Abstract
INTRODUCTION:
Fiber-optic bronchoscopy (FOB) with bronchoalveolar lavage (BAL) is a common procedure performed in immunocompromised patients with undiagnosed pulmonary pathology. Identifying patients with the highest potential diagnostic yield may help to avoid morbidity in patients unlikely to benefit from the procedure. We sought to determine which patient factors, specifically chest computed tomography (CT) findings, affected diagnostic yield of BAL.
METHODS:
Retrospective chart review of immunocompromised patients who underwent FOB with BAL from 01/01/2010 to 12/31/2011 at an academic medical center was performed. The lung lobe lavaged, characteristics of pulmonary infiltrate on radiograph, patient symptoms, and diagnostic yield were collected. A positive diagnostic yield was defined as a positive microbiological culture, finding on cytopathologic staining, diffuse alveolar hemorrhage, alveolar eosinophilia or a positive immunologic or nucleic acid assay.
RESULTS:
The overall diagnostic yield was 52.6%. Infiltrates that were predominantly reticular or nodular by CT had a lower diagnostic yield than predominantly consolidated, ground-glass, or tree-in-bud infiltrates (36.5% vs. 61.2%, P = 0.0058). The diagnostic yield was significantly improved in patients with both fever and chest symptoms compared to patients without symptoms (61.3% vs. 29.6%, P = 0.0066).
CONCLUSION:
CT findings of reticular and nodular infiltrates portend a worse diagnostic yield from BAL than those that are alveolar in nature. Symptomatic patients are more likely to have diagnostic FOB with BAL than asymptomatic patients.
Keywords: Bronchoscopy, computed tomography, immunodeficiency, respiratory infection, respiratory symptoms
Fiber-optic bronchoscopy (FOB) with bronchoalveolar lavage (BAL) is a common diagnostic tool employed by pulmonologists in the evaluation of unidentified pulmonary pathology. This procedure provides valuable information regarding the cellular components and potential pathogens within the alveolar space.[1] BAL is commonly utilized in the diagnostic evaluation of immunocompromised patients with pulmonary infiltrates.[2–8]
However, FOB is not a benign procedure, and it has been associated with complications.[9] FOB may not necessarily add information beyond what a non-invasive diagnostic strategy might provide.[10] Selecting patients for FOB with BAL who are most likely to have a positive diagnostic yield may mitigate or eliminate unnecessary procedural-related morbidity or mortality. Patient factors, which might influence diagnostic yield includes symptoms and radiographic studies. Although previous studies have examined the relationship between radiographic findings on plain chest films and diagnostic yield from FOB,[3,6,7] no studies have examined the relationship between diagnostic yield and the more specific findings obtained from dedicated chest computed tomography (CT). Reticular and nodular infiltrates are characteristic of abnormalities that are extra-alveolar as opposed to consolidated infiltrates, ground glass opacities and tree-in-bud pulmonary infiltrates, where the abnormality is predominantly bronchiolar and alveolar in location. We hypothesized then that alveolar infiltrates on chest CT would be associated with a higher diagnostic yield on BAL.
The proportion of lavage fluid recovered is higher in the right middle lobe and in the lingula than in the lower lobes.[11] It is also established that the cellular component of lavage fluid may have significant interlobar variation in various disease processes,[12] and that yield may be higher for certain infectious agents when BAL is performed in the area of most disease involvement by radiograph.[13] Although it has been recommended to perform BAL in the area of abnormality in localized disease,[14] the diagnostic yield may not be uniform for all lobes in a heterogeneous population of immunocompromised patients with a low incidence of Pneumocystis jirovecci infection.
We sought to retrospectively evaluate our large population of immunocompromised patients who have undergone FOB with BAL to search for specific factors associated with a higher diagnostic yield, including chest CT findings, lobe of lung lavaged, and patient symptoms.
Methods
A retrospective review of all patients at the University of Kansas Medical Center who underwent FOB with BAL from January 1, 2010 to December 31, 2011 was performed. The patients′ medical records were screened for the presence of conditions associated with a compromised immune system, and those patients were included in this study. Such conditions included the presence of a hematogenous or solid organ malignancy for which the patient was currently receiving chemotherapy within 14 days of bronchoscopy, receipt of a bone marrow or hematopoietic stem cell transplantation, receipt of a solid organ transplantation, infection with human immunodeficiency virus (HIV), neutropenia, and diagnosis of an autoimmune disorder for which the patient was being treated with immune suppressants.
After patients were appropriately identified, their medical records were evaluated for patient characteristics, procedural details, symptoms prior to FOB, imaging characteristics, results of diagnostic studies obtained by FOB with BAL, diagnostic studies obtained non-invasively and eventual diagnosis. A febrile patient was defined by the presence of a temperature of 38.0°C or greater within 48 h of FOB. All data was collected with the approval of the University of Kansas Medical Center institutional review board, project Human Subjects Committee (HSC) #12949.
All patients selected for this study had a CT of the chest performed prior to FOB with BAL. All of the CT scans were interpreted by an attending radiologist as well as a pulmonologist at the time of performance. The entire chest CT scans was characterized as having either a focal or a diffuse abnormality. In addition, the scans were interpreted as having one of five predominant radiographic abnormalities: Airspace consolidation, ground-glass opacities, tree-in-bud infiltrates, nodular infiltrates or reticular infiltrates. When more than one pattern was present in a chest CT, the pattern that was deemed most prevalent was chosen as the predominant pattern. Reticular and nodular infiltrates were grouped together as predominantly extra-alveolar findings. Consolidated infiltrates, tree-in-bud opacities, and ground-glass opacities were grouped together as findings within the alveoli or terminal bronchioles.
All BAL fluid was collected via FOB performed by members of the Division of Pulmonary and Critical Care Medicine at the University of Kansas Medical Center. All patients or their surrogate decision maker signed informed consent prior to procedural initiation. The patients had a new finding of pulmonary infiltrate(s) and were selected for FOB with BAL at the discretion of the attending physician based on patient symptoms, medical history, and differential diagnosis. FOB with BAL was performed per accepted guidelines as published previously.[15] BAL was performed in the lung lobe deemed to be the most afflicted based on radiographic analysis of the chest and the opinion of the performing physician. Procedural details may have varied as dictated by patient tolerance of the procedure. The volume of BAL fluid instilled and subsequently collected was not routinely recorded. Trans-bronchial biopsies were performed at the discretion of the proceduralist when it was deemed necessary to enhance diagnostic yield and only when they presented minimal risk to the patient. The results of trans-bronchial biopsies were not included in the diagnostic yield of this manuscript as such findings were outside the primary focus of this study.
Diagnostic studies performed on BAL fluid were ordered at the discretion of the proceduralist performing the bronchoscopy, in conjunction with input from the referring physician and an infectious diseases consultant, when involved with the case. There was some variability in diagnostic studies obtained as dictated by the patients′ clinical presentation and the differential diagnosis at the time of FOB. All BAL specimens were evaluated by bacterial culture, cytology with silver stain, and cell count. Specific other studies that were commonly selected to be performed on BAL fluid included fungal and viral cultures, P. jirovecci polymerase chain reaction (PCR), cytomegalovirus PCR, herpes simplex virus PCR, galactomannan (GM) antigen assay and respiratory viral panel (RVP). The RVP (Luminex xTAG, Viracor-IBT Laboratories, Lee′s Summit, MO, USA), is a qualitative nucleic acid multiplex test designed to detect common respiratory viruses, which include influenza virus, adenovirus, coronavirus, metapneumovirus, parainfluenzavirus, rhinovirus, and respiratory syncytial virus. This RVP has been shown to have exceptional sensitivity when used in BAL samples.[16,17] In addition, we analyzed studies collected non-invasively during the diagnostic evaluation, including peripheral blood cultures, sputum cultures, nasal washing RVP, serum GM, and serum 1-3-β-D-glucan assay.
A positive diagnostic yield was defined as a positive microbiological culture, a positive finding on cytopathologic staining, presence of diffuse alveolar hemorrhage, alveolar eosinophilia, a positive immunologic assay, RVP assay or PCR study. In the scenario in which two diagnostic studies were positive, the study that most closely correlated with the clinical presentation was chosen as the principal diagnosis.
Statistical analysis was performed using a statistical software program (GraphPad Prism 5; GraphPad Software Inc., La Jolla, CA). Proportional outcomes were compared using the Fisher′s exact test or a 1-sided χ2-test where appropriate. A P < 0.05 was considered to indicate statistical significance. For continuous variables, we report mean plus and minus standard deviation.
Results
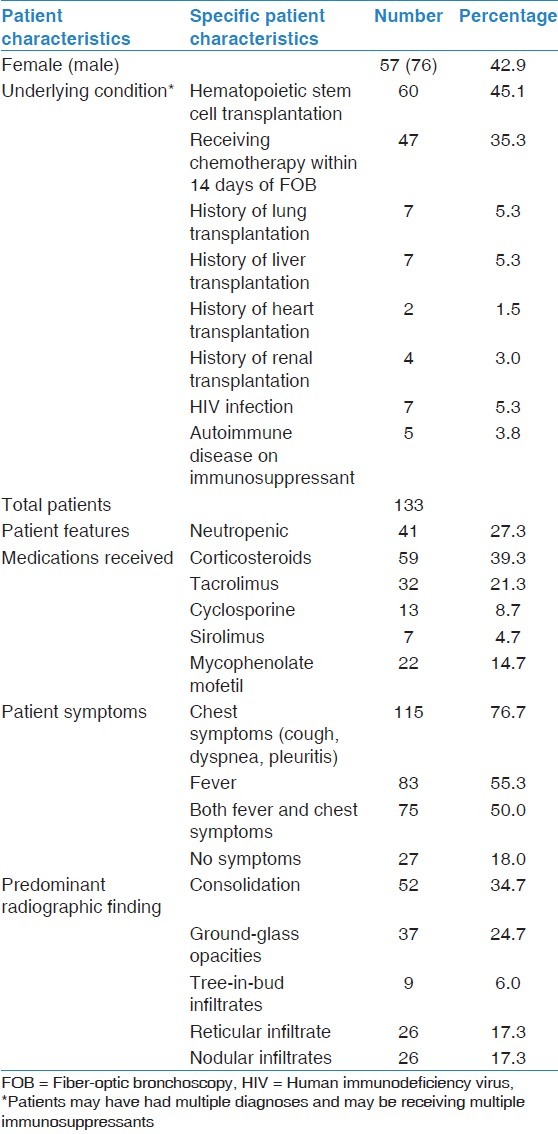
There were 133 patients who underwent 150 separate FOB with BAL during the 2-year study period. 57 of the patients were female and 76 were male, with a mean age at the time of bronchoscopy of 50.4 years (standard deviation ± 14.6 years). Sixty patients received hematopoietic stem cell transplantation, 20 patients received a solid organ transplant, 47 patients were receiving chemotherapy, seven patients had HIV, and five patients had autoimmune disease and were receiving immunosuppressants. Some of the patients who had undergone stem cell transplantation subsequently had a relapse of their underlying malignancy and were undergoing chemotherapy, leading to an overlap in patients and their conditions. For full details on patient background, please see Table 1.
Table 1.
Background patient characteristics
Of the 133 patients who underwent FOB with BAL, 13 had FOB twice, and two patients had FOB on three separate occasions. The mean time between the separate bronchoscopies was 94.4 days (standard deviation ± 92.3 days). Thirteen of the 15 patients (86.7%) had a change in chest symptoms or in the presence or absence of fevers at the time of their subsequent FOB with BAL. A different type of infiltrate was noted in 9 of the 15 patients (60%) at the time of their second FOB, and a different lobe was lavaged in 14 of the 15 patients (93.3%). In only two instances (13.3%) was the same diagnosis achieved during the subsequent bronchoscopy.
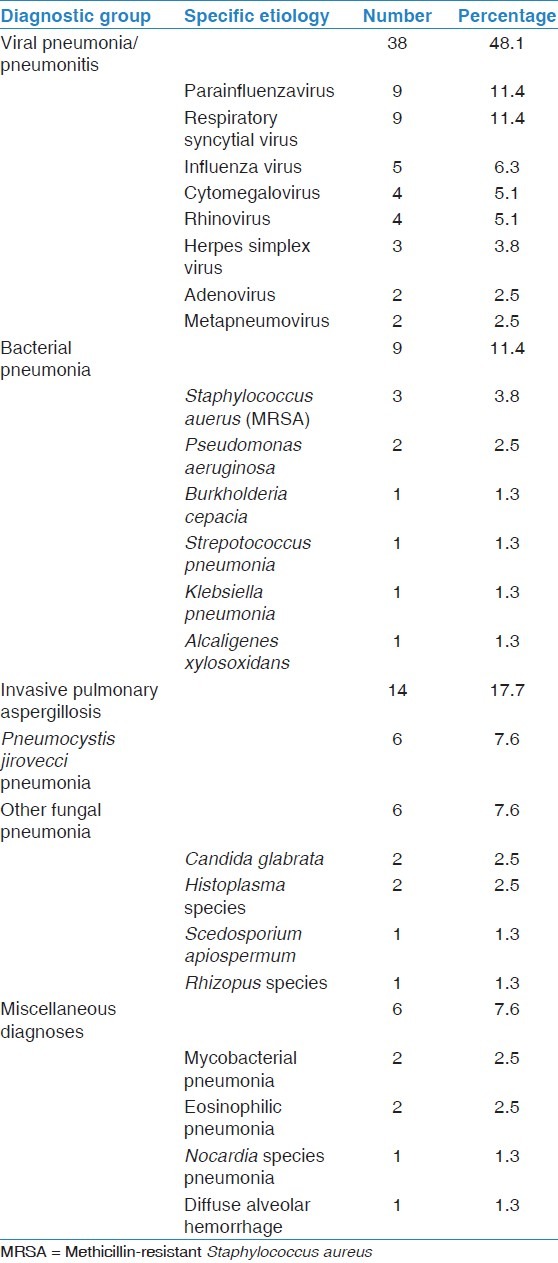
Of the 150 FOB with BAL, 79 resulted in diagnoses, giving an overall diagnostic yield of 52.6%. The number needed to perform FOB to achieve a diagnosis was 1.9. Specific diagnoses included viral pneumonia/pneumonitis in 38 patients (48.1%), bacterial pneumonia in 9 patients (11.4%), invasive pulmonary aspergillosis in 14 patients (17.7%), P. jirovecci in 6 patients (7.6%), other fungal pneumonias in 6 patients (7.6%) and miscellaneous diagnoses in six patients (7.6%). For full details of specific diagnoses, see Table 2. There was no difference in diagnostic yield among the subgroups of immunosuppressed patients.
Table 2.
Specific patient diagnoses made by bronchoalveolar lavage
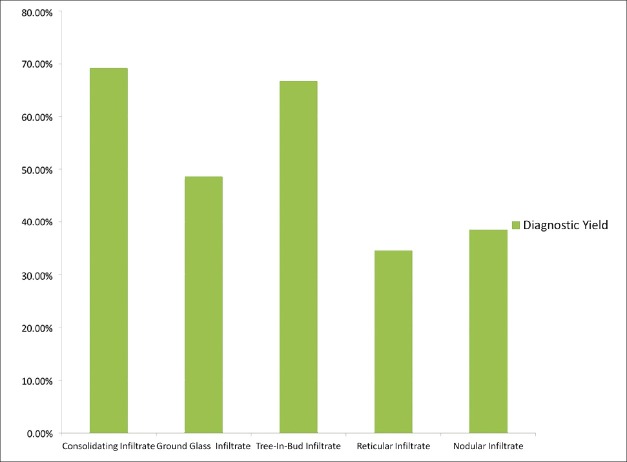
Forty-three patients had focal infiltrates on imaging (28.7%) while 107 patients had diffuse infiltrates involving two or more lobes (71.3%). There was no difference in diagnostic yield between focal and diffuse infiltrates (53.5% vs. 52.3%). The diagnostic yield with respect to each predominant pulmonary imaging abnormality is shown in Figure 1. There was no difference in prevalence between the specific infiltrate type and location of BAL. Infiltrates where the abnormality was extra-alveolar had a lower diagnostic yield than those where the abnormality was bronchiolar or alveolar in location, 36.5% versus 61.2% respectively (P = 0.0058).
Figure 1.
A higher diagnostic yield was demostrated in patients whose predominant radiographic infiltrate is within the alveoli or airways, as compared to infiltrates were the abnormality is predominantly extra-alveolar
There was significant variation in diagnostic yield based on the lobe of the lung that was lavaged as shown in Figure 2. A diagnosis was made when the upper lobes were lavaged in 38 of 72 bronchoscopies (52.8%), when the right middle lobe was lavaged in 16 of 42 cases (38.1%), and when the lower lobes were lavaged in 25 of 36 cases (69.4%). There were no significant differences in frequency of the various diagnoses based on the lobe of lung lavaged.
Figure 2.
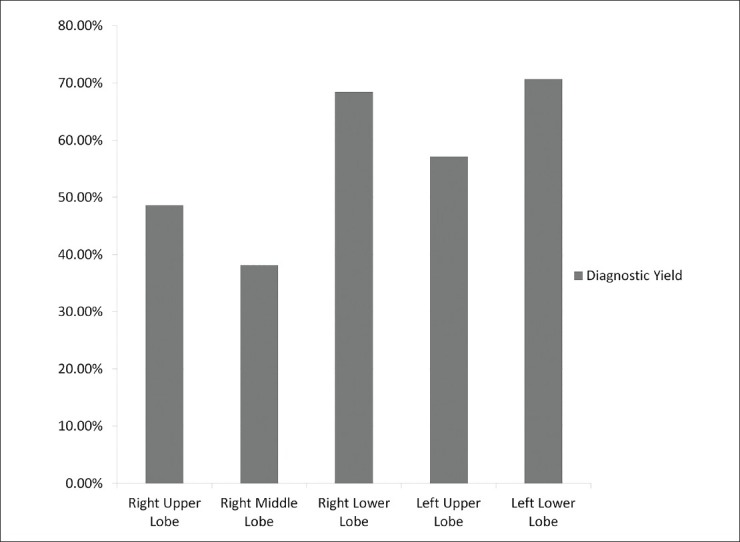
There is significantly higher diagnostic yield of bronchoscopy with bronchoalveolar lavage in the lower lobes than in the middle or upper lobes in immunocompromised patients
Some asymptomatic patients were referred for FOB with BAL when abnormalities were discovered incidentally on chest imaging while other patients had a fever or chest symptoms, such as cough, sputum production, dyspnea and pleuritis. Diagnostic yield was collected and compared to the presence or absence of chest symptoms and fevers and is displayed in Figure 3. The diagnostic yield was significantly improved in patients with both fever and chest symptoms compared with patients who had neither finding (61.3% vs. 29.6%, P = 0.0066).
Figure 3.
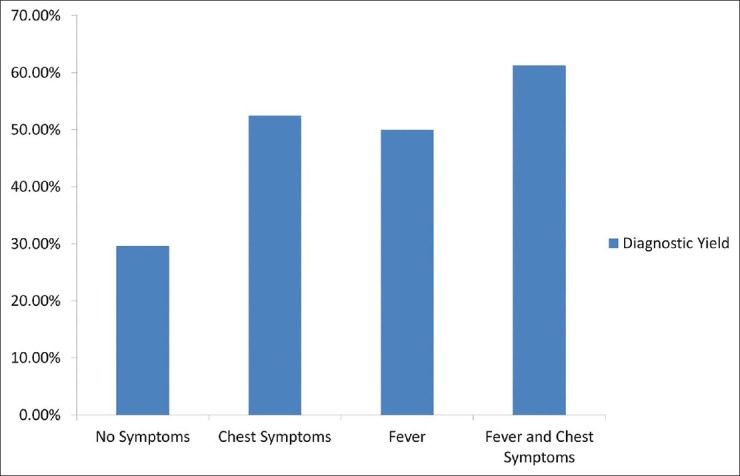
Immunocompromised patients with fever and chest symptoms have a higher diagnostic yield from fiber-optic bronchoscopy with bronchoalveolar lavage than asymptomatic patients
Complications occurred as a result of 11 of our cohort of 150 bronchoscopies, with a complication rate of 7.3%. Two patients had minor hemorrhage, 1 patient had a pneumothorax requiring chest tube placement, 4 patients had sustained hypoxemia for 24 h following bronchoscopy, and 4 patients required intubation and mechanical ventilation following bronchoscopy. There were no sustained arrhythmias during or following FOB. The number needed to harm with bronchoscopy was 13.6.
Of the 79 cases with a positive diagnostic yield, the diagnosis would have only been made by FOB with BAL in 58 of the cases (73.4%). In the 21 cases where a diagnosis was also made by non-invasive testing, 16 of the cases had a positive nasal wash RVP and five of the patients had a positive serum GM antigen.
Discussion
In this study, we examined the relationship between patient symptoms, radiographic findings and diagnostic yield in immunocompromised patients undergoing FOB with BAL. We had demonstrated a statistically significant improvement in diagnostic yield when BAL was performed on patients with respiratory symptoms and fever and in patients whose predominant pulmonary abnormality on imaging occurred in an alveolar or bronchiolar location.
FOB with BAL has been to shown to be more likely to establish a diagnosis in immunocompromised patients with pulmonary infiltrates when the cause of the pulmonary infiltrate is infectious in nature.[8] Therefore, identifying patients who have a higher pretest probability of an infectious etiology should theoretically improve diagnostic yield from FOB with BAL. It stands to reason that patients with fever and symptoms of chest infection are more likely to have an infectious cause of their pulmonary imaging abnormality than those without symptoms, which explains the higher diagnostic yield.
With regards to imaging characteristics, we examined the relationship between the predominant radiographic characteristic on chest CT scan and diagnostic yield. In those findings that are associated with a better diagnostic yield, there appears to be a higher likelihood of infectious etiology, as well. Tree-in-bud infiltrates typically represent dilated centrilobular bronchioles with lumens obstructed with pus or other fluids,[18] and are associated with airway infection in most cases.[19] Consolidation is commonly seen in infectious pneumonia, though it may also be seen in diseases such as chronic eosinophilic pneumonia or organizing pneumonia. Ground-glass opacification, which is defined by increased attenuation of the of the lung parenchyma but without obscuration of the vascular structures or of the air bronchograms seen in consolidating infiltrates,[20] may be due to infectious causes,[21] but may also be seen in interstitial lung disease with active inflammation.[22]
In comparison, those predominant imaging characteristics that did not correlate favorably with diagnostic yield included reticular and nodular infiltrates. Reticular infiltrates occur within the interlobular or intralobular septa and the bronchovascular interstitium. Nodular opacities may occur in any anatomic area of the lung, with common distributions including in the interlobar septa, centrilobular or in random distribution. The differential diagnosis for pulmonary nodules is broad and dependent on the distribution of nodules, but includes lymphangitic spread of malignancy, pneumoconiosis, infection and hypersensitivity pneumonitis.[18] Two possible reasons that this group of radiographic patterns may not be associated with a higher diagnostic yield would be the anatomic association with a process occurring outside of the airway or alveoli, and the likely higher prevalence of non-infectious causes in reticular and nodular infiltrates. Because not all patients in our study underwent high-resolution CT imaging, we were unable to further characterize the nodular infiltrates by area within the secondary pulmonary lobule or the reticular infiltrates as being smooth or nodular in appearance.
We found a significant difference in diagnostic yield based on the lobe of the lung that was lavaged. While the volume of fluid returned is typically greater from the right middle lobe and lingula than from other lobes,[11] the increased volume did not correlate with a higher diagnostic yield in our patient population. This finding may reflect the evolving population of immunocompromised patients, and their infectious etiologies. During the era previous to anti-retroviral therapy, when patients with HIV were the predominant immunocompromised patients undergoing FOB with BAL, P. jirovecci infection was one of the dominant infectious etiologies diagnosed by BAL.[23] The majority of immunocompromised patients in this study undergoing FOB with BAL had either received hematopoietic stem cell tr ansplantation or were receiving chemotherapy, and the majority of our patients were not diagnosed with P. jirovecci pneumonia. Likewise, in the era before HIV, sensitive diagnostic studies such as PCR and GM antigen detection were not available. Given our higher prevalence of viral infections diagnosed on FOB with BAL, the finding of increased diagnostic yield when FOB with BAL was performed in the lower lobes could represent a greater affinity of viruses to involve the lower lobes preferentially, much as tuberculosis and P. jirovecci involve the upper lobes preferentially.[13,24]
The focus of our study was to evaluate the ability of BAL to diagnose pulmonary complications of immunosuppression. Jain et al. showed that transbronchial biopsy added to the diagnostic yield from bronchoscopy.[8] However, this study was performed without the aid of now widely available assays that improve diagnostic yield from BAL, including GM assay, RVP, and PCR detection of various viruses. Given the availability of these new diagnostic assays, we determined that studying the diagnostic yield of BAL alone in immunosuppressed patients would be clinically useful.
Our study has some limitations. First, the retrospective nature of the study resulted in a non-uniform protocol for diagnostic evaluation of patients and non-uniform patient selection criteria for undergoing FOB. Because of the common use of empiric antibiotics and anti-fungal agents in our cohort, diagnostic yield may have been limited in patients who were receiving antimicrobials at the time of FOB; likewise it is impossible to control for the duration of antibiotic therapy or of infiltrates prior to the procedure. Although FOB with BAL is performed in a standard manner by all members of this division, there was no protocol to ensure that every procedure was completed in exactly the same manner. Likewise, we were unable to control for variability of physicians in the interpretation of CT imaging.
In summary, we have shown a statistically significant association between diagnostic yield from FOB with BAL in patients whose radiographic infiltrates on CT scan involve the airways or alveoli. This study emphasizes the role of CT scanning in patient selection and procedural planning for immune suppressed patients with undiagnosed pulmonary pathology.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hunninghake GW, Gadek JE, Kawanami O, Ferrans VJ, Crystal RG. Inflammatory and immune processes in the human lung in health and disease: Evaluation by bronchoalveolar lavage. Am J Pathol. 1979;97:149–206. [PMC free article] [PubMed] [Google Scholar]
- 2.Azoulay E, Mokart D, Lambert J, Lemiale V, Rabbat A, Kouatchet A, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: Randomized controlled trial. Am J Respir Crit Care Med. 2010;182:1038–46. doi: 10.1164/rccm.201001-0018OC. [DOI] [PubMed] [Google Scholar]
- 3.Gruson D, Hilbert G, Valentino R, Vargas F, Chene G, Bebear C, et al. Utility of fiberoptic bronchoscopy in neutropenic patients admitted to the intensive care unit with pulmonary infiltrates. Crit Care Med. 2000;28:2224–30. doi: 10.1097/00003246-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Hohenthal U, Itälä M, Salonen J, Sipilä J, Rantakokko-Jalava K, Meurman O, et al. Bronchoalveolar lavage in immunocompromised patients with haematological malignancy: Value of new microbiological methods. Eur J Haematol. 2005;74:203–11. doi: 10.1111/j.1600-0609.2004.00373.x. [DOI] [PubMed] [Google Scholar]
- 5.Joos L, Chhajed PN, Wallner J, Battegay M, Steiger J, Gratwohl A, et al. Pulmonary infections diagnosed by BAL: A 12-year experience in 1066 immunocompromised patients. Respir Med. 2007;101:93–7. doi: 10.1016/j.rmed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Shannon VR, Andersson BS, Lei X, Champlin RE, Kontoyiannis DP. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:647–55. doi: 10.1038/bmt.2009.203. [DOI] [PubMed] [Google Scholar]
- 7.Dunagan DP, Baker AM, Hurd DD, Haponik EF. Bronchoscopic evaluation of pulmonary infiltrates following bone marrow transplantation. Chest. 1997;111:135–41. doi: 10.1378/chest.111.1.135. [DOI] [PubMed] [Google Scholar]
- 8.Jain P, Sandur S, Meli Y, Arroliga AC, Stoller JK, Mehta AC. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest. 2004;125:712–22. doi: 10.1378/chest.125.2.712. [DOI] [PubMed] [Google Scholar]
- 9.White P, Bonacum JT, Miller CB. Utility of fiberoptic bronchoscopy in bone marrow transplant patients. Bone Marrow Transplant. 1997;20:681–7. doi: 10.1038/sj.bmt.1700957. [DOI] [PubMed] [Google Scholar]
- 10.Azoulay E, Mokart D, Rabbat A, Pene F, Kouatchet A, Bruneel F, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: Prospective multicenter data. Crit Care Med. 2008;36:100–7. doi: 10.1097/01.CCM.0000295590.33145.C4. [DOI] [PubMed] [Google Scholar]
- 11.Pingleton SK, Harrison GF, Stechschulte DJ, Wesselius LJ, Kerby GR, Ruth WE. Effect of location, pH, and temperature of instillate in bronchoalveolar lavage in normal volunteers. Am Rev Respir Dis. 1983;128:1035–7. doi: 10.1164/arrd.1983.128.6.1035. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JG, Wolven RG, Garcia PL, Keogh BA. Assessment of interlobar variation of bronchoalveolar lavage cellular differentials in interstitial lung diseases. Am Rev Respir Dis. 1986;133:444–9. doi: 10.1164/arrd.1986.133.3.444. [DOI] [PubMed] [Google Scholar]
- 13.Baughman RP, Dohn MN, Shipley R, Buchsbaum JA, Frame PT. Increased Pneumocystis carinii recovery from the upper lobes in Pneumocystis pneumonia. The effect of aerosol pentamidine prophylaxis. Chest. 1993;103:426–32. doi: 10.1378/chest.103.2.426. [DOI] [PubMed] [Google Scholar]
- 14.Helmers RA, Hunninghake GW. Bronchoalveolar lavage in the nonimmunocompromised patient. Chest. 1989;96:1184–90. doi: 10.1378/chest.96.5.1184. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein RA, Rohatgi PK, Bergofsky EH, Block ER, Daniele RP, Dantzker DR, et al. Clinical role of bronchoalveolar lavage in adults with pulmonary disease. Am Rev Respir Dis. 1990;142:481–6. doi: 10.1164/ajrccm/142.2.481. [DOI] [PubMed] [Google Scholar]
- 16.Pabbaraju K, Tokaryk KL, Wong S, Fox JD. Comparison of the Luminex xTAG respiratory viral panel with in-house nucleic acid amplification tests for diagnosis of respiratory virus infections. J Clin Microbiol. 2008;46:3056–62. doi: 10.1128/JCM.00878-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadsby NJ, Hardie A, Claas EC, Templeton KE. Comparison of the Luminex Respiratory Virus Panel fast assay with in-house real-time PCR for respiratory viral infection diagnosis. J Clin Microbiol. 2010;48:2213–6. doi: 10.1128/JCM.02446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb WR. Thin-section CT of the secondary pulmonary lobule: Anatomy and the image - The 2004 Fleischner lecture. Radiology. 2006;239:322–38. doi: 10.1148/radiol.2392041968. [DOI] [PubMed] [Google Scholar]
- 19.Aquino SL, Gamsu G, Webb WR, Kee ST. Tree-in-bud pattern: Frequency and significance on thin section CT. J Comput Assist Tomogr. 1996;20:594–9. doi: 10.1097/00004728-199607000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Miller WT, Jr, Shah RM. Isolated diffuse ground-glass opacity in thoracic CT: Causes and clinical presentations. AJR Am J Roentgenol. 2005;184:613–22. doi: 10.2214/ajr.184.2.01840613. [DOI] [PubMed] [Google Scholar]
- 21.Reittner P, Müller NL, Heyneman L, Johkoh T, Park JS, Lee KS, et al. Mycoplasma pneumoniae pneumonia: Radiographic and high-resolution CT features in 28 patients. AJR Am J Roentgenol. 2000;174:37–41. doi: 10.2214/ajr.174.1.1740037. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Im JG, Ahn JM, Kim YM, Han MC. Fibrosing alveolitis: Prognostic implication of ground-glass attenuation at high-resolution CT. Radiology. 1992;184:451–4. doi: 10.1148/radiology.184.2.1620846. [DOI] [PubMed] [Google Scholar]
- 23.Wolff AJ, O′Donnell AE. Pulmonary manifestations of HIV infection in the era of highly active antiretroviral therapy. Chest. 2001;120:1888–93. doi: 10.1378/chest.120.6.1888. [DOI] [PubMed] [Google Scholar]
- 24.Farman DP, Speir WA., Jr Initial roentgenographic manifestations of bacteriologically proven Mycobacterium tuberculosis. Typical or atypical? Chest. 1986;89:75–7. doi: 10.1378/chest.89.1.75. [DOI] [PubMed] [Google Scholar]