Abstract
OBJECTIVES:
This study compares early and late outcomes for treatment by video-assisted thoracic surgery (VATS) versus treatment by thoracotomy for clinical N0, but post-operatively unexpected, pathologic N2 disease (cN0-pN2).
METHODS:
Clinical records of patients with unexpected N2 non-small cell lung cancer (NSCLC) who underwent VATS were retrospectively reviewed, and their early and late outcomes were compared to those of patients undergoing conventional thoracotomy during the same period.
RESULTS:
VATS lobectomy took a longer time than thoracotomy (P < 0.001), but removal of thoracic drainage and patient discharge were earlier for patients in the VATS group (P < 0.001). There was no difference in lymph node dissection, mortality and morbidity between the two groups (P > 0.05). The median follow-up time for 287 patients (89.7%) was 37.0 months (range: 7.0-69.0). The VATS group had a longer survival time than for the thoracotomy group (median 49.0 months vs. 31.7 months, P < 0.001). The increased survival time of the VATS group was due to patients with a single station of N2 metastasis (P = 0.001), rather than to patients with multiple stations of N2 metastasis (P = 0.225).
CONCLUSIONS:
It is both feasible and safe to perform VATS lobectomy on patients with unexpected N2 NSCLC. VATS provides better survival rates for those patients with just one station of metastatic mediastinal lymph nodes.
Keywords: Non-small cell lung cancer, outcomes, staging, surgery
Since the introduction of video-assisted thoracic surgery (VATS) lobectomy at the beginning of the 1990s, follow-up research has consistently demonstrated its feasibility and safety.[1–3] However, questions still remain about the oncological effectiveness of VATS lobectomy and systematic lymph node dissection in patients with non-small cell lung cancer (NSCLC), limiting its use to patients with early-stage NSCLC. However, accurate clinical N staging is difficult for NSCLC patients, and false negatives exist in both pre-operative non-invasive workups, such as computed tomography (CT) and positron emission tomography (PET), and invasive workups, such as endobronchial ultrasonography guided transbronchial needle aspiration (EBUS-TBNA) and mediastinoscopy. The possible differences between the pre-operative clinical stage and the post-operative pathological stage make some NSCLC patients with mediastinal lymph nodes metastasis (N2) candidates for VATS lobectomy. In addition, because there is few literature references concerning the difference in long-term results between open thoracotomy and VATS lobectomy, surgeons may be faced with a dilemma as to whether it is necessary to convert VATS to open thoracotomy during the operation. To address these questions, our study compares the early and long-term results between the VATS group and the open thoracotomy group for patients who had no clinical metastatic lymph nodes, but who were later found to have unexpected pathologic mediastinal lymph node metastasis (cN0-pN2) post-operatively.
Methods
Patients
We retrospectively reviewed and analyzed the clinical data of 4431 patients with cT1-2N0 stage NSCLC who underwent surgery between January 2005 and December 2010 at our institution. A subset of 320 patients (7.2%) with cN0-pN2 (IIIa stage) NSCLC was selected according to the criteria of the seventh edition of the TNM classification of IASLC Staging Project.[4] Patients were included only if they met the following conditions: (1) diagnosed with early stage NSCLC before the operation (cT1-2N0M0, stage I), but found to have unexpected minimal mediastinal lymph node metastasis post-operatively (pT1-3N2M0, stage IIIa); (2) not previously treated with any radiotherapy or chemotherapy before the operation; (3) between 18 years old and 75 years old; (4) without any residual tumors after the operation. The pre-operative chest enhanced CT scans for all 320 patients showed that the long axis of the mediastinal lymph nodes was less than 1 cm. The pre-operative PET/CT scans for 43 patients showed normal concentration (standard uptake value <2.5) of 2-deoxy-2-(18F) fluoro-D-glucose 2 in the mediastinal lymph node area. Twelve patients underwent one of the following workups: Mediastinoscopy biopsy or EBUS-TBNA, and no malignant cell metastases were found in the lymph nodes. The pre-operative clinical stage for 320 patients was confirmed to be cT1-2N0M0.
Patients were divided into two groups. The VATS group consisted of 101 patients who underwent VATS lobectomy and systematic lymph node dissection. The other 219 patients, who underwent open thoracotomy lobectomy and systematic lymph nodes dissection, comprised the thoracotomy group. Paraffin pathological examination of lymph nodes after the operations confirmed oncologic metastasis in the mediastinal lymph nodes (pN2).
Surgical considerations
Routine pre-operative workups included a pulmonary function test and an electrocardiogram to check the surgical tolerance of the patients, clinical staging, such as a CT scan with contrast enhancement of the chest and abdomen, a head enhanced magnetic resonance imaging (MRI) scan, a whole-body isotope bone scan and a fiberbronchoscopy to aid in the design of the method of bronchi resection. Lobectomy was the optimal surgical technique. All patients underwent double-lumen intubation and whole-body anesthesia in the lateral position. For the VATS group, a 15 mm trocar for 30° thoracoscopy was inserted through the seventh or eighth intercostal space in the median axillary line. A 2.5-3 cm manipulation incision was made through the fourth intercostal space in the intersection of the anterior axillary line and the front edge of the latissimus dorsi. A 2 cm utility incision was made through the seventh intercostal space in the auscultatory triangle. A rib retractor was not utilized during the VATS operation. The artery and vein branches and bronchi of the target pulmonary lobe, where the lesion was located, were dissected using a linear stapling device. Specimens of lung tissue were placed in an impermeable bag and removed from the thorax through the manipulation incision. According to the mountain regional lymph node classification, mediastinal lymph node dissection consisted of en bloc mandatory resections of all nodes at stations 2 R, 4 R, 7, 8, 9, 10 R, 11 for right-sided tumors and nodes at stations 5, 6, 7, 8, 9, 10 L, 11 for left-sided tumors. All specimens were removed through the manipulation incision. When lymph node metastasis was suspected, frozen pathology was performed during the operation. For the thoracotomy group, lobectomy, and systematic lymph node dissection were performed through a conventional posterolateral incision.
After the operation, the patients remained under intensive care until their vital signs stabilized without the need for mechanical ventilation support and vessel activity drugs. Thoracic drainage was removed when the drainage volume was less than 150 ml with no sign of bleeding, air leakage, and infection. After removal of the chest tube, the patients were discharged when X-ray and routine blood analysis showed no obvious abnormalities.
Four to six weeks after the operation, patients with a performance status score of 0-1 and who did not refuse chemotherapy underwent adjunctive chemotherapy. The initial regime was either navelbine with cisplatin or gemcitabine with cisplatin. Adjustment of the chemotherapy regime in the case of intolerance, side-effects or disease recurrence was determined by the oncologists.
Follow-up
All patients were evaluated with enhanced chest CT every 3 months for the first 2 years after surgery, and by means of abdominal CT scan, head MRI scan, whole-body isotope bone scan every 6 months to detect any regional recurrence or distant metastasis. Thereafter, the patients were followed-up by their oncologists. The evaluation period was defined as the day of the operation to December 2012. A monthly out-patient interview or telephone interview was conducted as a follow-up. Survival, the only parameter followed in this study, was defined as the period from the date of surgery to either death or the last interview.
Statistics
Statistics were analyzed and relevant curves were constructed using the SPSS 17.0 Statistics Software Package (SPSS Inc., Chicago, Illinois). Unless stated otherwise, mean values and standard deviations are reported. A Student t-test was used for the comparison between subgroups. In case of categorical variables, a χ2 test or a Fisher exact test was used when appropriate. Survival curves were constructed by using the Kaplan-Meier method; differences between the curves were analyzed with a log-rank test. For all statistical analyses, P values of less than 0.05 were considered to be significant.
Results
Pre-operative characteristics
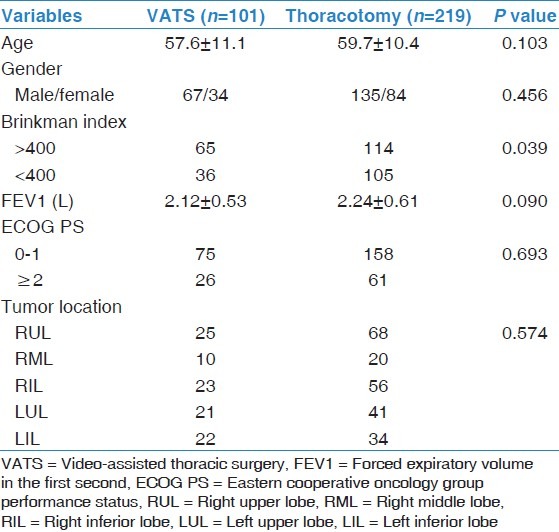
Pre-operative clinical features were comparable between the VATS group and the thoracotomy group except for the Brinkman Index [Table 1].
Table 1.
Comparison of clinical features for the VATS group and the thoracotomy group
Surgical features
No patients in the VATS group underwent conversion to thoracotomy during the operation. The operation time for the VATS group was significantly longer than that for the orthoracotomy group [P < 0.001, Table 2] while the difference in blood loss between the two groups was not significant [P = 0.098, Table 2]. Thoracic drainage was removed earlier in the VATS group than in the thoracotomy group [P < 0.001, Table 2] and the patients in the VATS group were discharged earlier than the patients in the thoracotomy group [P < 0.001, Table 2].
Table 2.
Comparison of operational features for the VATS group and the thoracotomy group
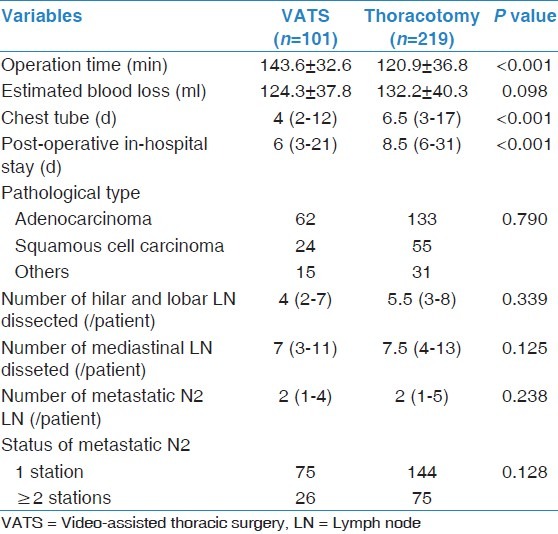
There were no significant differences by pathology in the distribution or type of the tumors between two groups [P = 0.790, Table 2 or the number of dissected lymph nodes in either the hilar and lobar areas or the mediastinal area [P > 0.05, Table 2]. Both number and the status of the metastatic mediastinal lymph node showed no differences between two groups [P > 0.05, Table 2].
In-hospital mortality and morbidity
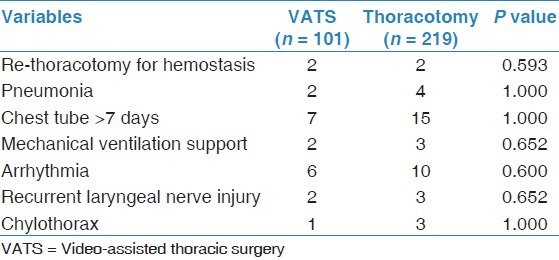
There was no in-hospital mortality for either groups, and the difference in major post-operative complications between the two groups was not significant [P > 0.05, Table 3].
Table 3.
Comparison of morbidity between VATS group and thoracotomy group
Survival analysis
Survival data were obtained for 287 patients (89.7%), with a median follow-up time of 37 months (range: 7-69 months). Adjuvant therapy was received by 74 out of 101 patients (73.3%) in the VATS group and 156 out of 219 patients (71.2%) in the thoracotomy group (P = 0.707). The overall 1-year survival rate, 3-year survival rate and 5-year survival rate were 95.6%, 50.7%, and 24.3%, respectively [Figure 1]. The mean survival times for the VATS group and the thoracotomy group were 47.5 ± 2.2 months (median 49.0 months) and 37.0 ± 1.4 months (median 31.7 months), respectively, which are statistically different [P < 0.001, Figure 2].
Figure 1.
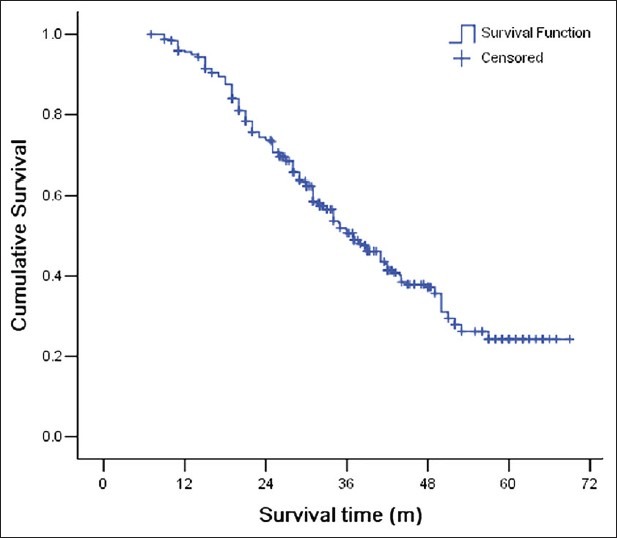
Overall patient survival curve
Figure 2.

Comparison of survival curves for the video-assisted thoracic surgery group and the thoracotomy group
Stratum analysis based on the N2 station number showed that for patients with a single station of N2 metastasis, the survival time for patients in the VATS group was significantly longer that for patients in the thoracotomy group [P = 0.001, Figure 3a], while for patients with multiple stations of N2 metastasis, there was no statistical difference in survival times [P = 0.225, Figure 3b].
Figure 3.
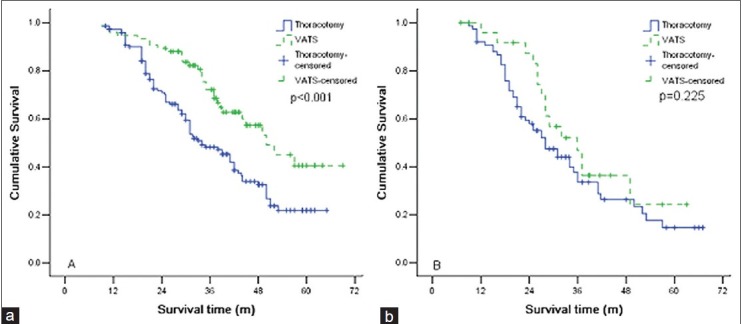
(a) Comparison of survival curves for patients with a single station of metastatic mediastinal lymph node for the video.assisted thoracic surgery (VATS) group and the thoracotomy group, (b) Comparison of survival curves for patients with multiple station of metastatic mediastinal lymph node for the VATS group and the thoracotomy group.
Discussion
Previous large-scale randomized clinical trials[5–7] and a meta-analysis study[8] have demonstrated the safety and feasibility of VATS lobectomy plus systematic lymph node dissection in treating patients with early stage NSCLC disease. Compared to conventional open thoracotomy lobectomy, VATS lobectomy reduces the rate of complications, decreases recovery time, and improves post-operative quality of life. However, it remains controversial whether the VATS or open thoracotomy lobectomy has better long-term oncologic efficacy. Extensive attention has been paid to whether the VATS is effective in lymph node dissection, which plays a vital role in post-surgery recovery and subsequent treatment of patients with NSCLC disease.
There is a possibility that clinical staging using the pre-operative oncological determination with CT, PET, MRI, mediastinoscopy, and EBUS-TBNA is inconsistent with post-operative staging by pathology. D′Cunha et al.[9] investigated 502 NSCLC patients and showed that the pathologic stage of 38.3% patients was inconsistent with the clinical stage determined with CT, PET, and other photographic tools. Although, it was once thought that mediastinal lymph nodes biopsy is the gold standard for NSCLC mediastinal lymph nodes metastasis,[10] a recent review by Whitson et al.[11] showed that the sensitivity, specificity, positive and negative predictive values, and accuracy of mediastinoscopy for lymph node staging were 66-93%, 100%, 100% 88-92%, and 90-95.2%, respectively. The low sensitivity and false negative rates are due to the inaccessibility of the anterior and inferior lymph nodes, such as No. 5, 6, 8, 9 stations, to mediastinoscopy. The same problems also exist in EBUS-TBNA. Thus, some of the patients with N2 disease were selected for VATS lobectomy.
Since, the patients in the current study were in the clinical early stage with tumors in peripheral areas in most cases, the mortality and morbidity were low, and no patients in the VATS group needed conversion to thoracotomy during the VATS procedure.
Analysis of lymph node dissection statistics shows that the number of hilar, mediastinal lymph nodes and the number of positive lymph nodes were similar for VATS lobectomy and open thoracotomy lobectomy. Thus, we conclude that VATS is feasible for lymph node dissection, which is consistent with the results of other studies.[2] Although, the number of dissected lymph nodes was somewhat smaller in our study, it is comparable to some results in the literature.[7]
It should be noted that the patients in the VATS group exhibited longer survival times, which reflects a less impaired immune system for patients in the VATS group than in the thoracotomy group.[12] The immune system plays a vital role in the destruction of potential residual cancer cells after an operation. In addition, post-operative subsequent adjuvant chemotherapy is dependent upon the patients′ overall health, and patients undergoing VATS lobectomy recover more quickly, leading to longer survival times. Since our study was retrospective, the operation technique depended on the surgeons′ preference and experience. Generally speaking, VATS was performed for earlier and less invasive diseases, which might explain the difference in survival times. However, the patients in both groups were at the same stage, and the baseline conditions were comparable between two groups. Moreover, since it is difficult to identify cN0-pN2 patients pre-operatively, a prospective study is not feasible for this population.
Rocco et al.[13] proposed that mediastinal lymph nodes metastasis is an ongoing dynamic process, developing from micro-metastatic focus within mediastinal lymph nodes under microscopic observation, to hidden minimal N2 disease, to N2 at single station under radioscopic observation, to N2 disease at multi-level stations, and finally forming mediastinal lymph nodes cohesion and spreading into surrounding tissues. The corresponding outcomes and post-operative treatments for patients in each stage are different. Kim et al.[14] defined minimal N2 as unexpected medistinal lymph nodes metastasis observed during an operation, but undetected in pre-operative evaluations. Minimal N2 patients have better and clearer surgical results than multi-level N2 patients. Decaluwé et al.[15] reported that the 5-year survival rates for patients who underwent inductive chemotherapy with lymph node metastasis in a single-level station and multi-level stations were 37% and 7.1%, respectively. These results show that patients with N2 disease in a single-level station survive longer in comparison to patients with N2 disease in multi-level stations. In the current study, the stratum analysis based on the status of metastatic mediastinal lymph nodes shows that VATS mainly benefits patients with a single station of metastatic mediastinal lymph node. This is related to the fact that mediastinal lymph nodes with a minimal metastasis of one station are likely to be dissected completely through surgery, and the chance of tumor cells spreading into the surrounding soft-tissue through capsules is low while mediastinal lymph nodes with a minimal metastasis of two or more stations are likely to offset the survival benefits of VATS.
Kim et al.[14] reported a 3-year overall survival rate of 89% for patients with unexpected N2 disease, which approximates the survival rate for patients with stage I cancer. In our research, the overall 5-year survival rate is somewhat lower than those reported in the literature, which may be related to differences in the inclusion and exclusion criteria, or in the surgical aspect (e.g., the surgical technique) or post-operative aspect (e.g., the proportion of patients receiving adjuvant chemotherapy). Although, it is possible that some cases in our study might have been diagnosed as cN2 patients (advanced stage) if pre-operative mediastinoscopy, PET/CT and/or EBUS had been routinely used, we do not think that the routine use of such procedures in the absence of suspicious targets is necessary for the following reasons: (1) The positive rate in such patients is very low; a prospective study[16] applied routine mediastinoscopy and EUS-FNA to patients who were N2 negative by PET/CT, and found a very low incidence of unsuspected N2 disease (2.9% by mediastinoscopy and a 3.7% positive rate by EUS-FNA). A 2007study by Sawhney[17] of 44 patients showed a very low incidence of unsuspected N2 disease by EUS (3%) when only a CT scan was employed for preoperative staging. (2) The efficacy of neoadjuvant chemotherapy has not been well established by randomized controlled trials for unexpected N2 patients, so the routine use of mediastinoscopy or EUS-FNA without a suspicious target has little clinical significance. These reasons may be responsible for the variation of survival rates reported in the literature.
Due to the fact that this is a retrospective study, our findings should be interpreted with caution. Selection bias could not be averted completely, and differences in pre-operative workups might have resulted in a difference in outcomes, which complicates comparison between our results and other studies. Furthermore, survival was the only follow-up parameter in the current study, and there is no information on other parameters, such as disease-free survival. Finally, there is little detailed information on adjuvant chemotherapy and pathology.
Conclusions
We conclude that it is both feasible and safe to perform VATS lobectomy on patients with unexpected N2 NSCLC. Significant survival benefits are obtained from VATS by those patients with single station mediastinal lymph node metastasis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: Experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–5. doi: 10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 2.Onaitis MW, Petersen RP, Balderson SS, Toloza E, Burfeind WR, Harpole DH, Jr, et al. Thoracoscopic lobectomy is a safe and versatile procedure: Experience with 500 consecutive patients. Ann Surg. 2006;244:420–5. doi: 10.1097/01.sla.0000234892.79056.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitson BA, Andrade RS, Boettcher A, Bardales R, Kratzke RA, Dahlberg PS, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83:1965–70. doi: 10.1016/j.athoracsur.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 4.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 5.Shigemura N, Akashi A, Funaki S, Nakagiri T, Inoue M, Sawabata N, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg. 2006;132:507–12. doi: 10.1016/j.jtcvs.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 6.Swanson SJ, Herndon JE, 2nd, D′Amico TA, Demmy TL, McKenna RJ, Jr, Green MR, et al. Video-assisted thoracic surgery lobectomy: Report of CALGB 39802 - A prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993–7. doi: 10.1200/JCO.2007.12.6649. [DOI] [PubMed] [Google Scholar]
- 7.Scott WJ, Allen MS, Darling G, Meyers B, Decker PA, Putnam JB, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: A secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg. 2010;139:976–81. doi: 10.1016/j.jtcvs.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2553–62. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 9.D′Cunha J, Herndon JE, 2nd, Herzan DL, Patterson GA, Kohman LJ, Harpole DH, et al. Poor correspondence between clinical and pathologic staging in stage 1 non-small cell lung cancer: Results from CALGB 9761, a prospective trial. Lung Cancer. 2005;48:241–6. doi: 10.1016/j.lungcan.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Hammoud ZT, Anderson RC, Meyers BF, Guthrie TJ, Roper CL, Cooper JD, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg. 1999;118:894–9. doi: 10.1016/s0022-5223(99)70059-0. [DOI] [PubMed] [Google Scholar]
- 11.Whitson BA, Groth SS, Maddaus MA. Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg. 2007;84:1059–65. doi: 10.1016/j.athoracsur.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Walker WS, Codispoti M, Soon SY, Stamenkovic S, Carnochan F, Pugh G. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg. 2003;23:397–402. doi: 10.1016/s1010-7940(02)00814-x. [DOI] [PubMed] [Google Scholar]
- 13.Rocco G, Perrone F, Rossi A, Gridelli C. Surgical management of non-small cell lung cancer with mediastinal lymphadenopathy. Clin Oncol (R Coll Radiol) 2010;22:325–33. doi: 10.1016/j.clon.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim HK, Choi YS, Kim J, Shim YM, Kim K. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:1288–93. doi: 10.1016/j.jtcvs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Decaluwé H, De Leyn P, Vansteenkiste J, Dooms C, Van Raemdonck D, Nafteux P, et al. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg. 2009;36:433–9. doi: 10.1016/j.ejcts.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Cerfolio RJ, Bryant AS, Eloubeidi MA. Routine mediastinoscopy and esophageal ultrasound fine-needle aspiration in patients with non-small cell lung cancer who are clinically N2 negative: A prospective study. Chest. 2006;130:1791–5. doi: 10.1378/chest.130.6.1791. [DOI] [PubMed] [Google Scholar]
- 17.Sawhney MS, Bakman Y, Holmstrom AM, Nelson DB, Lederle FA, Kelly RF. Impact of preoperative endoscopic ultrasound on non-small cell lung cancer staging. Chest. 2007;132:916–21. doi: 10.1378/chest.06-2571. [DOI] [PubMed] [Google Scholar]


