Abstract
Human epithelial cancers are defined by a recurrent distribution of specific chromosomal aneuploidies, a trait less typical for murine cancer models induced by an oncogenic stimulus. After prolonged culture, mouse epithelial cells spontaneously immortalize, transform and become tumorigenic. We assessed genome and transcriptome alterations in cultures derived from bladder and kidney utilizing spectral karyotyping, array CGH, FISH and gene expression profiling. The results show widespread aneuploidy, yet a recurrent and tissue-specific distribution of genomic imbalances, just as in human cancers. Losses of chromosome 4 and gains of chromosome 15 are common and occur early during the transformation process. Global gene expression profiling revealed early and significant transcriptional deregulation. Chromosomal aneuploidy resulted in expression changes of resident genes and consequently in a massive deregulation of the cellular transcriptome. Pathway interrogation of expression changes during the sequential steps of transformation revealed enrichment of genes associated with DNA repair, centrosome regulation, stem cell characteristics and aneuploidy. Genes that modulate the epithelial to mesenchymal transition and genes that define the chromosomal instability phenotype played a dominant role and were changed in a directionality consistent with loss of cell adhesion, invasiveness and proliferation. Comparison with gene expression changes during human bladder and kidney tumorigenesis revealed remarkable overlap with changes observed in the spontaneously transformed murine cultures. Therefore, our novel mouse models faithfully recapitulate the sequence of genomic and transcriptomic events that define human tumorigenesis, hence validating them for both basic and preclinical research.
Introduction
Human cancers of epithelial origin invariably display chromosomal copy number changes as a defining feature (1–3) and the resulting genomic imbalances directly affect the transcription levels of resident genes (4). In order to dissect the contribution of these genome mutations on tumorigenesis, it requires models that recapitulate the sequential destabilization of the human genome that is so characteristic for human carcinogenesis. Murine cancer models have emerged as invaluable tools for discovery and analysis of genes and pathways associated with tumorigenesis (5). Based on our extensive profiling of mouse models for breast and colorectal cancer using molecular cytogenetic techniques as part of the Mouse Model of Human Cancer Consortium (MMHCC), we have demonstrated that strong oncogenic stimuli resulting from overexpression of multiple copies of oncogenes, such as Myc, Erbb2, Kras and Septin-9 (6–8), override the requirement for the acquisition of tissue-specific patterns of genomic imbalances that so clearly define human carcinomas. From these studies, it appears that mouse tumor models induced by the deletion of tumor suppressor genes are more similar to human cancers in terms of the distribution of chromosomal imbalances (9,10).
We recently developed and described a methodology to isolate and transform normal murine epithelial cells from bladder, cervix, colon, kidney, lung and mammary glands excised from female and male C57BL/6 mice (11). Without viral infection, chemical induction or genetic manipulation, the primary epithelial cell cultures spontaneously progressed through three distinct morphologically defined stages designated as preimmortal, immortal and transformed. The transformed cells were tumorigenic when injected into nude mice.
Our initial investigations revealed that kidney and bladder cells often became tetraploid during the preimmortal stage, accompanied by chromosomal aneuploidies and centrosomal instabilities; at the immortal stage, the mitotic rates of the primary cultures accelerated, accompanied by increased chromosomal instability (CIN) and alterations of telomerase enzyme activity. At the transformed stage, we observed several focal genomic amplifications as a consequence of the formation of double minute (dmin) chromosomes and/or homo-geneously staining regions. Furthermore, at the transformation stage, 50% of cell lines developed tumors when subcutaneously injected into nude mice (11).
We now present an extensive molecular genetic characterization of five bladder and six kidney cell cultures and their derived cell lines using gene expression profiling and array CGH (aCGH). We were interested in answering the following questions: (i) what are the gene expression patterns found in our spontaneously transformed epithelial cell lines at the earliest stages of cellular transformation, (ii) how do the patterns change throughout progression, (iii) what are the similarities and differences between the different cell lines and (iv) how do the genomic imbalances and gene expression profiles compare with what has been observed in human bladder and kidney cancers? The results reveal a remarkable similarity with genome and transcriptome aberrations in human tumorigenesis, hence validating our newly derived cancer models.
Materials and methods
Tissue culture
Normal murine bladder and kidney epithelial cells from 5- to 6-week-old male and female C57BL/6 mice were cultured as described (11). All animals were killed following the protocols outlined in the NIH Animal Protocol Study: NCI-ASP-MB-045.
Spectral karyotyping
Preparation of metaphase chromosome suspension, spectral karyotyping (SKY) probes, slide pretreatment, slide denaturation, detection and imaging have been described previously (12). Karyotypes were interpreted as presented earlier (11) and can be viewed at http://www.ncbi.nlm.nih.gov/projects/sky/ (NCI45 Mouse Cell Line Panel-HPN) (13).
FISH
BAC clones for gene-specific loci Aurka (Aurora kinase A, RP23-358I19, Chr 2H3), Cdkn2a/p16 (RP23-166I8, Chr 4C4), Mdm2 (RP23-186D15, Chr 10D2), Hras1 (RP23-179K7, Chr 7F5), Ccnd1 (Cyclin D1, RP23-107I11, Chr 7F5) and Myc (D15Mit17, Chr 15D1) were purchased from Qiagen (Alameda, CA). Hmga2 (RP23-392I12, Chr10D2) was purchased from BACPAC Resources Center at Children’s Hospital Oakland Research Institute in Oakland, CA. Labeling of DNA for all FISH probes used in this study was performed by Nick translation following protocols described in http://www.riedlab.nci.nih.gov/protocols. FISH images were acquired using a Leica 4000 microscope (Leica, Wetzlar, Germany) and Q-FISH™ software (Leica).
RNA extraction
Mouse bladder and kidney cells were grown in vitro and sequentially recovered at three different time points referred to as preimmortal, immortal and transformed. RNA was extracted from five different bladder samples and six kidney samples and from normal uncultured epithelial bladder and kidney cells. Cells were stored in TRIzol at −80°C until RNA isolation using the Qiagen RNAeasy Kit (Midi #75144, Qiagen, Valencia, CA). The concentrations of RNA were quantitated using a NanoDrop® ND-1000 (Thermo Scientific, Wilmington, DE), and RNA quality was assessed by gel electrophoresis and a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). The RIN value of the extracted RNA for microarray and quantitative real-time PCR (qRT-PCR) assay was >7 for all samples.
DNA extraction
DNA was extracted from murine epithelial cells, primary cultures and derived cell lines derived from five bladders and six kidneys from C56BL/6 mice. Normal control DNA was extracted from sex-matched mouse livers of the same mouse strain.
Protocols for extraction of DNA are available at http://www.riedlab.nci.nih.gov/protocols. The quality of the DNA was assessed by gel electrophoresis and the DNA concentration was measured using a NanoDrop® ND-1000 (Thermo Scientific).
Agilent aCGH
DNA was isolated following standard protocols and purified with the QIAprep Spin Miniprep Kit (Qiagen). Seven micrograms of the digested DNA was prepared for labeling with the BioPrime Array Genomic Labeling Kit (Invitrogen, Carlsbad, CA) using Cy3-dUTP for the test DNA and Cy5-dUTP for the reference DNA. DNA hybridization to the CGH array was performed using the Agilent Mouse Genome CGH 44K Microarray Kit (Agilent Technologies, Santa Clara, CA). The microarrays were hybridized in a rotisserie Microarray Oven, Model 777 (SciGene, Sunnyvale, CA) at 65°C at 10 r.p.m. for 40 h. The arrays were scanned using a Microarray Scanner System (G2565BA, Agilent Technologies). Extraction of scanned images was performed with Agilent Feature Extraction Software 8.1.
Affymetrix gene expression microarray
RNA was extracted from each of the cultured bladder and kidney samples and normal uncultured bladder and kidney epithelial cells. The cDNA was generated using a GeneChip Two-Cycle cDNA Synthesis Kit (Affymetrix, Santa Clara, CA) and a T7-Oligo (dT) Promoter Primer. The in vitro transcription of the cDNA to cRNA was carried out with the T7 Megascript Kit (Ambion, Austin, TX) following manufacturer’s instructions. The purified biotin-labeled cRNA was quantified using the NanoDrop® ND-1000 spectrophotometer (Thermo Fisher Scientific, Fair Lawn, NJ).
Gene expression levels were determined using Affymetrix Mouse Genome 430 2.0 GeneChip microarrays by hybridizing for 16h at 45°C at 60 r.p.m. using the Hybridization Oven 640 (Affymetrix). Washing and staining with streptavidin-R–phycoerythrin was performed in an automatic fluidics station using the EukGE-WS2v5 protocol (GeneChip Fluidics Station 450, Affymetrix). The quality of the RNA hybridization was evaluated by a GeneChip Scanner 3000 (Affymetrix), using internal control genes, β-actin and Gapdh. The gene expression data were processed with the Affymetrix GCOS (Gene Chip Operating Software v1.2) analysis program.
Immunofluorescence assay
The immunofluorescence staining method used followed the protocol described in http://www.riedlab.nci.nih.gov/protocols. Slides were incubated with fluorescein isothiocyanate-conjugated monoclonal anti-β-actin (Sigma–Aldrich, St Louis, MO) for visualization of cytoskeleton structures.
Histology
Transformed murine epithelial cells from bladder and kidney were injected subcutaneously into the interscapular region as described previously (11). All animals were killed following the protocols outlined in the NIH Animal Protocol Study: NCI-ASP-MB-045. Tumors were prepared for histological evaluation using hematoxylin and eosin staining (Histoserve, Germantown, MD).
Western blot analysis
Five bladder and six kidney transformed cell lines, and normal bladders from five male and five female C57BL/6 mice were prepared in lysis buffer [10 mM Tris–Cl (pH 7.4); 150mM NaCl; 1% sodium dodecyl sulfate, and protease inhibitors cocktail, Roche Diagnostics, Indianapolis, IN]. Forty micrograms of protein was loaded on gels and transferred onto polyvinylidene difluoride membranes. Antibodies specific for Cdkn2a/p16 (Santa Cruz Biotechnology, Santa Cruz, CA), Myc (Epitomics, Burlingame, CA) and β -actin (Sigma–Aldrich) were used. Signals were detected using Super Signal West Pico (Thermo Scientific Pierce, Rockford, IL) and the membranes stripped with Restore Plus (Thermo Scientific Pierce).
Migration assay
All five bladder and six kidney transformed cultures were grown to 100% confluency in six-well collagen-coated plates (BD Biosciences, Rockville, MD). The in vitro scratch assay was used following established protocols (14) to measure the migratory potential of each cell line. Each cell line was assayed in triplicate and monitored at 0 h and maintained for 30 h. The cultures were washed with 1× phosphate-buffered saline twice after the scratch was made to eliminate any floating cells or spheres and then photographed at time 0 h (before the scratch), after the scratch and after 30 h for all 11 transformed bladder and kidney cell lines.
Fluorescence-activated cell sorting
Data were collected with an FACS Calibur flow cytometer (BD Biosciences) and analyzed with FlowJo software (Treestar, Ashland, OR). Non-viable cells were excluded after 7-AAD (BD Biosciences) staining before analysis. Directly labeled mouse antibodies were used for flow cytometry to detect Cd44, Cd133 and Oct 4 (Abcam, Cambridge, MA) in transformed bladder and kidney cell lines. Unstained cells were used as controls.
Quantitative real-time PCR
Total RNA was isolated from five bladder and six kidney transformed cell lines and cDNA was synthesized following protocols described at http://www.riedlab.nci.nih.gov/index.php/protocols. Primers were obtained from Eurofins MWG Operon Technologies (Huntsville, AL). Additional information and primer sequences used for validation of gene expression for selected genes are provided in Supplementary Methods, available at Carcinogenesis Online.
Mouse bladder and kidney microarray data analysis
Microarray results were analyzed using multiple statistical analyses including t-test under analysis of variance. For additional details, refer to the Supplementary Methods, available at Carcinogenesis Online. The gene expression and aCGH data have been deposited into Gene Expression Omnibus (GEO) and the GEO records were made public on 14 March 2013. The GEO accession number is GSE45129 and is the reference series for this publication: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE45129. This superseries record provides access to all of our gene expression and aCGH data. For information on GEO linking and citing, please refer to http://www.ncbi.nlm.nih.gov/geo/info/linking.html. In addition, we refer to the SubSeries that are linked to GSE45129: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE45127 and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE45128.
Results
Morphological changes associated with spontaneous transformation of epithelial cells
In order to develop cancer models that recapitulate the genome mutation spectrum that defines human carcinomas, i.e. specific chromosomal imbalances and consequent changes to the transcriptome, we established cultures from epithelial cells isolated from bladder, cervix, colon, kidney, lung and mammary glands from wild-type C57BL/6 mice (11). After continued culturing, the cells bypassed senescence, became immortal and transformed. This sequence of events is presented in Figure 1A–C for cultures derived from bladder cells. The tumors that resulted from injection of the transformed cell lines into immunocompromised Nu/Nu mice revealed features characteristic of human carcinogenesis, such as angiogenesis (Figure 1D), aneuploidy, high mitotic indices (Figure 1E) and invasiveness shown here as cells migrating through the muscle layer of the host animal (Figure 1F and G). Experimental procedures and morphological changes that occur during spontaneous transformation of all six organs have been described elsewhere (11). Here, we present a comprehensive and systematic characterization of genome and transcriptome aberrations, and the signaling pathways that are affected during this process, which revealed remarkable similarity to the sequential changes observed during human carcinogenesis.
Fig. 1.
Morphology of spontaneously transformed bladder and kidney epithelial cells and tumor sections. Morphological changes were documented throughout the stages of transformation and defined as preimmortal (A), immortal (B) and transformed (C) for the bladder culture and derivative cell line NCI-HPN-M1C Blad. The preimmortal stage is when cells first become adherent and converted to enlarged quiescent cells; the immortal stage was defined by the presence of actively dividing cells and lack of contact inhibition; the first appearance of foci and loss of anchorage dependence defined the transformed stage. Panels D–G are representative photographs of hematoxylin and eosin-stained tissue sections of bladder and kidney tumors resulting from injection of transformed cells into nude mice. In (D), arrows identify several blood vessels that developed within a tumor, indicative of angiogenesis. In (E), the black arrow shows an enlarged nucleus and the white arrows identify mitotic figures in a bladder tumor, and (F) shows the invasion of murine transformed kidney cells into the host’s skin layers. (G) Enlarged image of black framed area in (F).
Specific cytogenetic changes associated with spontaneous transformation
We used SKY (15) and aCGH to characterize chromosomal aberrations that occurred during spontaneous transformation of the bladder and kidney cultures. Figure 2A presents the karyotype for a near-tetraploid kidney cell line NCI-HPN-M1C Kid, whereas Figure 2B shows the near-triploid bladder cell line NCI-HPN-M1C Blad. Both cell lines were derived from the identical male mouse and contain many numerical chromosomal aberrations. Structural chromosomal aberrations were more common in the bladder cell line, which also contained more than 100 dmin chromosomes, the cytogenetic correlates of oncogene amplification, in this case, derived from chromosome 15 (MMU15). Most of these initial chromosomal aneuploidies were maintained throughout progression despite increasing CIN and regional amplifications on the chromosomes that were present early in extra copy numbers (Supplementary Table I, available at Carcinogenesis Online) and microdeletions on chromosomes that were lost (MMU4).
Fig. 2.
Genomic imbalances identified by SKY, aCGH and FISH. The genomic imbalances in spontaneously transformed bladder and kidney cell lines were determined by SKY (A, B) and by aCGH (C). The SKY karyotype of a near-tetraploid transformed cell line NCI-HPN-M1C Kid (A) reveals numerical aberrations, including gains of MMU10 and loss of MMU9. The SKY karyotype of a near-triploid cell line NCI-HPN-M1C Blad (B) identified copy number gains of MMU5, MMU13 and two unbalanced translocations T(8;6) and T(8;X), resulting in gains of the distal end of MMU6. (C) Results of the aCGH analysis. For each chromosome, green shading represents copy number gains, and red represents losses. Five spontaneously transformed bladder cell line lines were analyzed by aCGH, and six cell lines for the kidney. Recurrent chromosome imbalances were detected in both organs, loss of MMU4, gains of MMU10 and MMU15. Tissue-specific chromosomal imbalances were detected for bladder (gain of MMU3, MMU6 and MMU8) and for kidney (increased copy number gains of MMU19, loss of MMU13). aCGH (D) and FISH (E) results for transformed cell line NCI-HPN-M1C Blad. Over 50 dmin chromosomes derived from MMU15 (first observed by SKY) were identified as amplifications of the oncogene Myc by FISH. Chromosomes were stained with 4′,6-diamidino-2-phenylindole (pseudocolored red). Chromosome MMU15 is shown in blue and yellow signals identify copies of Myc. The arrow indicates a cluster of dmin chromosomes.
We then used aCGH to map chromosomal gains and losses in the transformed cell line populations. The results were consistent with SKY and are presented in Figure 2C. Note that the bladder lines gained chromosomes MMU3, MMU8 and the distal end of MMU6, whereas extra copy numbers of MMU19 and loss of MMU13 were specific to the kidney cultures. SKY and aCGH complement one another, as SKY discerns the sources of the chromosomal imbalances and reveals heterogeneity and the extent of chromosome instability so characteristic of human carcinomas, whereas aCGH reveals global chromosomal imbalances of the entire cell population.
aCGH was also used to map the precise location of amplified or deleted regions in five bladder and six kidney transformed cell lines (Supplementary Table II, available at Carcinogenesis Online). Recurrent amplifications involved the oncogenes Myc (located at MMU15, band D2; Figure 2D) and Mdm2 (MMU10D1); both oncogenes were contained within dmin chromosomes, which was confirmed by targeted FISH analysis (Figure 2E and Supplementary Figure 1J and K, available at Carcinogenesis Online). Several tumor suppressor genes were subject to focal deletions in both bladder and kidney cell lines such as Cdkn2a and Cdkn2b (MMU4C4) and other recently described tumor suppressor genes Opcml, Dcc, Ptprs and Ptprd (3,16–19).
The loss of chromosome 13 was a recurrent and prominent feature of the kidney cell lines. This loss was accompanied by microdeletions of two genes, Cast and Naip3, in 50% of the kidney cell lines. Novel focal deletions for Trim17/Terf and Kcnc3 were detected in two bladder and four kidney transformed cell lines. Examples of amplification of genes within clusters, focal amplifications and microdeletions are shown in Supplementary Figure 1 and Supplementary Table III, available at Carcinogenesis Online.
aCGH identified numerous focal amplifications in both bladder and kidney transformed cell lines, including the genes Mapk15/Erk7-8, Bcl2l12, Mthfd2 and Trim 59. However, only two of the focal amplifications, Puf60 and 1700029J11Rik, were recurrent (Supplementary Table III, available at Carcinogenesis Online). The majority of gene amplifications consisted of gene clusters within either the same chromosome (50 genes including Mdm2 in NCI-HPN-M1I Blad) or several clusters on the same chromosome; three clusters with (i) 4 genes at MMU19B, (ii) 41 genes at MMU19C3 and (iii) 13 genes at MMU19D2, respectively, in NCI-HPN-F1B Kid. In addition, gene clusters were detected on two different chromosome bands (MMU9A1 and MMU2G1, both in NCI-HPN-M2G Kid; Supplementary Table IV, available at Carcinogenesis Online). Human chromosome arm 5p is one of the most commonly gained regions in human bladder and kidney cancers (3). It is orthologous to mouse chromosome 15, which is gained in both of our models, and contains the gene Skp2 in a region of minimal overlap. This gene was highly overexpressed in transformed mouse cells of both kidney and bladder (Supplementary Figure 2, available at Carcinogenesis Online) and was associated with cellular proliferation and tumorigenesis (20).
Gene expression
In order to assess the consequences of specific genomic imbalances on the transcriptome, to discover potential candidate oncogenes and tumor suppressor genes and to identify deregulated signaling pathways at defined steps of spontaneous cellular transformation, we studied global gene expression profiles using whole genome arrays. Five bladder and six kidney cell lines were analyzed at the preimmortal, immortal and transformed stages and compared with normal bladder or kidney uncultured epithelial cells from sex-matched isogenic mice.
The results of the gene expression analyses were visualized as a principal component analysis in Figure 3A and B. In this analysis, the different stages were compared with the control samples, which consisted of normal uncultured epithelial cells from bladder and kidney. The principal component analysis demonstrated that the gene expression for each organ cluster together, as do the three stages. There are significantly more genes deregulated at the transition from normal to preimmortal compared with subsequent stages. This pattern follows the evolution of the karyotypes (11). The gene expression results are also displayed as Venn diagrams (P = 0.01, >3-fold change; Figure 3C and D), but in this analysis, the gene expression profiles are shown for the transition between the stages. For the bladder samples, the fol-lowing were differentially expressed: 611 genes when comparing the preimmortalized samples with the controls, 31 genes when comparing immortalized cells with preimmortal cultures and 51 genes when comparing the transformed cell lines with the immortalized cell lines. For the kidney samples, 908 genes were deregulated at the preimmortal stage, 24 at the immortal stage, with no additional changes compared with the transformed stage. The hierarchical cluster analysis of the gene expression data is provided in Supplementary Figure 3A–C, available at Carcinogenesis Online. Venn diagrams (P = 0.01, >3-fold change) presenting the number of genes deregulated at all three stages compared with the normal controls for both bladder and kidney samples, rather than during progression, are shown in Supplementary Figure 3D–E, available at Carcinogenesis Online. In the bladder samples, more genes were upregulated than downregulated (156 versus 95) at the transformed stage, and for the kidney samples, more genes were upregulated at the preimmortal stage than downregulated (121 versus 52).
Fig. 3.
Gene expression analysis of spontaneously transformed bladder and kidney epithelial cells. Principle component analysis of the gene expression for normal controls, primary cultures and their respective immortal and transformed cell lines for five bladder samples (A) and six kidney samples (B). Blue, normal controls (uncultured epithelial bladder and kidney cells); green, preimmortal; yellow, immortal; red, transformed. The gene expression patterns for all three stages are statistically different and separable from the normal uncultured epithelial cells (controls). Venn diagrams display the gene expression patterns occurring during the progression of spontaneous transformation for bladder (C) and kidney (D). The preimmortal stage (P) is compared with the normal controls (N) (blue circle), immortal stage (I) to preimmortal (red circle) and transformed stage (T) to immortal (green circle). The number of genes that are significantly upregulated (red arrow) and downregulated (blue arrow) (>3-fold, P = 0.01) are provided within each circle. Note that the most profound changes occur when comparing the preimmortal cultures to the normal controls for both organs. (E and F) Box plots display the gene expression of the oncogenes Myc and Mdm2 for the normal (N) uncultured epithelial cells and in five bladder and six kidney cultures/cell lines at each stage of spontaneous transformation. Blue, normal; green, preimmortal (P); yellow, immortal (I); red, transformed (T). Black arrows identify cell lines containing dmin chromosomes for each oncogene revealing that the increased copy number gains resulted in increases in gene expression levels.
Next, we analyzed the expression levels of those genes that were contained in dmin chromosomes, such as Myc and Mdm2 (Supplementary Table IV, available at Carcinogenesis Online). Two bladder samples (NCI-HPN-M1C Blad and NCI-HPN-M2C Blad) both contained Myc amplifications and showed dramatically increased (more than 30-fold) expression levels for this oncogene. However, the early low-level copy number increases of MMU15, where Myc resides, also resulted in overexpression of this oncogene (2- to 3-fold), as shown in Figure 3E. In addition, two other bladder cell lines NCI-HPN-F1G Blad and NCI-HPN-M1I Blad contained genomic amplification of Mdm2, also in the form of dmin chromosomes, which coincided with >10 and >100 increased expression (Figure 3F). FISH confirmed the presence of Mdm2 in the dmin chromosomes (Supplementary Figure 1J and K, available at Carcinogenesis Online).
The analysis of known tumor suppressor genes revealed a profound expression decrease of Wt1 (6-fold) and Fh1/fumarate, both involved in human renal development and cancer, specifically in the kidney model; however, the Vhl1 gene was not significantly altered in expression. Rb1, a gene whose loss is associated with immortalization in numerous cancer types, was significantly downregulated in both bladder and kidney cells. The expression level of Cdkn2a/p16, which was subject to microdeletions in two bladder and three kidney cell lines, followed genomic copy number, both when measured by the RNA and by protein levels. Two bladder cell lines revealed no loss of Cdkn2a/p16 gene expression and in fact contained higher levels of p16 protein as detected by western blot analysis (Supplementary Figure 4). Supplementary Table III summarizes the expression values. The expression of the tumor suppressor gene Trp53 was not reduced (data not shown), but its early inactivation resulted via overexpression of Mdm2 (Figure 3F).
Interestingly, relatively few of the genes associated with focal amplifications correlated with increased expression relative to controls with the exception of Trim59, Tmem5 and Mthfd2. Those genes that were upregulated have been recently described as being involved with cellular proliferation and transformation (21–23) (Supplementary Table III, available at Carcinogenesis Online).
qRT-PCR confirmation
Genes that were either highly expressed or downregulated in five bladder and six kidney transformed cell lines were validated and confirmed using RT-PCR, including Aurka, Cd44, Cenpa, Mdm2, Myc, Upk3a, Cpe, Pten, S100a6 and Glyat (Supplementary Table V, available at Carcinogenesis Online). The qRT-PCR results confirmed the majority of the gene expression changes detected in the messenger RNA (mRNA) microarray, in particular in the cell lines containing amplifications for Myc and Mdm2 (Supplementary Table V, available at Carcinogenesis Online). Our previous literature searches of spontaneous transformation of mammalian cells and immunofluorescence staining for actin revealed that many of the housekeeping genes, such as actin, β-tubulin and Gapdh, show increases in their gene and protein expression (11). Therefore, when performing qRT-PCR, we elected to use genes from the mRNA microarray data set that exhibit the same gene expression values for the controls (normal uncultured cells) and transformed cell lines to normalize our data. Our qRT-PCR samples were normalized to the gene Hrk. Two main discrepancies were found involving Pten and S100a6. In the mRNA microarray, Pten expression was upregulated, but in the transformed bladder (5/5) and kidney cell lines (5/6), the qRT-PCR assay found Pten to be downregulated. The RNA used in the mRNA microarrays was evaluated by a GeneChip Scanner 3000 (Affymetrix) using the internal control genes, β-actin and Gapdh. Also, different primers were used in the qRT-PCR assay. These facts may have contributed to the differences of gene expression observed between the two methods.
Correlation of genomic copy number and gene expression
We were then interested in determining (i) the consequences of the observed widespread aneuploidy of specific chromosomes on gene expression levels in general and (ii) of the localized amplicons targeting Myc and Mdm2 in particular. We observed a genome-wide transcriptional deregulation in which expression levels of genes residing on aneuploid chromosomes followed genomic copy numbers, i.e. we observed an average message increase of genes on chromosomes that were gained, and reduced expression on chromosomes that were lost (Figure 4A). Therefore, genomic imbalances resulted in a massive disturbance of the transcriptional equilibrium in our models. This is consistent with observations in human carcinomas (for review, see ref. 4). The consequences of high-level genomic amplification of Myc and Mdm2 were similar: the expression levels of both oncogenes closely followed genomic copy number, as visualized in correlation plots displayed in Figure 4B for Myc and Figure 4C for Mdm2.
Fig. 4.
Correlations of genomic copy number and gene expression levels. (A) Correlation of average genomic copy number and average gene expression for all genes for all chromosomes in five bladder (gray triangles) and six kidney (black circles) cell lines. Copy number gains and losses of all 20 chromosomes (1–19, and X, excluding the Y) as assessed by aCGH followed the same directionality as the average of the gene expression for genes analyzed by the mRNA microarray. (B and C) Correlation of genomic copy number and gene expression for Myc (upper panel) and Mdm2 (lower panel). The gene expression versus the copy numbers for these two oncogenes was plotted for five bladder and six kidney transformed cell lines and displayed as black diamonds. The cell lines containing dmin chromosomes (the two outliers on each graph) displayed the highest degree of correlation between copy number increases determined by aCGH compared with their gene expression values assessed by mRNA microarrays.
Pathway analyses
As the epithelial cells progressed through immortalization and entered into the transformation stage (Figure 1A–C), we also observed an accumulation of actin and multiple protrusions (lamellipodia and pseudopodia) at the surface of the kidney cells (Figure 5A, right panel), which are common features of epithelial to mesenchymal transition (EMT), a process associated with invasiveness and metastasis (24). Consistent with the process of EMT was also our observation that the cells that were tumorigenic showed profoundly higher migratory potential when assayed in a scratch assay (Figure 5B). The ability of the transformed cell lines to migrate corresponded with the ability of cells to produce tumors in the nude mouse assay (11).
Fig. 5.
Morphology of spontaneously transformed cells, scratch assay, gene expression of Cd44, keratins and gene expression of EMT and CIN25 signature during transformation process. In order to visualize changes in the cytoskeleton during the transformation process, here kidney cells (A) undergoing thetransformation process were stained with a fluorescein isothiocyanate-labeled antibody for actin (green), and DNA stained with the counterstain 4′,6-diamidino-2-phenylindole (orange pseudocolor). The cells became flat at the preimmortal stage, became spindle shaped at the immortal stage and exhibited multiple aggregates of actin at the transformed stage, with cellular projections resembling pseudopodia, often associated with invasiveness in human tumors. (B) The scratch assay was used to determine the level of invasiveness for of non-tumorigenic and tumorigenic cell lines (as determined previously with the nude mouse assay). Shown here are two kidney cell lines: NCI-HPN-M1C Kid (upper row) and NCI-HPB-F1B Kid (lower row). Photographs were taken at 0 h (left panels—both rows) and 30 h (right panels—both rows). The degree of invasiveness correlated well with the nude mouse assay results (for nude mouse assay, see Padilla-Nash et al. (11)). (C) Expression changes of 12 genes involved in EMT in bladder and kidney samples. Genes associated with cell adhesion (Ocln, Dsp) were lost as early as the preimmortal stage and metastatic genes (Fn1, Twist 2) were highly upregulated at the transformed stage. Blue diamonds = normal uncultured cells; yellow triangles = preimmortal; green squares = immortal; red circles = transformed. (D) Gene expression values for Cd44 in normal uncultured epithelial cells compared with cells at the preimmortal, immortal and transformed stages. Cd44 was highly upregulated as early as the preimmortal stage and remained high throughout progression in five bladder and six kidney cultures/cell lines relative to controls (normal uncultured cells). Blue, controls; yellow, preimmortal; green, immortal; red, transformed. (E) Gene expression changes for a set of keratin genes in controls (uncultured normal bladder cells), five bladder cultures and derivative cell lines. Blue diamonds = normal uncultured cells; yellow triangles = preimmortal; green squares = immortal; red circles = transformed. (F) Gene expression changes for genes involved in CIN derived from the Carter CIN gene list in five bladder and six kidney cultures and cell lines. All values reflect log2 ratios. Blue diamonds = normal uncultured cells; yellow triangles = preimmortal; green squares = immortal; red circles = transformed.
In order to explore whether these morphological changes were reflected by specific alterations of the transcriptome, we first extracted the expression levels of specific genes known to be both upregulated and downregulated during EMT in human cancers. This gene list was not based on existing databases because databases usually only show an association, but not directionality. The method utilized for obtaining a list of the gene expression patterns of EMT genes was performed first by reading numerous reviews about the EMT process and next locating information about EMT-related genes using Entrez Gene: http://www.ncbi.nlm.nih.gov/gene/. The last step involved literature searches using PubMed: http://www.ncbi.nlm.nih.gov/pubmed and reviewing the data to determine if during EMT the genes being queried were upregulated or downregulated. As shown in Figure 5C, among the 12 genes given as examples, genes that code for adherent cell proteins (Cdh1, Cldn7, Cldn8, Ocln and Dsp) exhibited significantly lower expression levels in transformed cells compared with normal control than genes coding for proteins that promote invasiveness or genes associated with a mesenchymal phenotype (Smad2, Snai2, Mmpl11, Col1a1, Fn1, Twist1 and Twist2). This observation applied to both bladder and kidney tissues. Cd44, a potential marker for tumor initiating cells and for EMT, was significantly increased in expression as well throughout all stages (Figure 5D). Fluorescence-activated cell sorting for stem cell markers Cd44, Cd133 and Oct4 revealed that 90–99% of the transformed cell populations was positive for these three markers (the results for Cd44 are shown in Supplementary Figure 5, available at Carcinogenesis Online). This overexpression was coincidental with the downregulation of specific cytokeratins, consistent with EMT (Figure 5E) (25). The complete list of genes that we comprised in our EMT gene set is provided as Supplementary Table VI, available at Carcinogenesis Online.
The cells in our study are characterized by profound CIN, as shown by aCGH, SKY and FISH. To further validate our models, we therefore queried to which extent genes known to define the expression signature of CIN in human cancers were deregulated during spontaneous transformation of murine cells. This comparison was based on the gene expression signature identified by Carter et al. (26); our results revealed a remarkable overlap (Figure 5F). Their list includes genes involved in DNA repair, mitosis, centrosome regulation and aneuploidy. The majority of these genes were upregulated in our models as early as the preimmortal stage. For instance, Aurka (aurora kinase A) (bladder: log2-fold change = 2.99, P = 0.000001; kidney: log2-fold change = 2.24, P = 0.00054) and Tpx2 (bladder: log2-fold change = 3.06, P = 0.000001) were highly upregulated. The expression levels of the ‘Carter CIN genes’ are provided in Supplementary Table VII.
Discussion
The distribution of chromosomal aneuploidies and the landscape of resulting genomic imbalances are defining features of human carcinomas; in general, the pattern of genomic imbalances is sufficient to discern distinct entities of epithelial tumors (1–3). These cancer-specific genomic imbalances directly affect the expression levels of resident genes, hence resulting in a massive, genome-wide transcriptional deregulation (4). To which extent such aneuploidy-dependent expression changes contribute to tumorigenesis is not clear; however, it is a fundamental question in tumor biology, which could be addressed with the help of suitable mouse models. Mouse tumors induced by strong oncogenic stimuli, however, tend to have lower levels of CIN relative to human tumors, thus rendering them less valuable to study the role of aneuploidy in malignant transformation (10). We, therefore, took advantage of the propensity of murine epithelial cells to spontaneously transform and to become tumorigenic and characterized this process with respect to phenotype, genome mutations and alterations of the cellular transcriptome. Our study was focused on epithelial cells from bladder and kidney from which we established five and six transformed cell lines, respectively. The molecular cytogenetic characterization and details of the methodologies used to establish these lines (in addition to bladder and kidney, we also established cell lines from lung, colon, cervix and mammary gland) have been published elsewhere (11).
Genomic profiling by SKY and aCGH revealed several recurrent genomic imbalances: gains of MMU5, 10, 11, 15 and 17 and losses of MMU4, 9 and 12 are required for the transformation process in both organs, but the gains of MMU3, MMU8 and the distal region of MMU6 are specific for bladder, whereas increased copy number gains of MMU19 and the loss of MMU13 are specific for kidney. These findings are reminiscent of the patterns observed in human tumorigenesis. Also consistent with the sequential steps of human tumorigenesis, as seen during the development of colorectal or cervical cancers through defined stages of increasing cellular dysplasia, is the observation that chromosomal aneuploidies occur early in the transformation process and that high-level oncogene amplifications occur on those chromosome that are gained at early stages (27). MMU10 and MMU15 serve as examples: Mdm2 and Myc were subject to enormous copy number increases at the transformed stage, exhibited as dmin chromosomes in the bladder cell lines. The question to which extent genes on the gained chromosomes other than the obvious candidates are drivers of tumorigenesis remains open, but can now be addressed experimentally.
Gene expression levels follow genomic copy number. This has now been established in yeast, in experimental systems in which chromosomes were introduced artificially, in mammalian cell lines and in primary tumors (for review, see ref. 4). The models that we present here are no exception. We observed a genome-wide correlation, which resulted in chromosome-wide low-level gene expression changes as a consequence of genomic imbalances. This similarity with human tumors now affords us the possibility to use our novel models to study whether the aneuploidy-dependent transcriptional deregulation can be pursued as a therapeutic target, by, e.g., manipulating the chromatin and histone code and analyzing the consequences of such manipulations with respect to global gene expression levels (Figure 4).
All 11 cell lines were profiled by array-based gene expression analysis at three defined stages of preimmortalization, immortalization and transformation and the results compared with uncultured, organ- and sex-matched controls as well as among the distinct stages. We observed by far the most pronounced changes in gene expression when comparing the earliest stage of immortalization to controls. This is reflected in the principal component analysis shown in Figure 3A and B, in which the controls cluster most tightly and are furthest apart from the immortalized and transformed samples. This would be indicative of the fact that the phenotypic changes between normal cells and cells that are in the process of immortalization are most profound. It would also indicate that the cellular gene expression program, which is eventually responsible for transformation, changes early. It is, though, consistent with the early acquisition of specific aneuploidies, which have, as discussed above, global effects on the transcriptome. Gene expression changes that define the transition from an immortalized to a transformed stage appear to be subtle (Figure 3D).
Two characteristics of the transformation process were most prominent: (i) the obvious change in morphology from an epithelial to a more mesenchymal phenotype and (ii) the early acquisition of pronounced CIN. We, therefore, were curious to which extent these changes were evident in the cancer transcriptome. Toward that goal, we generated a list of genes from the literature involved in EMT and observed a highly significant overlap with gene expression changes in our models. Of note, the directionality of changes was as would have been expected from loss of cell adhesion, increased invasiveness and metastasis, and included loss of Cdh1/E-cadherin, gains of Fn1, and Twist2, which have been observed in both human bladder and kidney cancers (28,29). Recently, Carter et al. (26) reported a CIN25 signature in multiple human cancers. Similar to the EMT signature, the list of genes responsible for CIN in human cancer showed a remarkable overlap with genes deregulated in our models. In particular, genes involved in the regulation of centrosome duplication, e.g. Aurka and Tpx2 (30–33), were highly upregulated very early in the transformation process and accompanied the aneuploidy and centrosome abnormalities observed in our cultures as early as 48 h (11). We submit that these findings provide credence to our models.
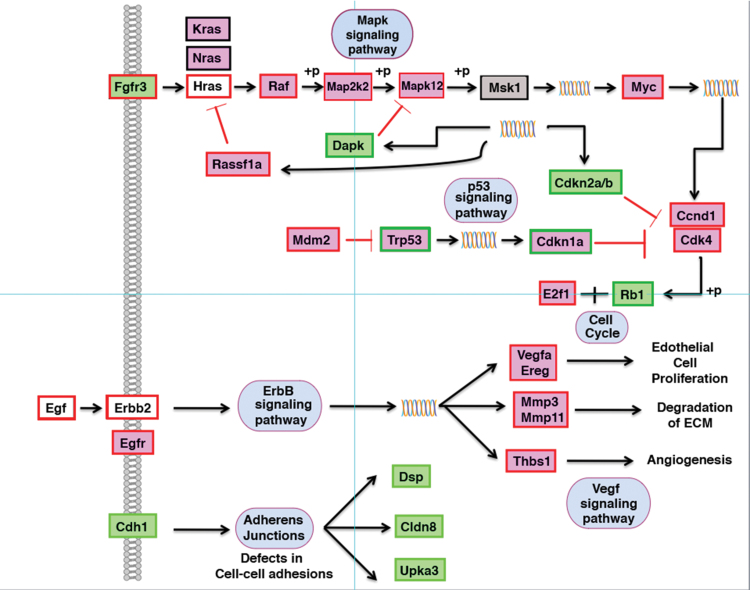
The validation of our models was supported by a comprehensive and systematic comparison with deregulated pathways in human bladder and kidney cancers. This comparison was based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for human bladder and kidney cancers (34). When we overlapped the gene expression data from our murine model onto the human KEGG cancer pathways, we observed that the superimposition of gene expression changes overlapped with several of the homologous genes in the same directionality (Figure 6 and Supplementary Figure 6, available at Carcinogenesis Online). A recently published review of bladder cancer murine models describes two major pathways associated with human bladder cancer. One involving the FGFR3/HRAS pathway, the other is the TRP53 signaling pathway, which includes MDM2, TRP53, CDKN2A, CDKN1A, CCND1, CDK4, RB1 and E2F1 (35). In our murine bladder model, Rb1 is statistically significantly downregulated (P = 0.00100178, log2-fold = 1.27075), in the transformed cell lines relative to the controls (normal bladder epithelial cells), and Ccnd1, Cdk1 and E2f1 all follow the same directionality of gene expression patterns as those observed in human bladder cancers. In addition, Egfr was highly upregulated in the bladder cell lines. This gene has been recently described as inducing human bladder cancer (36).
Fig. 6.
Comparison of gene expression changes in human bladder cancer and our mouse model based on KEGG pathway analysis. The information derived from the gene expression analysis of the transformed bladder cell lines has been superimposed onto the human KEGG pathway display. Red border, genes upregulated in human; green border, genes downregulated. Pink boxes, genes upregulated in mouse; green boxes, genes downregulated in mouse. No shading, no change in expression of this gene in mouse; gray shading, gene was not on the array. The majority of genes deregulated in human bladder cancer is also deregulated in the mouse model for bladder cancer (Myc, Mdm2, Ccnd1, Cdkn2a/b, Rb1, etc.) and followed the same directionality as the human genes.
EGFR3 mutations have been frequently reported in human bladder cancers; however, in our model, Egfr3 was downregulated rather than being activated. With respect to the lack of correlation for FGFR3 between the two species, there are several reports indicating that the presence of mutations of FGFR3 and increased copy numbers of human chromosome arm 5p in bladder cancer are mutually exclusive (37,38). In our mouse bladder model, we have detected copy number gains for MMU15 using SKY and aCGH. MMU15 contains regions homologous to human 5p (Supplementary Figure 2, available at Carcinogenesis Online).
VHL is an important tumor suppressor found in renal cancers; however, in our model, this gene, Vhlh1, was not downregulated. Interestingly, the elongin-BC ubiquitinin complex (includes CUL2, RBX1), which is regulated by VHL, was upregulated in our model (39). Our data revealed that two important tumor suppressors found in different types of renal cancers, Fh1/fumarate and Wt1/Wilm’s tumor, were statistically significantly downregulated. In addition, the transformed kidney cell lines displayed upregulation of Raf1, Akt1 and Shp2, which all have been associated with human renal cancers (40–42). Global gene expression profiles revealed the involvement of genes that resulted in an EMT-like phenotype during transformation in both models (Figure 5 and Supplementary Table VI, available at Carcinogenesis Online). The overexpression or downregulation of several genes known to be involved in human bladder and kidney cancers matched what we observed in the mouse models in the same directionality.
Review of the literature regarding spontaneous transformation described that human cells were very resistant to in vitro immortalization owing to the Hayflick limit, which was less restrictive in rodent cells (11). In addition, when human epithelial tumors are cultured in vitro and develop into cell lines, they often have been derived from advanced stages of cancer.
The sequential transformation of murine epithelial cells requires the acquisition of transformation and tissue-specific chromosomal aneuploides. The resulting genomic imbalances led to a massive, aneuploidy-dependent deregulation of the cellular transcriptome. Our murine cell lines represent earlier time points for cells that have become immortalized and most interestingly exhibit significant deregulation of several genes associated with centrosome regulation, DNA repair, chromosome segregation, EMT and CIN with the expected directionality well before their ability to form tumors. Taken together, our results show that both morphological and genetic changes during spontaneous transformation of murine epithelial cells reveal remarkable similarity with the respective aberration profiles and affected cellular signaling pathways observed during human carcinogenesis. As an extension to this, we propose that our models are suitable for preclinical assessment of the efficacy of candidate drugs.
In summary, we established new cancer models for bladder and kidney tumors, analyzed their genomic mutation profile and identified a set of deregulated genes, which comprise the Carter et al. (26) CIN25 signature and genes involved in EMT, which overlap with genes deregulated in the human counterpart of these tumor entities. These findings validate our models.
Supplementary material
Supplementary Methods, Tables I–VII and Figures 1–6 can be found at http://carcin.oxfordjournals.org/
Funding
Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Supplementary Material
Acknowledgements
We would like to acknowledge the following colleagues for their contributions to this project: Darawalee Wangsa (SKY and FISH probes), Raluca Yonescu (FISH hybridizations), Linda Barenboim (FISH probes and RT-PCR), Timo Gaiser and Maria Becker (flow-sorting), Jordi Camps (aCGH human bladder cell lines), Kathleen Dorritie (RNA extraction), Joseph Cheng and Buddy Chen (IT support).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- aCGH
array CGH
- CIN
chromosomal instability
- dmin
double minute
- EMT
epithelial to mesenchymal transition
- GEO
Gene Expression Omnibus
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- mRNA
messenger RNA
- qRT-PCR
quantitative real-time PCR.
References
- 1. Ried T. (2009). Homage to Theodor Boveri (1862–1915): Boveri’s theory of cancer as a disease of the chromosomes, and the landscape of genomic imbalances in human carcinomas. Environ. Mol. Mutagen., 50, 593–601 [DOI] [PubMed] [Google Scholar]
- 2. Heim S., et al. (2009). Cancer Cytogenetics. John Wiley & Sons: Hoboken: [Google Scholar]
- 3. Beroukhim R., et al. (2010). The landscape of somatic copy-number alteration across human cancers. Nature, 463, 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ried T., et al. (2012). The consequences of chromosomal aneuploidy on the transcriptome of cancer cells. Biochim. Biophys. Acta, 1819, 784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Green J., et al. (2011). Genetically Engineered Mice for Cancer Research: Design, Analysis, Pathways, Validation and Pre-clinical Testing. Springer, New York, NY; [Google Scholar]
- 6. Weaver Z.A., et al. (1999). A recurring pattern of chromosomal aberrations in mammary gland tumors of MMTV-cmyc transgenic mice. Genes Chromosomes Cancer, 25, 251–260 [PubMed] [Google Scholar]
- 7. Montagna C., et al. (2002). Centrosome abnormalities, recurring deletions of chromosome 4, and genomic amplification of HER2/neu define mouse mammary gland adenocarcinomas induced by mutant HER2/neu. Oncogene, 21, 890–898 [DOI] [PubMed] [Google Scholar]
- 8. Montagna C., et al. (2003). The Septin 9 (MSF) gene is amplified and overexpressed in mouse mammary gland adenocarcinomas and human breast cancer cell lines. Cancer Res., 63, 2179–2187 [PubMed] [Google Scholar]
- 9. Weaver Z., et al. (2002). Mammary tumors in mice conditionally mutant for Brca1 exhibit gross genomic instability and centrosome amplification yet display a recurring distribution of genomic imbalances that is similar to human breast cancer. Oncogene, 21, 5097–5107 [DOI] [PubMed] [Google Scholar]
- 10. Ried T., et al. (2004). Molecular cytogenetics of mouse models of breast cancer. Breast Dis., 19, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Padilla-Nash H.M., et al. (2012). Spontaneous transformation of murine epithelial cells requires the early acquisition of specific chromosomal aneuploidies and genomic imbalances. Genes Chromosomes Cancer, 51, 353–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Padilla-Nash H.M., et al. (2006). Spectral karyotyping analysis of human and mouse chromosomes. Nat. Protoc., 1, 3129–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knutsen T., et al. (2005). The interactive online SKY/M-FISH & CGH database and the Entrez cancer chromosomes search database: linkage of chromosomal aberrations with the genome sequence. Genes Chromosomes Cancer, 44, 52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang C.C., et al. (2007). In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro . Nat. Protoc., 2, 329–333 [DOI] [PubMed] [Google Scholar]
- 15. Liyanage M., et al. (1996). Multicolour spectral karyotyping of mouse chromosomes. Nat. Genet., 14, 312–315 [DOI] [PubMed] [Google Scholar]
- 16. Duarte-Pereira S., et al. (2011). Prognostic value of opioid binding protein/cell adhesion molecule-like promoter methylation in bladder carcinoma. Eur. J. Cancer., 47, 1106–1114 [DOI] [PubMed] [Google Scholar]
- 17. Morris L.G., et al. (2011). Genomic dissection of the epidermal growth factor receptor (EGFR)/PI3K pathway reveals frequent deletion of the EGFR phosphatase PTPRS in head and neck cancers. Proc. Natl. Acad. Sci. USA., 108, 19024–19029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castets M., et al. (2012). DCC constrains tumour progression via its dependence receptor activity. Nature, 482, 534–537 [DOI] [PubMed] [Google Scholar]
- 19. Clark O., et al. (2012). Functional analysis of the putative tumor suppressor PTPRD in neuroblastoma cells. Cancer Invest., 30, 422–432 [DOI] [PubMed] [Google Scholar]
- 20. Inuzuka H., et al. (2012). Acetylation-dependent regulation of Skp2 function. Cell, 150, 179–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valiyeva F., et al. (2011). Characterization of the oncogenic activity of the novel TRIM59 gene in mouse cancer models. Mol. Cancer Ther., 10, 1229–1240 [DOI] [PubMed] [Google Scholar]
- 22. Colecchia D., et al. (2012). MAPK15/ERK8 stimulates autophagy by interacting with LC3 and GABARAP proteins. Autophagy, 8, 1724–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kontos C.K., et al. (2012). Molecular cloning of novel alternatively spliced variants of BCL2L12, a new member of the BCL2 gene family, and their expression analysis in cancer cells. Gene, 505, 153–166 [DOI] [PubMed] [Google Scholar]
- 24. Lim J., et al. (2012). Epithelial-mesenchymal transitions: insights from development. Development, 139, 3471–3486 [DOI] [PubMed] [Google Scholar]
- 25. Thiery J.P., et al. (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139, 871–890 [DOI] [PubMed] [Google Scholar]
- 26. Carter S.L., et al. (2006). A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat. Genet., 38, 1043–1048 [DOI] [PubMed] [Google Scholar]
- 27. Ried T., et al. (1999). Genomic changes defining the genesis, progression, and malignancy potential in solid human tumors: a phenotype/genotype correlation. Genes., Chromosomes Cancer., 25, 195–204 [DOI] [PubMed] [Google Scholar]
- 28. van der Horst G., et al. (2012). Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol. Cancer Res., 10, 995–1009 [DOI] [PubMed] [Google Scholar]
- 29. Bai S., et al. (2012). Cdc42-interacting protein-4 promotes TGF-Β1-induced epithelial-mesenchymal transition and extracellular matrix deposition in renal proximal tubular epithelial cells. Int. J. Biol. Sci., 8, 859–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fraizer G.C., et al. (2004). Aurora-A/STK15/BTAK enhances chromosomal instability in bladder cancer cells. Int. J. Oncol., 25, 1631–1639 [PubMed] [Google Scholar]
- 31. Yamamoto Y., et al. (2009). Intercellular centrosome number is correlated with the copy number of chromosomes in bladder cancer. Cancer Genet. Cytogenet., 191, 38–42 [DOI] [PubMed] [Google Scholar]
- 32. Bufo P., et al. (2010). Expression of mitotic kinases phospho-aurora A and aurora B correlates with clinical and pathological parameters in bladder neoplasms. Histol. Histopathol., 25, 1371–1377 [DOI] [PubMed] [Google Scholar]
- 33. Aguirre-Portoles C., et al. (2012). Tpx2 controls spindle integrity, genome stability, and tumor development. Cancer Res., 72, 1518–1528 [DOI] [PubMed] [Google Scholar]
- 34. Kanehisa M., et al. (2012). KEGG for integration and interpretation of large-scale molecular datasets Nucleic Acids Res ., 40, D109–D114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahmad I., et al. (2012). Exploring molecular genetics of bladder cancer: lessons learned from mouse models. Dis. Model. Mech., 5, 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Izumi K., et al. (2012). Epidermal growth factor induces bladder cancer cell proliferation through activation of the androgen receptor. Int. J. Oncol., 41, 1587–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zieger K., et al. (2009). Consistent genomic alterations in carcinoma in situ of the urinary bladder confirm the presence of two major pathways in bladder cancer development. Int. J. Cancer, 125, 2095–2103 [DOI] [PubMed] [Google Scholar]
- 38. Lindgren D., et al. (2012). Integrated genomic and gene expression profiling identifies two major genomic circuits in urothelial carcinoma. PLoS One, 7, e38863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okumura F., et al. (2012). The role of elongin BC-containing ubiquitin ligases. Front. Oncol., 2, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuroda N., et al. (1998). Differential expression of SHP2, a protein- tyrosine phosphatase with SRC homology-2 domains, in various types of renal tumour. Virchows Arch., 433, 331–339 [DOI] [PubMed] [Google Scholar]
- 41. Hara S., et al. (2005). Akt activation in renal cell carcinoma: contribution of a decreased PTEN expression and the induction of apoptosis by an Akt inhibitor. Ann. Oncol., 16, 928–933 [DOI] [PubMed] [Google Scholar]
- 42. Cohen J.D., et al. (2011). cAMP-dependent cytosolic mislocalization of p27(kip)-cyclin D1 during quinol-thioether-induced tuberous sclerosis renal cell carcinoma. Toxicol. Sci., 122, 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.