Abstract
The morbidity and mortality of acute respiratory distress syndrome remain to be high. Over the last 50 years, the clinical management of these patients has undergone vast changes. Significant improvement in the care of these patients involves the development of mechanical ventilation strategies, but the benefits of these strategies remain controversial. With a growing trend of extracorporeal support for critically ill patients, we provide a historical review of extracorporeal membrane oxygenation (ECMO) including its failures and successes as well as discussing extracorporeal devices now available or nearly accessible while examining current clinical indications and trends of ECMO in respiratory failure.
Keywords: Acute respiratory distress syndrome, extracorporeal life support, extracorporeal membrane oxygenation
The initial mortality rates associated with acute respiratory distress syndrome (ARDS) were reported to be as high as 50% in 1967.[1] Published studies in the early 1990s demonstrated a reduction in mortality that continued to a nadir of 29-38% by the end of the decade.[2–6] More recently, the mortality associated with ARDS has remained steady at 25-30%.[7,8] In a recent study, there was no difference in mortality rates between early- and late-onset ARDS.[9] Although this reduction in ARDS mortality is not universally accepted, there has been some progress in the treatment of acute lung injury (ALI) and ARDS. This progress is directly related to the methods of respiratory support employed in the treatment of critically ill patients with ARDS. In this manuscript, we review the changes in conventional mechanical ventilation before moving onto methods of extracorporeal support. We discuss early failures and more recent success of extracorporeal membrane oxygenation (ECMO), while discussing alternative extracorporeal devices currently available or soon to be accessible. To conclude, we briefly discuss the current clinical applications and recent trends for the use of ECMO.
Conventional Respiratory Support for Acute Respiratory Distress Syndrome
Typically, therapeutic strategies hinge on the knowledge of the underlying disease process, but our understanding of ARDS is very limited. Despite our limited understanding of the pathophysiology, numerous clinical trials, including the ARDS network trial, suggested that specific ventilator management techniques could lead to superior outcomes.[10] Lung protective ventilation with adjusted positive end-expiratory pressure (PEEP) remains the most effective respiratory support method. It is now clear that high tidal volumes result in further lung injury and that the use of lower tidal volumes (6 ml/kg) may improve mortality.[10–14] Mechanical ventilation with lower tidal volumes, resulting in higher than normal CO2 partial pressures (permissive hypercapnia), is associated with not only a reduction in mortality but also a less number of days requiring ventilator use. The complications and mortality of severe lung injury and ARDS remain very high at 35-45%.[15]
A major reason for the slow progress in advancement of the treatment of ALI and ARDS is the lack of detailed knowledge of the pathophysiology of ARDS and the impact upon this physiology by the current treatment strategies. The current trend of permissive hypercapnia is constrained by the limits of tolerable respiratory acidosis which may cause substantial changes in hemodynamic function and organ blood flow unless the arterial pH is controlled.[16,17]
In refractory ARDS with profound hypoxemia or respiratory acidosis, additional non-pharmacological interventions are necessitated, such as positioning maneuvers, nitric oxide, reverse inspiratory:expiratory ratio ventilation strategies, airway pressure ventilation, partial liquid ventilation, or high-frequency ventilation techniques. Additionally, the use of extracorporeal support is now starting to be used more commonly. Extracorporeal technology may benefit this patient population by facilitating gas exchange without the harm associated with aggressive mechanical ventilation. Extracorporeal life support is a modified form of cardiopulmonary bypass used to provide prolonged gas exchange in patients with respiratory and/or cardiac failure. The devices require fairly large cannulas for continuous pumping of blood from the patient to a membrane oxygenator. In addition to oxygenation, CO2 can be efficiently removed with extracorporeal technology. A major limitation of this complicated form of intensive care is the need for anticoagulation to prevent blood clotting.
Extracorporeal membrane oxygenation in ARDS
In 1972, Hill et al. published the first case report that used ECMO in a patient who suffered from ARDS due to acute post-traumatic respiratory failure. At that time, mortality rates from ARDS were exceedingly high, and the idea of extracorporeal support in this population showed great promise.[18] However, a randomized controlled trial using ECMO in ARDS demonstrated mortality rates of >90% in both treatment arms.[19] Although this was a landmark paper by Zapol et al.[19] at that time, it suffered from significant flaws, including the use of only veno-arterial (VA) ECMO, termination of ECMO after 5 days was an option if no improvement occurred, significant problems with bleeding, the lack of “rest” ventilator settings, and the lack of experience of many participating centers. Despite these dismal results, several investigators continued to work in the area of extracorporeal devices and strategies. Extracorporeal CO2 removal paired with a novel ventilation strategy at that time, low-frequency positive pressure mechanical ventilation, demonstrated some improvement in results.[20–23] However, a randomized controlled trial failed to demonstrate an actual survival benefit from extracorporeal CO2 removal in ARDS, although the survival rates were substantially improved as compared to the 1970s.[24] Survival rates were 33% for the extracorporeal group and 42% for the conventional mechanical ventilation group.[24] The investigators were unable to show a survival benefit as the cohort study was small (n = 40) compared to the ARDS network trial on low tidal volumes that stopped recruitment after enrolling 861 patients.
Early poor outcomes with extracorporeal membrane oxygenation in ARDS
The lack of any substantial impact upon mortality in these early studies with extracorporeal CO2 removal in ARDS is multifactorial. Early extracorporeal CO2 devices were limited in their technology. In addition, ventilation strategies differed substantially at that time compared to today where low tidal volumes and airway pressure gradients are now used for protective ventilation.[10,11,25,26]
Extracorporeal devices continue to be used more frequently than ever before in intensive care units throughout the world. With advancements in technology, the new devices are less prone to complications. In addition to technological progress, there has been improvement related to the clinical application of extracorporeal support devices in individual patients, including early introduction and strategies for changing from devices as clinically indicated.[27,28]
Recent outcomes with ECMO in ARDS
The recent report of successful ECMO support in older patients inflicted with H1N1 influenza increased interests in the use of this mode of support in adult patients with severe respiratory failure.[29] Also that same year, the conventional ventilatory support versus ECMO for severe adult respiratory failure (CESAR) study was published. The investigators used a “pragmatic” study design and were criticized for the inability to standardize mechanical ventilation management in the conventional care group.[30] Importantly, the CESAR trial demonstrated that protocolized care that included ECMO in an expert center for ARDS care yielded higher survival than the best standard care in tertiary intensive care units in the UK.[30]
Innovations in ECMO
In the 1960s, a milestone in the technological evolution of ECMO was the development of membrane oxygenators, which began replacing bubble oxygenators. Membrane oxygenators provided gas exchange with the benefits of increased short- and long-term biocompatibility. The recently developed membrane-type oxygenators were less harmful as blood was exposed to oxygen through a gas-permeable membrane, which enhanced gas transfer compared to the bubble oxygenators. At the end of the 1990s, a silicone covering of the microporous polypropylene hollow fibers was used that had a heat exchanger and oxygenating compartment with a polymethylpentene (PMP) membrane in a small polycarbonate shell.[31] Development of the PMP oxygenator has proven to be an important advancement in ECMO technology as the PMP oxygenators have slowly replaced both the silicone membrane and polypropylene microporous oxygenators.[32,33] The PMP oxygenators result in a reduction of the need for red blood cell and platelet transfusions, provide better gas exchange, have lower resistance and priming volumes compared to silicone membrane oxygenators, and have less oxygenator failure compared to polypropylene microporous oxygenators.[34] The development of heparin-coated circuits allows for extracorporeal support with decreased platelet, complement, and granulocyte activation with reduced heparin requirements.[35,36] New generation centrifugal pumps permit support with essentially no risk of tubing rupture with a smaller priming volume and potentially reduced need for a reservoir.[37] Yet, another major innovation that has truly enhanced the capability of extracorporeal CO2 removal was the development of a bicaval dual-lumen catheter (Avalon Laboratories, Rancho Dominguez, CA, USA) that allows respiratory support through a single catheter for application of ECMO.[38]
Different modes of ECMO
In the setting of complete cardiopulmonary support, conventional VA ECMO is primarily used while primary respiratory failure, including severe oxygenation failure, is treated with veno-venous (VV) ECMO. Hypercapnic respiratory failure can be treated with either a VV or a VA approach. Both VV and VA approaches require a pump that is capable of generating flow rates of 3-5 l/min to assure sufficient organ perfusion and oxygenation in the adult size patient. Figure 1 illustrates the different cannulation strategies for the variable forms of ECMO support (VA, VV, and double lumen VV) currently used in patients. An arterio-venous (AV) approach can be used as well which does not require a pump. In specific circumstances, low-resistance devices can be used which allow sufficient blood flow driven by the systemic blood pressure from the patient. These pumpless extracorporeal lung assist (PECLA) devices achieve flow rates of 0.8-1.5 l/min allowing for sufficient CO2 removal.
Figure 1.
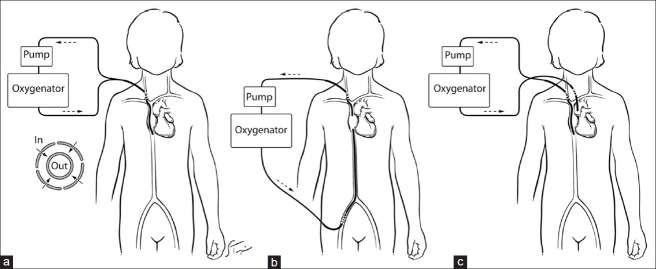
Three configurations of extracorporeal blood flow (a) single-site double lumen veno-venous (VV), (b) two-site VV, and (c) veno-arterial
Alternative extracorporeal devices
Arterio-venous CO2 removal
An AV shunt for extracorporeal gas exchange can potentially reduce the complexity of conventional ECMO, while allowing for gas exchange to achieve near total removal of CO2. The technique of extracorporeal AV CO2 removal (AVCO2R) was developed using a low-resistance, commercially available, hollow fiber gas exchanger to provide lung rest in the setting of severe respiratory failure.[39] Although AVCO2R is efficient in CO2 removal, it does not provide any substantial oxygen transfer. The initial human study with AVCO2R included five adult patients with ARDS and CO2 retention with percutaneous AVCO2R achieving approximately 70% CO2 removal in the cohort without hemodynamic compromise or instability, while oxygenation was successfully managed with gentle ventilation and near-apneic oxygenation.[40] All of these patients survived the study period without adverse sequelae and only minor complications.[40]
PECLA, Novalung,® and interventional lung assist (used interchangeably)
The early success of AVCO2R intensified the interest and increased the use of AVCO2R in Europe, which is termed PECLA, interventional lung assist (ILA) device, or Novalung® (Novalung GmbH, Talheim, Germany). These devices have a circuit that uses a hollow fiber gas exchanger without the need for a pump.
An in vivo study with an ovine model was undertaken to determine the efficacy of the Novalung® circuits in the short-term removal of CO2 and to assess hemodynamic responses.[41] The animal was cannulated in the jugular vein and carotid artery for 72 h.[41] The Novalung® device provided near total CO2 removal (mean: 119.3 ml/min) with device blood flow rates (Qb), 1 l/min; sweep gas flow rates (Qg), 5 l/min; and PaCO2, 40-50 mmHg.[41] The PaCO2 level was also found to be directly proportional to the CO2 clearance for the device.[41] In another in vivo study with a pig model, ILA was studied to determine the device′s ability to improve oxygenation with cannulation through both femoral arteries and one femoral vein with the animals anesthetized and mechanically ventilated.[42] With the application of ILA, the arterial partial pressure of oxygen was increased from 64 ± 13 mmHg to 71 ± 14 mmHg and 74 ± 17 mmHg with blood flow through one and two femoral arteries, respectively.[42] The increase in oxygenation was small, but was significant; thus, the results indicated that ILA may not be warranted if oxygenation is the primary therapeutic goal.[42] Two further studies demonstrated that the Novalung® device provided adequate gas exchange without hemodynamic instability with static ventilation at PEEP pressures ≥10 cm H2O.[43,44] A third animal study, using an ARDS model, was performed to prospectively evaluate the effects of ILA on hemodynamics and gas exchange in cardiopulmonary resuscitation.[45] Ventricular fibrillation was induced in the lung injured, mechanically ventilated pig with chest compressions starting immediately and continuing for 30 min in the anesthetized animals.[45] The experimental group (open ILA system) showed a marked decrease in PaCO2 and increase in PaO2 without a significant difference in systolic and mean blood pressure compared to the control group (clamped ILA system).[45]
The Novalung® system has been investigated in Europe since 1996 and has been established as a therapeutic measure for a variety of lung conditions. In one study, the Novalung® was used easily and inexpensively used in 1,800 patients for artificial lung assistance.[46] Furthermore, the PECLA device was effective at oxygenation and CO2 removal in 70 patients with severe respiratory failure of various etiologies.[47] A 10-year-review outlined experience in 159 patients ranging in age from 7 to 78 years who were treated with PECLA for ARDS (70.4%) and pneumonia (28.3%).[48] The study had a cumulative experience of over 1,300 days.[48] During the study period, the overall mortality was 48.7%, mostly attributed to multiorgan failure.[48] Inability to stabilize pulmonary function was noted in only 3% of patients, and the 30-day mortality after PECLA was 13.6%.[48] Numerous case reports, retrospective analyses, and prospective studies have validated the PECLA for use as a therapeutic measure for CO2 removal in a wide variety of etiologies of acute respiratory failure including ARDS[49–67] and as a bridge to lung transplantation.[52–56] The PECLA system has been proven to be superior to lung assist devices that require a pump because it significantly reduces bleeding, hemolysis, and mechanical trauma to the blood.
A multitude of studies have demonstrated that the Novalung® facilitates the reversal of hypercapnia, while stabilizing oxygenation with the only reported complication being reversible distal limb ischemia.[68–80] In a large cohort study with 96 patients with severe ARDS, the application of ILA significantly increased the PaO2/FiO2 ratio, while improving the PaCO2 and pH within 2 h in all patients.[81] The ILA eliminated approximately 50% of calculated total CO2 produced with rapid normalization of respiratory acidosis.[81]
Despite extensive study, the main disadvantages of ILA are arterial damage, immobilization, and cardiovascular steal. Therefore, newer technology was needed and has continued to evolve with the most device released being ILA activve® (Novalung GmbH, Talheim, Germany).
Intravenacaval (intravascular) oxygenator and CO2 removal device
Mortensen developed the concept of an intravenacaval (intravascular) oxygenator and CO2 removal device (IVOX) as an intracorporeal gas exchange device in patients with ARDS.[82,83] The components of IVOX include multiple hollow fibers, that are silicone coated and heparin bonded, to create a thin membrane, which are then placed in the vena cava to provide blood oxygenation and CO2 removal, without the need for extracorporeal circulation or blood transfusion. The fibers join together in a manifold that communicates with the dual-lumen gas conduit at both the proximal and distal ends.
In animal models, implantation of the IVOX device did not adversely affect hemodynamic function and there was no evidence of significant hemolysis, thromboembolism, foaming in the blood, catheter migration, or vena caval intimal injury.[84–87] In the initial design, the IVOX was capable of removing up to 30% of CO2 production in an ovine model (normal: 150-180 ml/min) of severe smoke inhalation injury.[84] The IVOX device was easy to use, but generated somewhat variable results, for instance when there were changes in cardiac output, instability in metabolism, or compensation in respiration. The IVOX device has had limited capacity in comparison to natural lungs.[87] The IVOX device in animal and human studies has demonstrated an average of 40 ml/min of CO2 removal and oxygen exchange, approximately 25-30% of the metabolic demands of the patients implanted with the device.[85,86] The end result was that IVOX could not be recommended as an alternative for ECMO or provide total support for patients with acute respiratory failure.
An international multicenter Phase I-II clinical trial of IVOX with a total of 164 IVOX devices used in 160 patients with acute respiratory failure, due to a variety of causes (lung infection, trauma, sepsis, and ARDS), found an immediate improvement in blood gas findings in a majority of patients, which allowed for a reduction in ventilator settings.[88] The overall survival of patients who were treated with the IVOX device was only 30% and was directly related to the severity of lung injury and patient selection. Device complications included mechanical and/or performance problems and user errors, whereas patient complications included bleeding, thrombosis, infection, venous occlusion, and arrhythmias.[88] Due to these experiences with IVOX, significant improvements are needed in the design and engineering of the device in order for it to become more clinically applicable.
Intravascular lung assist device
The intravascular lung assist device (ILAD) was designed by placing the membrane fibers into sub-units of rosette-like layers, with a surface area of 0.4-0.6 m2 perpendicular to blood flow with the same intravascular placement.[89] The device achieved 100 ml/min of both oxygen and CO2 exchange, but the blood pressure gradient required to overcome the resistance of the device to attain this gas exchange was high (23-105 mmHg). Further attempts were unsuccessful with the fibers in a helical form.[90] Unlike conventional devices which depend on passive bulk blood flow around them, this “pumping” ILAD causes an active driving force for the blood when rotated.
Hattler respiratory assist catheter
The Hattler respiratory assist catheter incorporates a small pulsating balloon into the middle of a hollow fiber bundle. Functioning characteristics of the device have been studied in vivo and in vitro studies.[91] The balloon allows for convective mixing of the blood and thus increases gas exchange. A larger balloon volume and higher pulsation rate have been shown to increase both oxygen loading and CO2 removal in a linear fashion in an in vitro model.[91] In another study, application of a random balloon pulsation did not significantly impact gas exchange within the respiratory assist catheter.[92] Despite these advances, the clinical use of an intravenous respiratory assist device is impeded by the insertion diameter of the catheter due to the catheter being dependent upon the critical number of hollow fiber membranes necessary to achieve gas exchange. The current catheters being prepared for human clinical trials require an insertion size of 32 Fr, even with optimal gas exchange efficiency of the pulsating balloon. Therefore, efforts are moving forward with the development of an impeller percutaneous respiratory assist catheters (iPRAC) with an insertion size <25 Fr.[93] The limitation to the clinical application of this sort of a catheter is the ability to protect the vascular endothelium from rotating fibers.
The latest concept in respiratory assist catheters is the development of iPRAC. The new design incorporates rotating impellers within a stationary bundle of hollow fiber membranes. Active mixing by rotating impellers produced 70% higher gas exchange efficiency than pulsating balloon catheters.[94] The iPRAC catheter has a diameter of 25 Fr and surface area of 0.07 m2 with no adverse effects on hemodynamic function in laboratory animals. Even though the CO2 removal efficiency of the iPRAC is currently the highest of any respiratory assist catheter, improvements are still needed before it can be used as a clinical device. Future studies will need to address optimal blood flow and gas exchange through the catheter and long-term efficacy of CO2 removal while assessing novel hollow fiber membrane coatings to facilitate additional CO2 removal.[94]
Decap and hemolung respiratory assist system®
More recently, the modification of a continuous VV hemodialysis machine was introduced that solely performs decapneization (CO2 removal) in conjunction with hemofiltration in a system called DECAP/DECAPsmart (Medica S.p.A., Medolla, Italy).[95] For intravascular access, a single double lumen cannula is inserted into the femoral vein with blood flow achieved by a non-occlusive roller pump with blood circulating through a membrane oxygenator then a hemofilter. Although this system does not allow for total gas exchange, it may augment CO2 removal that would further permit reduction of minute ventilation. Extracorporeal CO2 removal devices have been used in a Phase II study supporting the use of this technology in patients with severe ARDS who fail a trial of protective ventilation.[96] Similar dialysis-like systems are infiltrating the market now, including the Hemolung Respiratory Assist System®(ALung Technologies, Inc., Pittsburgh, PA, USA).
Clinical indications of ECMO
Figure 2 illustrates three key components of an ECMO circuit: The oxygenator, better bladder (venous compliance chamber) if used, and bubble detector. The oxygenator facilitates gas exchange and the better bladder controls pump flow as a function of inlet pressure, while bubble detection is a vital preventative measure. Guidelines describing indications and the practice of ECMO are published by the Extracorporeal Life Support Organization.[97] The generally accepted criterion for the initiation of ECMO is either acute severe cardiac or pulmonary failure or combination of both that is potentially reversible and unresponsive to conventional management. Examples of these clinical situations include the following etiologies: Hypoxic respiratory failure with a ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2) <100 mmHg despite optimal settings on mechanical ventilation, hypercapnic respiratory failure with an arterial pH < 7.20, refractory cardiogenic shock, cardiac arrest, failure to wean from cardiopulmonary bypass after cardiac surgery, and as a bridge to either heart or lung transplantation. Depending on the clinical situation, ECMO can even be implemented at the bedside of a patient [Figure 3].
Figure 2.
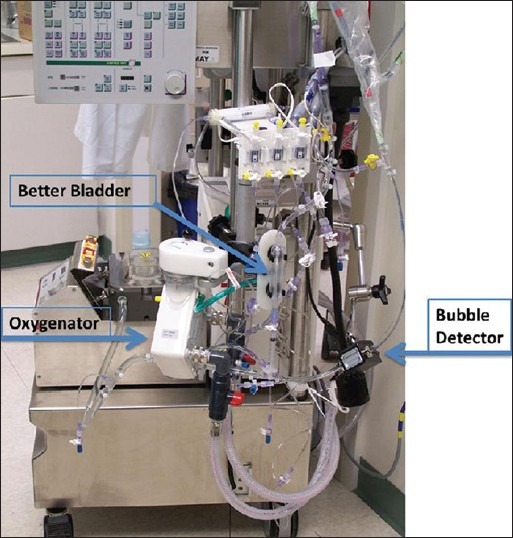
An extracorporeal membrane oxygenation circuit with the oxygenator, better bladder, and bubble detector
Figure 3.
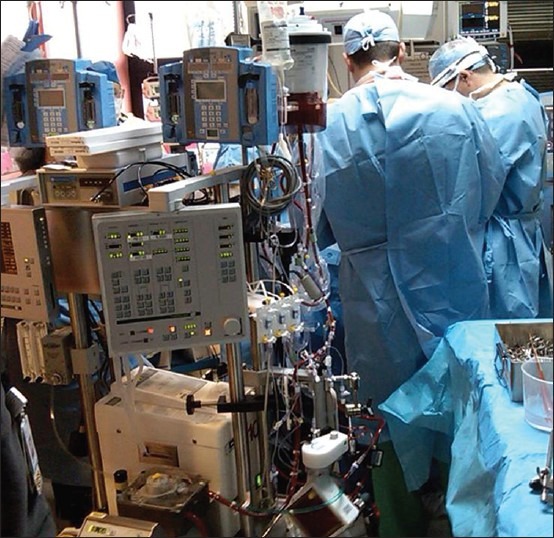
Implementation of veno-arterial extracorporeal membrane oxygenation at the beside of a patient
Clinically, VA ECMO provides complete cardiorespiratory support by extracting blood from the right atrium and returning it to the arterial system, therefore bypassing the heart and lungs. Contrasted to a VV approach, blood is extracted with VV ECMO from the vena cava or right atrium and returned to the right atrium with the patient still dependent on their intrinsic biventricular cardiac performance for hemodynamic support. Characteristically, VA ECMO is used for cardiac or combined cardiopulmonary failure, and VV ECMO is used for respiratory failure. The results of VV ECMO support for respiratory failure are similar to outcomes compared to VA ECMO, but with less morbidity secondary to improved neurological outcomes and preservation of arterial blood vessels.[98–102]
As discussed earlier, the development of a bicaval dual-lumen catheter (Avalon Laboratories, Rancho Dominguez, CA, USA) allows for respiratory support via application of ECMO through a single catheter site.[38] The catheter is inserted from the right jugular vein into the superior vena cava, traversing the right atrium to the inferior vena cava where it drains venous blood from both the superior and inferior vena cava and then directs oxygenated blood into the right atrium toward the tricuspid valve. The single site application of this method of VV ECMO in the neck permits ambulation and oral nutrition and has been used in patients as a bridge awaiting lung transplantation.[103–106] The theory behind this methodology of ambulatory ECMO [Figure 4] in patients with advanced lung disease requiring lung transplantation is to optimize both physical rehabilitation and nutrition prior to lung transplantation. To our knowledge, the use of ambulatory ECMO has not been attempted in ARDS, but the use of a simple circuit with the capability of total or near-total gas exchange should be considered in this population and may be a potential therapeutic option to liberate ARDS patients from mechanical ventilation.
Figure 4.
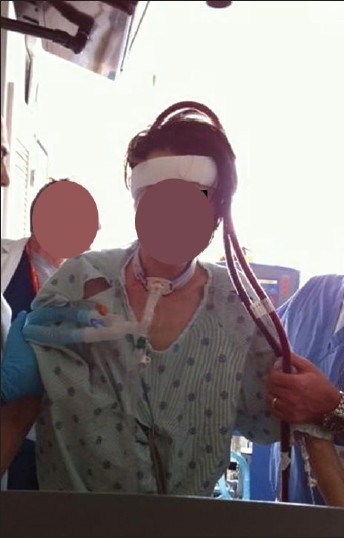
Ambulatory extracorporeal membrane oxygenation with single-site double lumen veno-venous approach being used in a patient as a bridge for lung transplantation
Conclusions
Extracorporeal treatment modalities are showing promise in the management of ARDS and are being increasingly used in intensive care units as rescue therapies in patients with ARDS who fail to respond to conventional mechanical ventilation. Patient selection and timing of the application of the extracorporeal device continue to be very important determining factors in the eventual outcome. The technology of the current devices is slowly evolving into smaller systems and will likely continue to shrink in size. As the risk of these devices decreases, their role in ARDS may increase but continues to not be well defined presently with further research needed in this patient population.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–23. [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983-1993. JAMA. 1995;273:306–9. [PubMed] [Google Scholar]
- 4.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–32. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 5.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 6.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD NIH NHLBI ARDS Network. Recent trends in acute lung injury mortality: 1996-2005. Crit Care Med. 2009;37:1574–9. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–24. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 8.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, Sakr Y, Groeneveld J, Zandstra DF, Hoste E, Malledant Y, et al. ARDS of early or late onset: Does it make a difference? Chest. 2010;137:81–7. doi: 10.1378/chest.09-0714. [DOI] [PubMed] [Google Scholar]
- 10.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distres syndrome The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 11.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–54. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 12.Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondéjar E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The multicenter trial group on tidal volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158:1831–8. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- 13.Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16:372–7. doi: 10.1007/BF01735174. [DOI] [PubMed] [Google Scholar]
- 14.Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and volume-limited ventilation strategy group. N Engl J Med. 1998;338:355–61. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- 15.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, et al. Has mortality from acute respiratory distress syndrome decreased over time.: A systematic review? Am J Respir Crit Care Med. 2009;179:220–7. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 16.Bidani A, Tzouanakis AE, Cardenas VJ, Jr, Zwischenberger JB. Permissive hypercapnia in acute respiratory failure. JAMA. 1994;272:957–62. [PubMed] [Google Scholar]
- 17.Cardenas VJ, Jr, Zwischenberger JB, Tao W, Nguyen PD, Schroeder T, Traber LD, et al. Correction of blood pH attenuates changes in hemodynamics and organ blood flow during permissive hypercapnia. Crit Care Med. 1996;24:827–34. doi: 10.1097/00003246-199605000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Hill JD, O′Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629–34. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 19.Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–6. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 20.Gattinoni L, Kolobow T, Tomlinson T, Iapichino G, Samaja M, White D, et al. Low-frequency positive pressure ventilation with extracorporeal carbon dioxide removal (LFPPV-ECCO2R): An experimental study. Anesth Analg. 1978;57:470–7. doi: 10.1213/00000539-197807000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Gattinoni L, Kolobow T, Agostoni A, Damia G, Pelizzola A, Rossi GP, et al. Clinical application of low frequency positive pressure ventilation with extracorporeal CO2 removal (LFPPV-ECCO2R) in treatment of adult respiratory distress syndrome (ARDS) Int J Artif Organs. 1979;2:282–3. [PubMed] [Google Scholar]
- 22.Gattinoni L, Agostoni A, Damia G, Cantaluppi D, Bernasconi C, Tarenzi L, et al. Hemodynamics and renal function during low frequency positive pressure ventilation with extracorporeal CO2 removal. A comparison with continuous positive pressure ventilation. Intensive Care Med. 1980;6:155–61. doi: 10.1007/BF01757297. [DOI] [PubMed] [Google Scholar]
- 23.Gattinoni L, Pesenti A, Pelizzola A, Caspani ML, Iapichino G, Agostoni A, et al. Reversal of terminal acute respiratory failure by low frequency positive pressure ventilation with extracorporeal removal of CO2 (LFPPV-ECCO2R) Trans Am Soc Artif Intern Organs. 1981;27:289–93. [PubMed] [Google Scholar]
- 24.Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF, Jr, Weaver LK, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305. doi: 10.1164/ajrccm.149.2.8306022. [DOI] [PubMed] [Google Scholar]
- 25.Villar J, Kacmarek RM, Pérez-Méndez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: A randomized, controlled trial. Crit Care Med. 2006;34:1311–8. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann M, Bein T, Arlt M, Philipp A, Rupprecht L, Mueller T, et al. Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: A prospective pilot study. Crit Care. 2009;13:R10. doi: 10.1186/cc7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshima K, Kunimoto F, Hinohara H, Okawa M, Mita N, Kanemaru Y, et al. Evaluation of prognosis in patients with respiratory failure requiring venovenous extracorporeal membrane oxygenation (ECMO) Ann Thorac Cardiovasc Surg. 2010;16:156–62. [PubMed] [Google Scholar]
- 28.Floerchinger B, Philipp A, Foltan M, Rupprecht L, Klose A, Camboni D, et al. Switch from venoarterial extracorporeal membrane oxygenation to arteriovenous pumpless extracorporeal lung assist. Ann Thorac Surg. 2010;89:125–31. doi: 10.1016/j.athoracsur.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 30.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 31.Mueller XM, Marty B, Tevaearai HT, Tozzi P, Jegger D, von Segesser LK. A siliconized hollow fiber membrane oxygenator. ASAIO J. 2000;46:38–41. doi: 10.1097/00002480-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Lawson DS, Lawson AF, Walczak R, McRobb C, McDermott P, Shearer IR, et al. North American neonatal extracorporeal membrane oxygenation (ECMO) devices and team roles: 2008 survey results of Extracorporeal Life Support Organization (ELSO) centers. J Extra Corpor Technol. 2008;40:166–74. [PMC free article] [PubMed] [Google Scholar]
- 33.Peek GJ, Killer HM, Reeves R, Sosnowski AW, Firmin RK. Early experience with a polymethyl pentene oxygenator for adult extracorporeal life support. ASAIO J. 2002;48:480–2. doi: 10.1097/00002480-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Khoshbin E, Roberts N, Harvey C, Machin D, Killer H, Peek GJ, et al. Poly-methyl pentene oxygenators have improved gas exchange capability and reduced transfusion requirements in adult extracorporeal membrane oxygenation. ASAIO J. 2005;51:281–7. doi: 10.1097/01.mat.0000159741.33681.f1. [DOI] [PubMed] [Google Scholar]
- 35.Moen O, Fosse E, Dregelid E, Brockmeier V, Andersson C, Høgåsen K, et al. Centrifugal pump and heparin coating improves cardiopulmonary bypass biocompatibility. Ann Thorac Surg. 1996;62:1134–40. doi: 10.1016/0003-4975(96)00492-4. [DOI] [PubMed] [Google Scholar]
- 36.Fosse E, Moen O, Johnson E, Semb G, Brockmeier V, Mollnes TE, et al. Reduced complement and granulocyte activation with heparin-coated cardiopulmonary bypass. Ann Thorac Surg. 1994;58:472–7. doi: 10.1016/0003-4975(94)92231-4. [DOI] [PubMed] [Google Scholar]
- 37.Lawson DS, Ing R, Cheifetz IM, Walczak R, Craig D, Schulman S, et al. Hemolytic characteristics of three commercially available centrifugal blood pumps. Pediatr Crit Care Med. 2005;6:573–7. doi: 10.1097/01.pcc.0000163282.63992.13. [DOI] [PubMed] [Google Scholar]
- 38.Bermudez CA, Rocha RV, Sappington PL, Toyoda Y, Murray HN, Boujoukos AJ. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg. 2010;90:991–5. doi: 10.1016/j.athoracsur.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Brunston RL, Jr, Zwischenberger JB, Tao W, Cardenas VJ, Jr, Traber DL, Bidani A. Total arteriovenous CO2 removal: Simplifying extracorporeal support for respiratory failure. Ann Thorac Surg. 1997;64:1599–604. doi: 10.1016/s0003-4975(97)01113-2. [DOI] [PubMed] [Google Scholar]
- 40.Zwischenberger JB, Conrad SA, Alpard SK, Grier LR, Bidani A. Percutaneous extracorporeal arteriovenous CO2 removal for severe respiratory failure. Ann Thorac Surg. 1999;68:181–7. [PubMed] [Google Scholar]
- 41.Zhou X, Loran DB, Wang D, Hyde BR, Lick SD, Zwischenberger JB. Seventy-two hour gas exchange performance and hemodynamic properties of NOVALUNG iLA as a gas exchanger for arteriovenous carbon dioxide removal. Perfusion. 2005;20:303–8. doi: 10.1191/0267659105pf838oa. [DOI] [PubMed] [Google Scholar]
- 42.Zick G, Frerichs I, Schädler D, Schmitz G, Pulletz S, Cavus E, et al. Oxygenation effect of interventional lung assist in a lavage model of acute lung injury: A prospective experimental study. Crit Care. 2006;10:R56. doi: 10.1186/cc4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen ND, Kjaergaard B, Koefoed-Nielsen J, Steensen CO, Larsson A. Apneic oxygenation combined with extracorporeal arteriovenous carbon dioxide removal provides sufficient gas exchange in experimental lung injury. ASAIO J. 2008;54:401–5. doi: 10.1097/MAT.0b013e31817e2b5f. [DOI] [PubMed] [Google Scholar]
- 44.Jungebluth P, Iglesias M, Go T, Sibila O, Macchiarini P. Optimal positive end-expiratory pressure during pumpless extracorporeal lung membrane support. Artif Organs. 2008;32:885–90. doi: 10.1111/j.1525-1594.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 45.Zick G, Schädler D, Elke G, Pulletz S, Bein B, Scholz J, et al. Effects of interventional lung assist on haemodynamics and gas exchange in cardiopulmonary resuscitation: A prospective experimental study on animals with acute respiratory distress syndrome. Crit Care. 2009;13:R17. doi: 10.1186/cc7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walles T. Clinical experience with the iLA membrane ventilator pumpless extracorporeal lung-assist device. Expert Rev Med Devices. 2007;4:297–305. doi: 10.1586/17434440.4.3.297. [DOI] [PubMed] [Google Scholar]
- 47.Liebold A, Philipp A, Kaiser M, Merk J, Schmid FX, Birnbaum DE. Pumpless extracorporeal lung assist using an arterio-venous shunt. Applications and limitations. Minerva Anestesiol. 2002;68:387–91. [PubMed] [Google Scholar]
- 48.Flörchinger B, Philipp A, Klose A, Hilker M, Kobuch R, Rupprecht L, et al. Pumpless extracorporeal lung assist: A 10-year institutional experience. Ann Thorac Surg. 2008;86:410–7. doi: 10.1016/j.athoracsur.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 49.Kopp R, Dembinski R, Kuhlen R. Role of extracorporeal lung assist in the treatment of acute respiratory failure. Minerva Anestesiol. 2006;72:587–95. [PubMed] [Google Scholar]
- 50.Iglesias M, Martinez E, Badia JR, Macchiarini P. Extrapulmonary ventilation for unresponsive severe acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg. 2008;85:237–44. doi: 10.1016/j.athoracsur.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Iglesias M, Jungebluth P, Petit C, Matute MP, Rovira I, Martínez E, et al. Extracorporeal lung membrane provides better lung protection than conventional treatment for severe postpneumonectomy noncardiogenic acute respiratory distress syndrome. J Thorac Cardiovasc Surg. 2008;135:1362–71. doi: 10.1016/j.jtcvs.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 52.Fischer S, Simon AR, Welte T, Hoeper MM, Meyer A, Tessmann R, et al. Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J Thorac Cardiovasc Surg. 2006;131:719–23. doi: 10.1016/j.jtcvs.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 53.Strueber M, Hoeper MM, Fischer S, Cypel M, Warnecke G, Gottlieb J, et al. Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant. 2009;9:853–7. doi: 10.1111/j.1600-6143.2009.02549.x. [DOI] [PubMed] [Google Scholar]
- 54.Taylor K, Holtby H. Emergency interventional lung assist for pulmonary hypertension. Anesth Analg. 2009;109:382–5. doi: 10.1213/ane.0b013e3181ac5461. [DOI] [PubMed] [Google Scholar]
- 55.Ricci D, Boffini M, Del Sorbo L, El Qarra S, Comoglio C, Ribezzo M, et al. The use of CO2 removal devices in patients awaiting lung transplantation: An initial experience. Transplant Proc. 2010;42:1255–8. doi: 10.1016/j.transproceed.2010.03.117. [DOI] [PubMed] [Google Scholar]
- 56.Haneya A, Philipp A, Mueller T, Lubnow M, Pfeifer M, Zink W, et al. Extracorporeal circulatory systems as a bridge to lung transplantation at remote transplant centers. Ann Thorac Surg. 2011;91:250–5. doi: 10.1016/j.athoracsur.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Zimmermann M, Bein T, Philipp A, Ittner K, Foltan M, Drescher J, et al. Interhospital transportation of patients with severe lung failure on pumpless extracorporeal lung assist. Br J Anaesth. 2006;96:63–6. doi: 10.1093/bja/aei274. [DOI] [PubMed] [Google Scholar]
- 58.Elliot SC, Paramasivam K, Oram J, Bodenham AR, Howell SJ, Mallick A. Pumpless extracorporeal carbon dioxide removal for life-threatening asthma. Crit Care Med. 2007;35:945–8. doi: 10.1097/01.CCM.0000257462.04514.15. [DOI] [PubMed] [Google Scholar]
- 59.Mallick A, Elliot S, McKinlay J, Bodenham A. Extracorporeal carbon dioxide removal using the Novalung in a patient with intracranial bleeding. Anaesthesia. 2007;62:72–4. doi: 10.1111/j.1365-2044.2006.04863.x. [DOI] [PubMed] [Google Scholar]
- 60.Twigg S, Gibbon GJ, Perris T. The use of extracorporeal carbon dioxide removal in the management of life-threatening bronchospasm due to influenza infection. Anaesth Intensive Care. 2008;36:579–81. doi: 10.1177/0310057X0803600424. [DOI] [PubMed] [Google Scholar]
- 61.Renner A, Neukam K, Rösner T, Elert O, Lange V. Pumpless extracorporeal lung assist as supportive therapy in a patient with diffuse alveolar hemorrhage. Int J Artif Organs. 2008;31:279–81. doi: 10.1177/039139880803100313. [DOI] [PubMed] [Google Scholar]
- 62.McKinlay J, Chapman G, Elliot S, Mallick A. Pre-emptive Novalung-assisted carbon dioxide removal in a patient with chest, head and abdominal injury. Anaesthesia. 2008;63:767–70. doi: 10.1111/j.1365-2044.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- 63.Haneya A, Philipp A, Foltan M, Mueller T, Camboni D, Rupprecht L, et al. Extracorporeal circulatory systems in the interhospital transfer of critically ill patients: Experience of a single institution. Ann Saudi Med. 2009;29:110–4. doi: 10.4103/0256-4947.51792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freed DH, Henzler D, White CW, Fowler R, Zarychanski R, Hutchison J, et al. Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A (H1N1) 2009 infection in Canada. Can J Anaesth. 2010;57:240–7. doi: 10.1007/s12630-009-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyer AL, Strueber M, Tomaszek S, Goerler A, Simon AR, Haverich A, et al. Temporary cardiac support with a mini-circuit system consisting of a centrifugal pump and a membrane ventilator. Interact Cardiovasc Thorac Surg. 2009;9:780–3. doi: 10.1510/icvts.2009.209783. [DOI] [PubMed] [Google Scholar]
- 66.Wiebe K, Poeling J, Arlt M, Philipp A, Camboni D, Hofmann S, et al. Thoracic surgical procedures supported by a pumpless interventional lung assist. Ann Thorac Surg. 2010;89:1782–7. doi: 10.1016/j.athoracsur.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Floerchinger B, Philipp A, Foltan M, Rupprecht L, Klose A, Camboni D, et al. Switch from venoarterial extracorporeal membrane oxygenation to arteriovenous pumpless extracorporeal lung assist. Ann Thorac Surg. 2010;89:125–31. doi: 10.1016/j.athoracsur.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 68.Reng M, Philipp A, Kaiser M, Pfeifer M, Gruene S, Schoelmerich J. Pumpless extracorporeal lung assist and adult respiratory distress syndrome. Lancet. 2000;356:219–20. doi: 10.1016/S0140-6736(00)02485-5. [DOI] [PubMed] [Google Scholar]
- 69.Grubitzsch H, Beholz S, Wollert HG, Eckel L. Pumpless arteriovenous extracorporeal lung assist: What is its role? Perfusion. 2000;15:237–42. doi: 10.1177/026765910001500309. [DOI] [PubMed] [Google Scholar]
- 70.Liebold A, Reng CM, Philipp A, Pfeifer M, Birnbaum DE. Pumpless extracorporeal lung assist-Experience with the first 20 cases. Eur J Cardiothorac Surg. 2000;17:608–13. doi: 10.1016/s1010-7940(00)00389-4. [DOI] [PubMed] [Google Scholar]
- 71.Bein T, Scherer MN, Philipp A, Weber F, Woertgen C. Pumpless extracorporeal lung assist (pECLA) in patients with acute respiratory distress syndrome and severe brain injury. J Trauma. 2005;58:1294–7. doi: 10.1097/01.ta.0000173275.06947.5c. [DOI] [PubMed] [Google Scholar]
- 72.Ruettimann U, Ummenhofer W, Rueter F, Pargger H. Management of acute respiratory distress syndrome using pumpless extracorporeal lung assist. Can J Anaesth. 2006;53:101–5. doi: 10.1007/BF03021536. [DOI] [PubMed] [Google Scholar]
- 73.von Mach MA, Kaes J, Omogbehin B, Sagoschen I, Wiechelt J, Kaiser K, et al. An update on interventional lung assist devices and their role in acute respiratory distress syndrome. Lung. 2006;184:169–75. doi: 10.1007/s00408-005-2577-9. [DOI] [PubMed] [Google Scholar]
- 74.Zimmermann M, Philipp A, Schmid FX, Dorlac W, Arlt M, Bein T. From Baghdad to Germany: Use of a new pumpless extracorporeal lung assist system in two severely injured US soldiers. ASAIO J. 2007;53:e4–6. doi: 10.1097/MAT.0b013e3180574b37. [DOI] [PubMed] [Google Scholar]
- 75.Muellenbach RM, Wunder C, Nuechter DC, Smul T, Trautner H, Kredel M, et al. Early treatment with arteriovenous extracorporeal lung assist and high-frequency oscillatory ventilation in a case of severe acute respiratory distress syndrome. Acta Anaesthesiol Scand. 2007;51:766–9. doi: 10.1111/j.1399-6576.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 76.Muellenbach RM, Kredel M, Wunder C, Küstermann J, Wurmb T, Schwemmer U, et al. Arteriovenous extracorporeal lung assist as integral part of a multimodal treatment concept: A retrospective analysis of 22 patients with ARDS refractory to standard care. Eur J Anaesthesiol. 2008;25:897–904. doi: 10.1017/S0265021508004870. [DOI] [PubMed] [Google Scholar]
- 77.Hommel M, Deja M, von Dossow V, Diemel K, Heidenhain C, Spies C, et al. Bronchial fistulae in ARDS patients: Management with an extracorporeal lung assist device. Eur Respir J. 2008;32:1652–5. doi: 10.1183/09031936.00021008. [DOI] [PubMed] [Google Scholar]
- 78.Weber-Carstens S, Bercker S, Hommel M, Deja M, MacGuill M, Dreykluft C, et al. Hypercapnia in late-phase ALI/ARDS: Providing spontaneous breathing using pumpless extracorporeal lung assist. Intensive Care Med. 2009;35:1100–5. doi: 10.1007/s00134-009-1426-3. [DOI] [PubMed] [Google Scholar]
- 79.Zimmermann M, Bein T, Arlt M, Philipp A, Rupprecht L, Mueller T, et al. Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: A prospective pilot study. Crit Care. 2009;13:R10. doi: 10.1186/cc7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bein T, Zimmermann M, Hergeth K, Ramming M, Rupprecht L, Schlitt HJ, et al. Pumpless extracorporeal removal of carbon dioxide combined with ventilation using low tidal volume and high positive end-expiratory pressure in a patient with severe acute respiratory distress syndrome. Anaesthesia. 2009;64:195–8. doi: 10.1111/j.1365-2044.2008.05735.x. [DOI] [PubMed] [Google Scholar]
- 81.Müller T, Lubnow M, Philipp A, Bein T, Jeron A, Luchner A, et al. Extracorporeal pumpless interventional lung assist in clinical practice: Determinants of efficacy. Eur Respir J. 2009;33:551–8. doi: 10.1183/09031936.00123608. [DOI] [PubMed] [Google Scholar]
- 82.Mortensen JD An intravenacaval blood gas exchange (IVCBGE) device. A preliminary report. ASAIO Trans. 1987;33:570–3. [PubMed] [Google Scholar]
- 83.Mortensen JD, Berry G. Conceptual and design features of a practical, clinically effective intravenous mechanical blood oxygen/carbon dioxide exchange device (IVOX) Int J Artif Organs. 1989;12:384–9. [PubMed] [Google Scholar]
- 84.Zwischenberger JB, Cox CS, Graves D, Bidani A. Intravascular membrane oxygenation and carbon dioxide removal: A new application for permissive hypercapnia? Thorac Cardiovasc Surg. 1992;40:115–20. doi: 10.1055/s-2007-1020127. [DOI] [PubMed] [Google Scholar]
- 85.Cox CS, Jr, Zwischenberger JB, Traber LD, Traber DL, Herndon DN. Use of an intravascular oxygenator/carbon dioxide removal device in an ovine smoke inhalation injury model. ASAIO Trans. 1991;37:M411–3. [PubMed] [Google Scholar]
- 86.Zwischenberger JB, Cox CS., Jr A new intravascular membrane oxygenator to augment blood gas transfer in patients with acute respiratory failure. Tex Med. 1991;87:60–3. [PubMed] [Google Scholar]
- 87.Cox CS, Jr, Zwischenberger JB, Graves DF, Niranjan SC, Bidani A. Intracorporeal CO2 removal and permissive hypercapnia to reduce airway pressure in acute respiratory failure. The theoretical basis for permissive hypercapnia with IVOX. ASAIO J. 1993;39:97–102. [PubMed] [Google Scholar]
- 88.Conrad SA, Bagley A, Bagley B, Schaap RN. Major findings from the clinical trials of the intravascular oxygenator. Artif Organs. 1994;18:846–63. doi: 10.1111/j.1525-1594.1994.tb03334.x. [DOI] [PubMed] [Google Scholar]
- 89.Vaslef SN, Mockros LF, Anderson RW. Development of an intravascular lung assist device. ASAIO Trans. 1989;35:660–4. doi: 10.1097/00002480-198907000-00160. [DOI] [PubMed] [Google Scholar]
- 90.Makarewicz AJ, Mockros LF, Anderson RW. A pumping intravascular artificial lung with active mixing. ASAIO J. 1993;39:M466–9. doi: 10.1097/00002480-199307000-00063. [DOI] [PubMed] [Google Scholar]
- 91.Hattler BG, Lund LW, Golob J, Russian H, Lann MF, Merrill TL, et al. A respiratory gas exchange catheter: In vitro and in vivo tests in large animals. J Thorac Cardiovasc Surg. 2002;124:520–30. doi: 10.1067/mtc.2002.123811. [DOI] [PubMed] [Google Scholar]
- 92.Eash HJ, Budilarto SG, Hattler BG, Federspiel WJ. Investigating the effects of random balloon pulsation on gas exchange in a respiratory assist catheter. ASAIO J. 2006;52:192–5. doi: 10.1097/01.mat.0000199752.89066.83. [DOI] [PubMed] [Google Scholar]
- 93.Eash HJ, Mihelc KM, Frankowski BJ, Hattler BG, Federspiel WJ. Evaluation of fiber bundle rotation for enhancing gas exchange in a respiratory assist catheter. ASAIO J. 2007;53:368–73. doi: 10.1097/MAT.0b013e318031af3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mihelc KM, Frankowski BJ, Lieber SC, Moore ND, Hattler BG, Federspiel WJ. Evaluation of a respiratory assist catheter that uses an impeller within a hollow fiber membrane bundle. ASAIO J. 2009;55:569–74. doi: 10.1097/MAT.0b013e3181bc2655. [DOI] [PubMed] [Google Scholar]
- 95.Gramaticopolo S, Chronopoulos A, Piccinni P, Nalesso F, Brendolan A, Zanella M, et al. Extracorporeal CO2 removal: A way to achieve ultraprotective mechanical ventilation and lung support: The missing piece of multiple organ support therapy. Contrib Nephrol. 2010;165:174–84. doi: 10.1159/000313757. [DOI] [PubMed] [Google Scholar]
- 96.Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, et al. Tidal volume lower than 6 ml/kg enhances lung protection: Role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–35. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- 97.ELSO General Guidelines. 2009. [Last accessed on 2012 Oct 1]. Available from: http://www.elso.med.umich.edu/WordForms/ELSO%20Guidelines%20General%20All%20ECLS%20Version1.1.pdf .
- 98.Lindén V, Palmér K, Reinhard J, Westman R, Ehrén H, Granholm T, et al. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med. 2000;26:1630–7. doi: 10.1007/s001340000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kugelman A, Gangitano E, Pincros J, Tantivit P, Taschuk R, Durand M. Venovenous versus venoarterial extracorporeal membrane oxygenation in congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:1131–6. doi: 10.1016/s0022-3468(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 100.Pettignano R, Fortenberry JD, Heard ML, Labuz MD, Kesser KC, Tanner AJ, et al. Primary use of the venovenous approach for extracorporeal membrane oxygenation in pediatric acute respiratory failure. Pediatr Crit Care Med. 2003;4:291–8. doi: 10.1097/01.PCC.0000074261.09027.E1. [DOI] [PubMed] [Google Scholar]
- 101.Guner YS, Khemani RG, Qureshi FG, Wee CP, Austin MT, Dorey F, et al. Outcome analysis of neonates with congenital diaphragmatic hernia treated with venovenous vs venoarterial extracorporeal membrane oxygenation. J Pediatr Surg. 2009;44:1691–701. doi: 10.1016/j.jpedsurg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 102.Turner DA, Rehder KJ, Peterson-Carmichael SL, Ozment CP, Al-Hegelan MS, Williford WL, et al. Extracorporeal membrane oxygenation for severe refractory respiratory failure secondary to 2009 H1N1 influenza A. Respir Care. 2011;56:941–6. doi: 10.4187/respcare.01066. [DOI] [PubMed] [Google Scholar]
- 103.Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: A new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139:e137–9. doi: 10.1016/j.jtcvs.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 104.Mangi AA, Mason DP, Yun JJ, Murthy SC, Pettersson GB. Bridge to lung transplantation using short-term ambulatory extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2010;140:713–5. doi: 10.1016/j.jtcvs.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 105.Turner DA, Cheifetz IM, Rehder KJ, Williford WL, Bonadonna D, Banuelos SJ, et al. Active rehabilitation and physical therapy during extracorporeal membrane oxygenation while awaiting lung transplantation: A practical approach. Crit Care Med. 2011;39:2593–8. doi: 10.1097/CCM.0b013e3182282bbe. [DOI] [PubMed] [Google Scholar]
- 106.Hayes D, Jr, Kukreja J, Tobias JD, Ballard HO, Hoopes CW. Ambulatory venovenous extracorporeal respiratory support as a bridge for cystic fibrosis patients to emergent lung transplantation. J Cyst Fibros. 2012;11:40–5. doi: 10.1016/j.jcf.2011.07.009. [DOI] [PubMed] [Google Scholar]


