliquot was used for each experiment and was not kept after standard reconstitution in Dulbecco's modified Eagle's medium/1% foetal calf serum.
Different multiplexed Cter assays, which allow the capture of peptides via their C-terminus, were designed to measure the concentration of truncated Aβ peptides. Carboxylated magnetic beads from different microsphere numbers were chemically coupled with anti-40 6H7, anti-42 12E8 or IRR (irrelevant) antibodies and coupling evaluated with phycoerythrin-coupled goat anti-mouse IgGs (Jackson Immunoresearch, Suffolk, UK). For truncated peptide detection, the anti-11 7H1 and anti-17 8H5 mAbs24 were used as detection antibodies after biotinylation (EZ-link Micro Biotinylation Kit, Pierce). Two different Cter sandwich assays were designed, based on the same bead combination (the 6H7/12E8/IRR triplex), to detect either 11-x or 17-x species (x=40 or 42) depending on the used detection antibody.
CSF Aβ1-42, T-Tau and P-Tau concentrations were measured with the AlzBio3 multiplex assay (Innotest, Innogenetics, Gent, Belgium).
CSF samples
Human CSF samples from age-matched patients with AD (n=23) or MCI (n=23) and donors with normal cognition (controls, n=21) were provided by PrecisionMed (San Diego, CA, USA31). The clinical protocol, consent forms and CSF registry were approved by the Western Institutional Review Board located in Washington, USA. Subjects with Mini-Mental State Examination (MMSE) score >13 to <28 signed the approved written informed consent and agreed to blood sampling by venipuncture (<90 ml blood) and CSF collection (<25 ml) by lumbar puncture every 6 months. The age at enrolment was >50 years and previous (within 2 years) brain scans excluded other pathologies as cause of dementia/memory disorder. Exclusion criteria were (1) evidence of multi-infarct dementia and drug intoxication, thyroid disease, pernicious anaemia, tertiary syphilis, chronic infections of the nervous system, normal pressure hydrocephalus, Huntington's disease, Creutzfeldt–Jakob disease, brain tumours, polypharmacy and Korsakoff's syndrome; (2) life expectancy <3 years; and (3) any contraindication to lumbar puncture, including anticoagulant therapy and subjects taking aspirin, aspirin-containing products or non-steroidal anti-inflammatory products, within 1 week from lumbar puncture. The probable AD classification was based on the NINCDS-ADRDA criteria:32 MMSE ⩾13 and ⩽26; deficit in two or more areas of cognition; no consciousness disturbance; onset between 40 and 90 years, generally after the age of 65; and absence of systemic disorders or other brain disease that could account for the cognitive impairment. MCI was diagnosed based on: MMSE ⩾21 and ⩽28; no dementia; memory complaint; preserved general cognitive function; intact daily living activities; problems with two or less of the following activities: phone calls, meal preparation, handling money, completing chores; abnormal memory function documented by scores below the education-adjusted cutoff at the logical Memory II subscale (delayed paragraph recall) from the Wechsler Memory Scale–Revised (maximum score=25). The ADAS-Cog33 and Cognitive Dementia Rating–Global Score (CDR-GS34, 35) scores were calculated for each participant. MMSE, ADAS-Cog and CDR sum of boxes (CDR-SB36) significantly discriminated the different groups with no gender-linked differences (Table 1a). Patients with MCI were divided as indicated in the validated interpretative guideline for the CDR-SB score,37 with a lower cutoff (1.5 instead of 2) for the detection of very early cognitive impairment. MCI patients with CDR-SB ⩽1.5 (MCI ⩽1.5 group, n=9) correspond to patients with ‘questionable impairment' and the MCI ⩾2 group (n=14) to patients with ‘very mild dementia' (Table 1b). Lumbar puncture, cognitive tests and diagnosis were all performed the same day to avoid any bias between clinical evaluation and CSF sampling. All CSF samples were stored in polypropylene tubes at −80 °C as previously described38 until thawing for immunoassays. All CSF Aβ11-x and Aβ17-x measurements were performed twice in two independent experiments to ensure the reliability of the conclusions.
Table 1. Demographic data and psychometric assessment of the patients.
| a | ||||||
|---|---|---|---|---|---|---|
|
Mean±s.d.
(min–max) |
P-value |
|||||
| CTRL (n=21) | MCI (n=23) | AD (n=23) | CTRL vs MCI | CTRL vs AD | MCI vs AD | |
| CDR-GS 0 | n=21 | n=1 | ||||
| CDR-GS 0.5 | n=22 | n=7 | ||||
| CDR-GS 1 | n=10 | |||||
| CDR-GS 2 | n=6 | |||||
| MMSE | 29.71±0.46 | 24.78±2.13 | 17.57±2.86 | <0.0001 | <0.0001 | <0.0001 |
| (29–30) | (21–28) | (13–24) | ||||
| ADAS-cog | 0 | 18.04±8.04 | 36.91±11.02 | <0.0001 | <0.0001 | <0.0001 |
| (2–36) | (22–59) | |||||
| CDR-SB | 0 | 2.3±1.35 | 6.5±3.81 | <0.0001 | <0.0001 | <0.0001 |
| (0–5.5) | (2–14) | |||||
| Mean age, year | 65.48±5.31 | 69.57±9.52 | 77.39±6.83 | 0.084 | <0.0001 | 0.0027 |
| (60–77) | (50–82) | (66–90) | ||||
| Sex, female/male | 10/11 | 10/13 | 15/8 | 0.55 | 0.37 | 0.56 |
| (%) | (47.62/52.38) | (43.48/56.52) | (65.22/34.78) | |||
| b | ||||||
|---|---|---|---|---|---|---|
|
Mean±s.d.
(min–max) |
P-value |
|||||
| CTRL (n=21) | MCI ⩽1.5 (n=9) | MCI ⩾2 (n=14) | CTRL vs MCI ⩽1.5 | CTRL vs MCI ⩾2 | MCI ⩽1.5 vs MCI ⩾2 | |
| MMSE | 29.71±0.46 (29–30) | 25.89±1.83 (23–28) | 24.07±2.06 (21–28) | <0.0001 | <0.0001 | 0.043 |
| ADAS-cog | 0 | 16±7.67 (2–27) | 19.36±8.27 (7–36) | <0.0001 | <0.0001 | 0.34 |
| CDR-SB | 0 | 1±0.5 (0–1.5) | 3.14±1 (2–5.5) | <0.0001 | <0.0001 | <0.0001 |
| Age, year | 65.48±5.31 (60–77) | 70.44±7.94 (58–81) | 69±10.67 (50–82) | 0.053 | 0.20 | 0.73 |
| Sex, female/male | 10/11 | 4/5 | 6/8 | 0.88 | 0.79 | 0.94 |
| (%) | (47.62/52.38) | (44.4/55.6) | (42.8/57.2) |
Abbreviations: AD, Alzheimer's disease; ADAS-cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale; CDR-GS, Cognitive Dementia Rating–Global Score; CDR-SB, Cognitive Dementia Rating–Sum of Boxes; CTRL, controls; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
Statistical analyses
Statistical analyses and figures were done using the ‘R/Bioconductor' statistical open source software39 or the SAS software v9.2 (SAS Institute, Cary, NC, USA). Univariate differential analysis was performed with the more appropriate statistical test (control of the normality and homoscedasticity hypotheses). Multiple testing corrections enabled to adjust the P-value of each marker to control the false discovery rate. The Benjamini and Hochberg approach40 was applied with the ‘multi-test' package. Adjusted P-values <0.05 were considered as statistically significant. All biomarker distributions are illustrated with boxplots and medians. The accuracy of each marker and its discriminatory power was evaluated using the Receiving Operating Characteristics (ROC) analysis. ROC curves are the graphical visualization of the reciprocal relation between sensitivity (Se) and specificity (Sp) of a test for various values. In addition to univariate analysis, all markers were combined to evaluate the potential increase in sensibility and specificity using two multivariate approaches (logistic regression41 and mROC method42). A logistic regression model was applied using biomarkers as categorical variables and the median values as cut-points. A backward selection process was considered in order to converge on the best multivariate model.43 The Wald statistic criterion of P-value <0.05 was used to keep variables in the final statistical model. Adjusted odds ratios and their 95% confidence intervals were computed for significant variables in the final model. The mROC program is dedicated to identifying the linear combination44 that maximizes the area under the ROC curve.45 The equation for the underlying combination is provided and can be used as a decision rule. The DeLong's test46 was also employed to compare several ROC curves.
Results
Antibody characterization
We produced specific anti-40 (6H7) and anti-42 (12E8) mAbs that displayed high affinity toward their corresponding synthetic peptides and exclusive specificity as no significant crossreaction toward other C-terminal truncated Aβ peptides was observed by surface plasmon resonance analyses (Supplementary Table S1). Thus, unlike 4G8 that, as expected, interacted similarly with both N-40 and N-42 peptides with affinities in the nanomolar range, 6H7 only bound to N-40 peptides, whereas 12E8 interacted with all tested N-42 peptides with high affinity. Surface-enhanced laser desorption/ionization analysis confirmed that 6H7 and 12E8 bound specifically to AβN-40 or N-42 peptides, respectively, in complex biological fluids. Accordingly, in supernatants from HEK293 APPwt+BACE1 cells that secrete high amounts of Aβ1-x and Aβ11-x peptides,24 the 6H7 and 12E8 antibodies captured only Aβ1-40 and Aβ11-40 or Aβ1-42 and Aβ11-42 peptides, respectively, without any crossreactivity toward other Aβ11-x or Aβ1-x (with x different from 40 or 42) variants (Supplementary Figure S1).
Characterization and validation of the 6H7/12E8/IRR triplex assays
We then developed two 6H7/12E8/IRR triplex assays in which AβN-40 and AβN-42 peptides are simultaneously captured via their C-terminus by the 6H7 and 12E8 antibodies. Aβ11-x or Aβ17-x peptides are then detected with the 7H1 or 8H5 mAbs that were previously characterized.24 Sandwich assays performed with all mAb combinations showed a detection limit of <10 pg ml−1 (Supplementary Figure S2). This rather high sensitivity allowed the accurate assessment of AβN-x peptides in complex media. The reproducibility of these assays was examined using supernatants from HEK293 cell lines that express wild-type AβPP (APPwt), wild-type APP and BACE1 (AβPPwt+BACE1) or AβPP with the Swedish mutation (APPsw) and that secrete various Aβ11-x and Aβ17-x peptides as well as in human control CSF samples (Supplementary Figure S3). Reproducibility was satisfactory for CSF Aβ11-x measurements (percent coefficient of variation <20%), and slightly more variable but still acceptable for Aβ17-x measurements (percent coefficient of variation between 20 and 30% for Aβ17-40 and ∼30% for Aβ17-42).
To further validate the assay specificity, we examined the ability of the multiplexed assays to discriminate between peptides differing by only one amino acid. Thus, we compared the reactivity of the 6H7/7H1 and 6H7/4G8 sandwich assays toward Aβ 9–40, 10–40, 11–40, 12–40 or 13–40 peptides (Supplementary Figure S4A) and the reactivity of the 6H7/8H5 and 6H7/4G8 sandwich assays toward Aβ 15–40, 16–40, 17–40, 18–40 or 19–40 peptides (Supplementary Figure S4B). The 6H7/7H1 and 6H7/8H5 assays clearly showed a restricted specificity toward Aβ11-40 and Aβ17-40 peptides, respectively. Conversely, the 4G8 antibody detected all tested peptides with different sensitivities, according to the peptide sequence.
As the concentrations of truncated fragments and flAβ in pathological conditions are unknown and could vary during the disease course, we examined whether high levels of flAβ could influence the detection of truncated fragments in the two assays. High concentrations of Aβ1-40 or Aβ1-42 (>100-fold above the affinity constant for the truncated fragments) did not significantly affect Aβ11-x or Aβ17-x detection, respectively (Supplementary Figure S5). We therefore conclude that, in these experimental conditions, the binding capacity of the beads remains sufficient to preclude any technical bias, thereby validating the use of the 6H7/12E8/IRR triplex assays for the detection and quantification of N-truncated Aβ peptides in human CSF samples.
Quantification of Aβ11-x and Aβ17-x peptides in CSF allows MCI detection at early stages
We then quantified the concentration of Aβ11-40, Aβ11-42, Aβ17-40 and Aβ17-42 peptides using the two Cter 6H7/12E8/IRR triplex assays and the concentration of Aβ1-42, T-Tau and P-Tau with the AlzBio3 assay in CSF samples from patients with AD or MCI (n=23 per group) and controls (n=21; Table 2a). As previously reported,10, 47 Aβ1-42 was significantly reduced whereas T-Tau and P-Tau concentrations were significantly higher in CSF samples from patients with MCI than from controls (P<0.05). These differences were further exacerbated at the AD stage (P<0.001). Aβ11-40 and Aβ11-42 concentrations were significantly lower in patients with MCI than in controls (P<0.01), whereas no significant difference was observed in Aβ17-x levels in the three groups, but for Aβ17-42 between controls and AD (P<0.05). Aβ11-40 and Aβ11-42 peptides discriminated more efficiently patients with MCI from controls, even when compared with the classical biomarkers Aβ1-42, T-Tau and P-Tau (Table 2b). Analysis of different marker combinations for discriminating patients with MCI from controls using the mROC program (Table 3) and logistic regression analysis (Table 4) indicated that the combination of Aβ11-40, Aβ17-40 and T-Tau allowed the best evaluation of the MCI risk (multivariate adjusted odds ratio: 15.30, P<0.05 for each biomarker). Accordingly, a person with a CSF Aβ11-40 level <147.6 pg ml−1 was 18.8 times more at risk to have MCI. This result was strengthened by the mROC approach, which confirmed that, compared with the reference Aβ1-42, T-Tau and P-Tau combination (sensitivity 60.87% specificity 66.67% area under the ROC curve 0.727), the Aβ11-40, Aβ17-40 and T-Tau combination better discriminated patients with MCI from controls (sensitivity 73.91% specificity 95.24% area under the ROC curve 0.890; Table 3 and Supplementary Figure S6).
Table 2a. Concentration of the different AD biomarkers and of N-truncated Aβ peptides in controls (CTRL) and patients with MCI or AD, and their significance in differentiating the three study groups.
| Mean (pg ml−1)±s.d. (min–max) | P-value | |||||
|---|---|---|---|---|---|---|
| |
CTRL (n=21) |
MCI (n=23) |
AD (n=23) |
CTRL vs MCI |
CTRL vs AD |
MCI vs AD |
| Aβ1-42 | 557.48±88.45 (380.21–699.62) | 468.20±152.09 (179.41–829.88) | 356.20±107.48 (186.35–588.39) | <0.05 | <0.001 | <0.01 |
| T-Tau | 54.72±20.13 (22.75–100.22) | 79.51±37.90 (29.95–174.34) | 145.40±89.10 (34.29–398.56) | <0.05 | <0.001 | <0.01 |
| P-Tau | 27.61±7.21 (15.27–41.10) | 36.85±16.99 (12.59–78.16) | 56.37±35.65 (13.75–171.99) | <0.05 | <0.001 | <0.05 |
| Aβ11-40 | 163.56±39.35 (95.75–230.23) | 133.10±28.76 (85.34–192.69) | 133.69±56.77 (30.77–235.95) | <0.01 | 0.051 | 0.97 |
| Aβ11-42 | 26.63±7.14 (15.67–40.99) | 22.23±7.01 (13.81–43.87) | 23.70±11.30 (5.02–50.02) | <0.01 | 0.32 | 0.60 |
| Aβ17-40 | 43.34±28.36 (9.65–98.65) | 45.58±25.49 (8.81–100.02) | 33.93±20.04 (5.19–66.03) | 0.79 | 0.20 | 0.09 |
| Aβ17-42 | 11.63±3.82 (5.58–18.80) | 11.51±7.12 (3.92–27.34) | 8.15±3.60 (1.35–15.25) | 0.94 | <0.05 | 0.051 |
Abbreviations: AD, Alzheimer's disease; CTRL, controls; MCI, mild cognitive impairment; P-Tau, phosphorylated Tau; T-Tau, total Tau.
The CSF biomarkers presented classical AD-like profiles with significant progressive decrease of Aβ1-42 and increase of both T-Tau and P-Tau concentration in accordance with the severity of the pathology. The concentration of the Aβ11-40 and Aβ11-42 peptides was lower in patients with MCI than in controls. Aβ17-40 level did not differ significantly in the three groups and Aβ17-42 concentration was lower in the AD group.
Table 2b. Diagnostic potential (mROC) of the different CSF biomarkers as univariate variables for MCI diagnosis.
| CTRL vs MCI | Aβ1-42 | T-Tau | P-Tau | Aβ11-40 | Aβ11-42 | Aβ17-40 | Aβ17-42 |
|---|---|---|---|---|---|---|---|
| Median (pg ml−1) | 535.14 | 56.6 | 27.47 | 147.58 | 22.21 | 37.85 | 11.15 |
| Cutoff (pg ml−1) | −490.47 | 64.16 | 27.26 | −146.66 | −21.87 | 46.15 | −11.05 |
| Sensitivity (%) | 60.87 | 60.87 | 69.57 | 69.57 | 65.22 | 52.17 | 60.87 |
| Specificity (%) | 76.19 | 76.19 | 66.67 | 71.43 | 76.19 | 66.67 | 61.9 |
| NPV (%) | 64.00 | 64.00 | 66.67 | 68.18 | 66.67 | 56.00 | 59.09 |
| PPV (%) | 73.68 | 73.68 | 69.57 | 72.73 | 75.00 | 63.16 | 63.64 |
| AUC | 0.704 | 0.706 | 0.684 | 0.739 | 0.708 | 0.537 | 0.594 |
| 95% Confidence interval | (0.547–0.861) | (0.548–0.864) | (0.522–0.847) | (0.588–0.890) | (0.548–0.868) | (0.360–0.715) | (0.419–0.769) |
| P-value (Delong's test) | 0.021 | 0.019 | 0.036 | 0.006 | 0.018 | 0.672 | 0.285 |
Abbreviations: AUC, area under the curve; CSF, cerebrospinal fluid; CTRL, controls; MCI, mild cognitive impairment; NPV, negative predictive value; PPV, positive predictive value; P-Tau, phosphorylated Tau; T-Tau, total Tau.
The cut-offs were chosen to yield the highest Youden's index. P-values (DeLong's test) represent the comparison of biomarkers with AUC=0.5.
Table 3. Diagnostic potential (mROC) of the different CSF biomarkers as multivariate variables for MCI diagnosis.
| CTRL vs MCI | T-Tau+P-Tau+Aβ1-42 (Z1) | T-Tau+Aβ11-40+Aβ17-40 (Z2) |
|---|---|---|
| Cutoff | −0.79 | −1.28 |
| Sensitivity (%) | 60.87 | 73.91 |
| Specificity (%) | 66.67 | 95.24 |
| NPV (%) | 60.87 | 76.92 |
| PPV (%) | 66.67 | 94.44 |
| AUC | 0.727 | 0.89 |
| 95% Confidence interval | (0.575–0.878) | (0.791–0.990) |
| P-value (Delong's test) | 0.01 | <0.0001 |
Abbreviations: AUC, area under the curve; CSF, cerebrospinal fluid; CTRL, controls; MCI, mild cognitive impairment; NPV, negative predictive value; PPV, positive predictive value; P-Tau, phosphorylated Tau; T-Tau, total Tau.
The cutoffs were chosen to yield the highest Youden's index. P-values (DeLong's test) represent the comparison of biomarkers with AUC=0.5.
Table 4. Logistic regression coefficient and odds ratios of the best multivariate model (controls vs MCI patients).
|
Logistic regression and odds ratio estimates | ||||
|---|---|---|---|---|
| Effect | Estimate | P-value | Odds ratio | 95% CI |
| Intercept | 0.49 | 0.45 | ||
| Tau | −1.75 | 0.03 | 0.17 | (0.035–0.85) |
| Aβ11-40 | 2.94 | 0.002 | 18.81 | (2.84–124.58) |
| Aβ17-40 | −1.89 | 0.038 | 0.15 | (0.025–0.90) |
| Intercept | −1.224 | 0.016 | ||
| T-Tau+Aβ11-40+Aβ17-40 | 2.73 | 0.0003 | 15.30 | (3.51–66.70) |
Abbreviations: CI, confidence interval; MCI, mild cognitive impairment; T-Tau, total Tau.
Variables were discretized using the median values as cut-points. The significant variables retained in the backward elimination model were T-Tau, Aβ11-40 and Aβ17-40. The model had a good fitness as estimated by the Hosmer–Lemeshow test (P>0.05).
Quantification of Aβ11-x and Aβ17-x peptides discriminate patients with MCI at different stages of severity
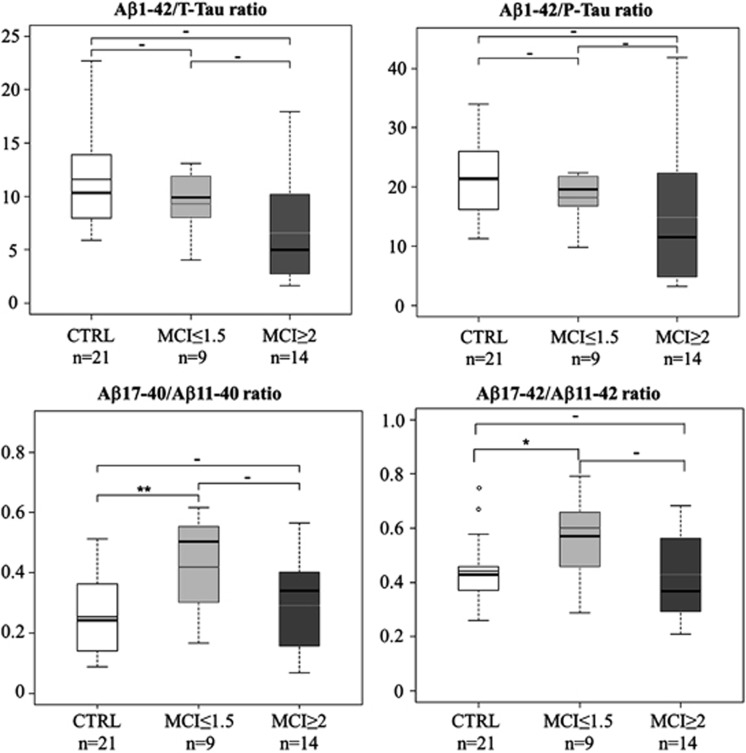
MCI is a complex concept that covers various stages characterized by distinct cognitive dysfunctions. Thus, to further investigate the potential interest of the measurements of N-truncated Aβ peptides for MCI diagnosis, we classified patients with MCI in two subgroups (MCI ⩽1.5 and MCI ⩾2) based on their CDR-SB score, because CDR-SB reliably measures AD clinical and pathological progression.37, 48, 49 Aβ1-42, T-Tau and P-Tau could not significantly discriminate patients with very early MCI (MCI ⩽1.5) from controls (Supplementary Figure S7A). Conversely, Aβ11-40 concentration was significantly lower (fold median=0.78; P<0.01) and Aβ17-x concentration tended to be higher (not significant) in CSF samples from patients with MCI ⩽1.5 than in controls (Supplementary Figures S7B and S7C). Accordingly, the Aβ17-40/Aβ11-40 ratio was significantly increased in the MCI ⩽1.5 group in comparison with controls (Figure 1, fold median=2.07; P<0.01), whereas the classical Aβ1-42/P-Tau and Aβ1-42/T-Tau ratios, which have potential predictive value, could not differentiate controls from patients with MCI ⩽1.5 (Figure 1). Noteworthy, the Aβ17-42/Aβ11-42 ratio also discriminated, although with lower significance, the MCI ⩽1.5 group from controls (Figure 1b). When analysing patients with very early cognitive impairment, the increase of the Aβ17-40/Aβ11-40 ratio becomes more significant when the used CDR-SB cutoff decreases, highlighting the potential value of this ratio for describing very early cognitive impairment, or categorizing the MCI status (Supplementary Figure S8).
Figure 1.
The Aβ1–42/P-Tau and Aβ1-42/T-Tau ratios do not allow discriminating between the mild cognitive impairment (MCI) ⩽1.5 group and controls (CTRL). P-Tau, phosphorylated Tau; T-Tau, total Tau. The Aβ17-40/Aβ11-40 ratio significantly differentiates the MCI ⩽1.5 group from controls. The symbol ‘–' indicates P>0.05; *P<0.05; **P<0.01.
Discussion
This study highlights for the first time the potential diagnostic value of the CSF concentration of Aβ11-40, Aβ11-42, Aβ17-40 and Aβ17-42 peptides for early AD detection and MCI characterization. These results are based on new sensitive multiplexed assays that were validated using different synthetic peptides and cell supernatants, before use in human CSF samples. We also show that the Aβ11-40, Aβ17-40 and T-Tau combination might better discriminate patients with MCI from controls than the currently used Aβ1-42, T-Tau and P-Tau combination.
The results obtained with these multiplexed assays in controls and patients with MCI or AD highlight several important points. First, the AβN-40 and AβN-42 diagnostic performances in controls and patients with MCI are not significantly different. This suggests that the subsequent cleavages of β'-secretase (C89) and α-secretase (C83)-derived AβPP fragments by γ-secretase leading to Aβ11-x or Aβ17-x peptides, respectively, does not account for the setting of early proteolytic alterations responsible for the generation of N-terminally truncated Aβ fragments during early MCI stages.
Second, the Aβ11-x levels in CSF samples from MCI patients were lower than in controls. Several previous studies demonstrated that BACE1 β'-cleavage between the Y10 and E11 residues of Aβ is dependent on the BACE1 activity level. Aβ11-x concentration is supposed to be lower in physiological conditions50 than in AD24, 51, 52, 53, 54, 55, 56 because BACE1 is upregulated in AD-affected brains, and could be associated with hippocampal atrophy.57 The apparent CSF reduction of Aβ11-x peptides in MCI could reflect their high hydrophobicity and aggregative properties, thus explaining their presence in plaques19, 58 and their reduced presence in CSF samples as previously described for pathogenic flAβ42. Alternatively, one cannot exclude the possibility of a modification of BACE1 activity/affinity toward other cleavage sites as previously suggested59, 60 that would favour breakdown at the β-site cleavage rather than at the β' one. A proteolytic shift between β and β' sites of cleavages was recently highlighted by the discovery of a new AβPP mutation at the E11 residue (E682K) in a Belgian patient with early-onset AD. This mutation prevents β'-cleavage and thus simultaneously decreases the production of C89 fragments and Aβ11-x peptides, while increasing that of C99 fragments and Aβ1-x peptides.61 This finding suggests that elevated Aβ11-x concentration in CSF samples represents a protective signature because AβPP cleavage at the β'-site has been considered nonamyloidogenic.61 It would be interesting to evaluate the CSF concentration of Aβ11-x in these patients and in patients with other APP mutations, such as the A673T mutation that has protective effect against AD by affecting directly the β-cleavage of AβPP by BACE1 and reducing Aβ1-x secretion.62
Third, Aβ17-x measurements, especially Aβ17-40, are of interest for discriminating between controls and patients with MCI, as shown by the mROC and logistic regression analyses. This may be explained by the heterogeneity of the MCI group. Indeed, this population could be divided in two subgroups (MCI ⩽1.5 and MCI ⩾2, based on their CDR-SB score). In our study, despite the low number of patients, the CDR-SB classification fitted very well with the scores of other cognitive tests, such as the MMSE or ADAS-cog (see Table 1b), thus strengthening our results. The Aβ1-42, T-Tau and P-Tau biomarkers and the Aβ1-42/T-Tau and Aβ1-42/P-Tau ratios, which were reported to have prognostic values,10, 11, 63, 64, 65 could not discriminate controls from the MCI ⩽1.5 group. Conversely, the Aβ17-40/Aβ11-40 ratio was significantly higher in the MCI ⩽1.5 group than in controls. Despite a lower discriminating value, the Aβ17-42/Aβ11-42 ratio allowed the identification of this subgroup as well. To our knowledge, this is the first report in which the modulation of α-secretase-derived products could be detected during the very first steps of AD. Indeed, previous studies aimed at detecting the CSF concentrations of secreted APPα, which derives from α-secretase cleavage of AβPP, did not find any significant variation in its level.28, 66 This may indicate that the first steps of AD could be characterized by an increase of the CSF concentration of Aβ17-x peptides, which are toxic component of diffuse amyloid deposits,67, 68, 69 probably because of a lack of degradation or modulation of their aggregation, rather than by an increase or decrease of α-secretase activity.
In conclusion, our results show differential CSF concentrations of β'- and α-secretase-derived peptides during AD progression and suggest the possible role of Aβ11-x and Aβ17-x peptides in the first steps of AD, highlighting key physiological aspects of the pathology. The clinical interest of Aβ11-x and Aβ17-x peptides as new biomarkers for improving MCI detection and characterization and as a consequence the stratification of MCI patients has to be further validated in longitudinal clinical studies, especially for delineating the outcome of the different MCI subpopulations. Our study adds new candidates to the cohort of Aβ-related fragments that could contribute to the aetiology of early-stage AD. Overall, it indicates that conclusions based on the monitoring of flAβ alone or on the results of immunological assays using antibodies that interact nonspecifically with all AβN-40/42 species should be reconsidered.
Acknowledgments
We thank Dominique Piquer, Audrey Malet, Julien Balicchi, Julia Mathieu, Isabelle Garric and Elisabeth Billy for their excellent technical assistance. The research leading to these results has received grants from the Agence Nationale de la Recherche (ANR-MNP Alzamyd) via the French Plan Alzheimer 2008–2012 and Eurobiomed funding. SR and CC are supported by the ANR-MNP Alzamyd grant. SysDiag high-throughput monoclonal antibody facility (HT-Mab) received grants from the Languedoc-Roussillon region via IBISA funding. FC is supported through the LABEX (excellence laboratory, program investment for the future) DISTALZ (Development of Innovative Strategies for a Transdisciplinary approach to ALZheimer's disease) and is recipient of a Hospital Contract for Translational Research (CHRT) between INSERM and the CHU of Nice.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, et al. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci USA. 1988;85:7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Sims NR, Bowen DM, Allen SJ, Smith CC, Neary D, Thomas DJ, et al. Presynaptic cholinergic dysfunction in patients with dementia. J Neurochem. 1983;40:503–509. doi: 10.1111/j.1471-4159.1983.tb11311.x. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Vanderstichele H, De Vreese K, Blennow K, Andreasen N, Sindic C, Ivanoiu A, et al. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer's disease and dementia with Lewy bodies. Clin Chem Lab Med. 2006;44:1472–1480. doi: 10.1515/CCLM.2006.258. [DOI] [PubMed] [Google Scholar]
- Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, et al. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- Buchhave P, Blennow K, Zetterberg H, Stomrud E, Londos E, Andreasen N, et al. Longitudinal study of CSF biomarkers in patients with Alzheimer's disease. PLoS One. 2009;4:e6294. doi: 10.1371/journal.pone.0006294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P, Mattsson N, Hansson O, Wallin A, Johansson JO, Andreasson U, et al. Cerebrospinal fluid biomarkers for Alzheimer's disease: diagnostic performance in a homogeneous mono-center population. J Alzheimers Dis. 2011;24:537–546. doi: 10.3233/JAD-2011-101878. [DOI] [PubMed] [Google Scholar]
- Schoonenboom NS, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- Abraham JD, Calvayrac-Pawlowski S, Cobo S, Salvetat N, Vicat G, Molina L, et al. Combined measurement of PEDF, haptoglobin and tau in cerebrospinal fluid improves the diagnostic discrimination between alzheimer's disease and other dementias. Biomarkers. 2011;16:161–171. doi: 10.3109/1354750X.2010.536995. [DOI] [PubMed] [Google Scholar]
- de Souza LC, Lamari F, Belliard S, Jardel C, Houillier C, De Paz R, et al. Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer's disease from other cortical dementias. J Neurol Neurosurg Psychiatry. 2011;82:240–246. doi: 10.1136/jnnp.2010.207183. [DOI] [PubMed] [Google Scholar]
- Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer's disease. J Neurochem. 1995;65:1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ma Q, Zhang YW, Xu H. Proteolytic processing of Alzheimer's beta-amyloid precursor protein. J Neurochem. 2012;120 (Suppl 1:9–21. doi: 10.1111/j.1471-4159.2011.07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, Kawashima S. Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3(pE), in senile plaques. Neuron. 1995;14:457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- Saido TC, Yamao-Harigaya W, Iwatsubo T, Kawashima S. Amino- and carboxyl-terminal heterogeneity of beta-amyloid peptides deposited in human brain. Neurosci Lett. 1996;215:173–176. doi: 10.1016/0304-3940(96)12970-0. [DOI] [PubMed] [Google Scholar]
- Sergeant N, Bombois S, Ghestem A, Drobecq H, Kostanjevecki V, Missiaen C, et al. Truncated beta-amyloid peptide species in pre-clinical Alzheimer's disease as new targets for the vaccination approach. J Neurochem. 2003;85:1581–1591. doi: 10.1046/j.1471-4159.2003.01818.x. [DOI] [PubMed] [Google Scholar]
- Portelius E, Tran AJ, Andreasson U, Persson R, Brinkmalm G, Zetterberg H, et al. Characterization of amyloid beta peptides in cerebrospinal fluid by an automated immunoprecipitation procedure followed by mass spectrometry. J Proteome Res. 2007;6:4433–4439. doi: 10.1021/pr0703627. [DOI] [PubMed] [Google Scholar]
- Sevalle J, Amoyel A, Robert P, Fournie-Zaluski MC, Roques B, Checler F. Aminopeptidase A contributes to the N-terminal truncation of amyloid beta-peptide. J Neurochem. 2009;109:248–256. doi: 10.1111/j.1471-4159.2009.05950.x. [DOI] [PubMed] [Google Scholar]
- Liu K, Doms RW, Lee VM. Glu11 site cleavage and N-terminally truncated A beta production upon BACE overexpression. Biochemistry. 2002;41:3128–3136. doi: 10.1021/bi015800g. [DOI] [PubMed] [Google Scholar]
- Ranaldi S, Caillava C, Prome S, Rubrecht L, Cobo S, Salvetat N, et al. N-truncated Abeta peptides in complex fluids unraveled by new specific immunoassays. Neurobiol Aging. 2013;34:523–539. doi: 10.1016/j.neurobiolaging.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Colciaghi F, Borroni B, Pastorino L, Marcello E, Zimmermann M, Cattabeni F, et al. [alpha]-Secretase ADAM10 as well as [alpha]APPs is reduced in platelets and CSF of Alzheimer disease patients. Mol Med. 2002;8:67–74. [PMC free article] [PubMed] [Google Scholar]
- Colciaghi F, Marcello E, Borroni B, Zimmermann M, Caltagirone C, Cattabeni F, et al. Platelet APP, ADAM 10 and BACE alterations in the early stages of Alzheimer disease. Neurology. 2004;62:498–501. doi: 10.1212/01.wnl.0000106953.49802.9c. [DOI] [PubMed] [Google Scholar]
- Ancolio K, Marambaud P, Dauch P, Checler F. Alpha-secretase-derived product of beta-amyloid precursor protein is decreased by presenilin 1 mutations linked to familial Alzheimer's disease. J Neurochem. 1997;69:2494–2499. doi: 10.1046/j.1471-4159.1997.69062494.x. [DOI] [PubMed] [Google Scholar]
- Sennvik K, Fastbom J, Blomberg M, Wahlund LO, Winblad B, Benedikz E. Levels of alpha- and beta-secretase cleaved amyloid precursor protein in the cerebrospinal fluid of Alzheimer's disease patients. Neurosci Lett. 2000;278:169–172. doi: 10.1016/s0304-3940(99)00929-5. [DOI] [PubMed] [Google Scholar]
- Alexander J, del Guercio MF, Maewal A, Qiao L, Fikes J, Chesnut RW, et al. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J Immunol. 2000;164:1625–1633. doi: 10.4049/jimmunol.164.3.1625. [DOI] [PubMed] [Google Scholar]
- Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, et al. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- Flax JS.Alzheimer's Disease and MCI: Banked Longitudinal CSF for Biomarker Discovery. . http://www.precisionmed.com/presos/sample_registry_poster_2010.pdf .
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Burch EA, Jr, Andrews SR. Comparison of two cognitive rating scales in medically ill patients. Int J Psychiatry Med. 1987;17:193–200. doi: 10.2190/gcvn-d2lj-aph3-kn0k. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryant SE, Lacritz LH, Hall J, Waring SC, Chan W, Khodr ZG, et al. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the national Alzheimer's coordinating center database. Arch Neurol. 2010;67:746–749. doi: 10.1001/archneurol.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewczuk P, Beck G, Esselmann H, Bruckmoser R, Zimmermann R, Fiszer M, et al. Effect of sample collection tubes on cerebrospinal fluid concentrations of tau proteins and amyloid beta peptides. Clin Chem. 2006;52:332–334. doi: 10.1373/clinchem.2005.058776. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE. Applied Regression Analysis and Other Multivariable Methods. Duxbury Press: Belmont, CA; 1988. [Google Scholar]
- Kramar A, Faraggi D, Fortune A, Reiser B. mROC: a computer program for combining tumour markers in predicting disease states. Comput Methods Programs Biomed. 2001;66:199–207. doi: 10.1016/s0169-2607(00)00129-2. [DOI] [PubMed] [Google Scholar]
- Agresti A.Categorical Data Analysis,2nd edn,2002
- Su JQ, Liu JS. Linear combinations of multiple diagnostic markers. J Am Stat Assoc. 1993;88:1350–1355. [Google Scholar]
- Staack A, Badendieck S, Schnorr D, Loening SA, Jung K. Combined determination of plasma MMP2, MMP9, and TIMP1 improves the non-invasive detection of transitional cell carcinoma of the bladder. BMC Urol. 2006;6:19. doi: 10.1186/1471-2490-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristics curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Palmqvist S, Hertze J, Minthon L, Wattmo C, Zetterberg H, Blennow K, et al. Comparison of brief cognitive tests and CSF biomarkers in predicting Alzheimer's disease in mild cognitive impairment: six-year follow-up study. PLoS One. 2012;7:e38639. doi: 10.1371/journal.pone.0038639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MM, Storandt M, Roe CM, Morris JC. Progression of Alzheimer's disease as measured by Clinical Dementia Rating Sum of Boxes scores. Alzheimers Dement. 2012;9 (1 Suppl:S39–S44. doi: 10.1016/j.jalz.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Nakatani E, Teramukai S, Nagai Y, Fukushima M. Risk classification in mild cognitive impairment patients for developing Alzheimer's disease. J Alzheimers Dis. 2012;30:367–375. doi: 10.3233/JAD-2012-112117. [DOI] [PubMed] [Google Scholar]
- Qahwash I, He W, Tomasselli A, Kletzien RF, Yan R. Processing amyloid precursor protein at the beta-site requires proper orientation to be accessed by BACE1. J Biol Chem. 2004;279:39010–39016. doi: 10.1074/jbc.M407101200. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- Holsinger RM, McLean CA, Collins SJ, Masters CL, Evin G. Increased beta-Secretase activity in cerebrospinal fluid of Alzheimer's disease subjects. Ann Neurol. 2004;55:898–899. doi: 10.1002/ana.20144. [DOI] [PubMed] [Google Scholar]
- Ewers M, Zhong Z, Burger K, Wallin A, Blennow K, Teipel SJ, et al. Increased CSF-BACE 1 activity is associated with ApoE-epsilon 4 genotype in subjects with mild cognitive impairment and Alzheimer's disease. Brain. 2008;131 (Pt 5:1252–1258. doi: 10.1093/brain/awn034. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, Andersson ME, et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol. 2008;65:1102–1107. doi: 10.1001/archneur.65.8.1102. [DOI] [PubMed] [Google Scholar]
- Mulder SD, van der Flier WM, Verheijen JH, Mulder C, Scheltens P, Blankenstein MA, et al. BACE1 activity in cerebrospinal fluid and its relation to markers of AD pathology. J Alzheimers Dis. 2010;20:253–260. doi: 10.3233/JAD-2010-1367. [DOI] [PubMed] [Google Scholar]
- Ewers M, Cheng X, Zhong Z, Nural HF, Walsh C, Meindl T, et al. Increased CSF-BACE1 activity associated with decreased hippocampus volume in Alzheimer's disease. J Alzheimers Dis. 2011;25:373–381. doi: 10.3233/JAD-2011-091153. [DOI] [PubMed] [Google Scholar]
- Tekirian TL, Saido TC, Markesbery WR, Russell MJ, Wekstein DR, Patel E, et al. N-terminal heterogeneity of parenchymal and cerebrovascular Abeta deposits. J Neuropathol Exp Neurol. 1998;57:76–94. doi: 10.1097/00005072-199801000-00009. [DOI] [PubMed] [Google Scholar]
- Barman A, Schurer S, Prabhakar R. Computational modeling of substrate specificity and catalysis of the beta-secretase (BACE1) enzyme. Biochemistry. 2011;50:4337–4349. doi: 10.1021/bi200081h. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Barman A, Chen Y, Nguyen PD, Wagner SL, Prabhakar R, et al. Loss of cleavage at beta'-site contributes to apparent increase in beta-amyloid peptide (Abeta) secretion by beta-secretase (BACE1)-glycosylphosphatidylinositol (GPI) processing of amyloid precursor protein. J Biol Chem. 2011;286:26166–26177. doi: 10.1074/jbc.M111.260471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Brouwers N, Benilova I, Vandersteen A, Mercken M, Van Laere K, et al. Amyloid precursor protein mutation E682K at the alternative beta-secretase cleavage beta'-site increases Abeta generation. EMBO Mol Med. 2011;3:291–302. doi: 10.1002/emmm.201100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- Vos S, van Rossum I, Burns L, Knol D, Scheltens P, Soininen H, et al. Test sequence of CSF and MRI biomarkers for prediction of AD in subjects with MCI. Neurobiol Aging. 2012;33:2272–2281. doi: 10.1016/j.neurobiolaging.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Tsolakidou A, Arnold A, Diehl-Schmid J, Grimmer T, Forstl H, et al. CSF soluble amyloid precursor proteins in the diagnosis of incipient Alzheimer disease. Neurology. 2011;77:35–38. doi: 10.1212/WNL.0b013e318221ad47. [DOI] [PubMed] [Google Scholar]
- Wei W, Norton DD, Wang X, Kusiak JW. Abeta 17-42 in Alzheimer's disease activates JNK and caspase-8 leading to neuronal apoptosis. Brain. 2002;125 (Pt 9:2036–2043. doi: 10.1093/brain/awf205. [DOI] [PubMed] [Google Scholar]
- Gowing E, Roher AE, Woods AS, Cotter RJ, Chaney M, Little SP, et al. Chemical characterization of A beta 17-42 peptide, a component of diffuse amyloid deposits of Alzheimer disease. J Biol Chem. 1994;269:10987–10990. [PubMed] [Google Scholar]
- Higgins LS, Murphy GM, Jr, Forno LS, Catalano R, Cordell B. P3 beta-amyloid peptide has a unique and potentially pathogenic immunohistochemical profile in Alzheimer's disease brain. Am J Pathol. 1996;149:585–596. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.