Abstract
Background:
Current research is inconclusive as to whether obstructive sleep apnea severity directly limits exercise capacity and lowers health-related quality of life (HRQoL).
Aims:
The aim of this study was to evaluate the association of obstructive sleep apnea severity with determinants of exercise capacity and HRQoL.
Subjects and Methods:
Subjects were evaluated by home somnography and classified as no obstructive sleep apnea (n = 43) or as having mild (n = 27), moderate or severe obstructive sleep apnea (n = 21). Exercise capacity was assessed by a ramping cycle ergometer test, and HRQoL was assessed with the SF-36 questionnaire.
Results:
Greater obstructive sleep apnea severity was associated with older age, higher body weight, higher body mass index, lower peak aerobic capacity, a higher percentage of peak aerobic capacity at a submaximal exercise intensity of 55 watts, and lower physical component summary score from the SF-36. None of these variables were statistically different among obstructive sleep apnea severity groups after controlling for age and body weight. Obstructive sleep apnea severity was not associated with any cardiorespiratory fitness or HRQoL parameter.
Conclusions:
Obstructive sleep apnea severity has no independent association with exercise capacity or HRQoL.
Keywords: Cardiorespiratory fitness, Health-related quality of life, Obstructive sleep apnea syndrome
Introduction
Obstructive sleep apnea syndrome (OSAS) is characterized by repetitive episodes of partial or complete upper airway obstruction that frequently results in sleep fragmentation, excessive daytime sleepiness, impaired concentration and memory, and reduced cognitive function.[1] OSAS contributes to decreased energy and motivation throughout the day, often characterized as lower health-related quality of life (HRQoL).[2] In addition, the presence of OSAS contributes to the development of hypertension, coronary artery disease, heart failure, and stroke.[3]
The most commonly prescribed treatment for OSAS is nasal continuous positive airway pressure (nCPAP), which has been shown to improve symptoms, increase vigilance, and reduce potentially negative cardiovascular outcomes (e.g., elevated blood pressure) known to be associated with OSAS.[4] However, the observed benefits of nCPAP are related to patient adherence. In this regard, nCPAP must be used consistently in order to maximize treatment benefit.[4] Unfortunately, adherence to nCPAP therapy is disappointingly low,[5] suggesting a need to identify other ancillary treatment options with potential to improve important health-related outcomes in OSAS.
The American Sleep Disorders Association endorses regular exercise as a non-pharmacological approach to improve sleep quality,[6] which may reduce daytime sleepiness and therefore promote improved quality of life in OSAS patients.[7,8] However, in addition to lack of time and motivation, which are two commonly reported barriers to exercise participation, the primary symptoms of OSAS (e.g., excessive daytime sleepiness and daytime fatigue) present additional challenges. Patients with these OSAS-related symptoms may not be psychologically motivated or physically able to follow a routine exercise program.[9] Furthermore, sleep-deprived patients in general have a decreased ability to perform maximal exercise, self-select a slower pace when walking, and report a higher perceived exertion at a given workload.[10] Thus, OSAS patients may not personally choose to engage in or otherwise receive encouragement to participate in regular exercise, which may increase cardiovascular risk due to a sedentary lifestyle.
Current research is inconclusive as to whether OSAS severity directly limits exercise capacity and lowers HRQoL. Relative to aerobic exercise capacity, five studies over the past decade showed peak aerobic capacity (VO2pk) was reduced in patients with untreated OSAS while six studies showed no difference.[11] The aim of the current study was to evaluate the independent association of OSAS severity on exercise capacity and HRQoL.
Subjects and Methods
Subjects
Subjects for this study were recruited from a group of volunteers referred for an overnight polysomnography study (PSG) to the Sleep Disorders Network of Southwest Virginia, Christiansburg, VA, USA. Data were collected and pooled from two protocols with similar methods and subject entry criteria. Eligible subjects were overweight or obese (body mass index [BMI] ≥ 25 kg/m2) adult males and females. Exclusions included a history of cardiovascular or pulmonary disease, current smoker, currently taking any prescriptive or over the counter medications known to affect cardiovascular or metabolic functions (e.g., anti-hypertensives, hypnotics, sedatives, analgesics, psychotropics, steroids or sympathomimetics), diabetes, musculoskeletal disorders that would preclude maximal aerobic exercise testing, or a recent history of regular participation (> 3 days/week, 30 min/day for previous 6 months) in moderate or vigorous physical activity. Each subject gave written informed consent and the protocols were authorized by the Institutional Review Board for Human Subjects Research at Virginia Tech. All research was in accordance with the Helsinki Declaration of 1975 as revised in 1983.
Study methods
Study methods and procedures used to assess OSAS and exercise capacity were identical to those described by Kaleth et al.[12] All subjects underwent a sleep evaluation by an attended overnight laboratory PSG or through a portable at-home diagnostic sleep system (Embletta PDS, Medcare, Reykjavik Iceland). The PSG studies were performed with standard instrumentation and procedures as recommended by the American Academy of Sleep Medicine.[13] The Embletta PDS has high sensitivity (92%) and specificity (86%) in quantifying apnea hypopnea index (AHI) when compared against PSG.[14] Subjects assessed with the portable sleep system completed testing on 1 weeknight with a minimum sleep period of 6 h and results then were processed automatically from digital data stored on the device using proprietary software (Somnologica, Version 3.1.2, Medcare®, Iceland). Scores were adjusted by a trained sleep technician to account for movement artifact and then verified by a sleep medicine physician. Measurements taken with this portable system included nasal flow, oxygen saturation, heart rate, snoring frequency and magnitude, and thoracic and abdominal breathing effort. Subjects with an AHI < 5 were classified as no OSAS, 5-14 as mild OSAS, 15-29 as moderate OSAS, and 30 or higher as severe OSAS.[15] Subjects with moderate or severe OSAS (AHI≥15) were combined into a single group for analysis.
All subjects completed questionnaires to assess daytime sleepiness and HRQoL. The epworth sleepiness scale (ESS)[8] was used to assess daytime sleepiness. Subjects rated the likelihood that they may doze off or fall asleep for eight different situations commonly encountered in daily life. The Rand 36-item short form health survey (SF-36),[16] was used to evaluate HRQoL by calculation of physical component summary (PCS) and mental component summary (MCS) scores.
All subjects completed a maximal exercise test on an electronically braked cycle ergometer (MedGraphics® CardioO2, St. Paul, MN or SensorMedics Vmax 229®, Yorba Linda, CA) using a standardized 15 watt/min ramping protocol. Prior to each test, height, weight, heart rate and blood pressure were obtained. The initial workload was set to 25 watts and then increased 5 watts every 20 s until volitional fatigue. Trained technicians monitored the exercise and recorded perceived exertion each minute using the 6-20 Borg rating of perceived exertion (RPE) scale. Respiratory gas exchange measurements were obtained during the ramp exercise test using a computer controlled breath-by-breath gas exchange system. Peak VO2 (VO2pk) was defined as the highest VO2 achieved during the final minute of exercise. All submaximal exercise parameters were recorded at the end of the 55 watt stage of the graded maximal exercise test.
Statistical analysis
Data were analyzed with the Statistical Package for the Social Sciences, version 18.0 (SPSS, Chicago, IL). Continuous data were reported as mean ± standard deviation and dichotomous data were reported as counts and percentages. One-way analysis of variance was used to detect differences in baseline characteristics, exercise capacity, and HRQoL by OSAS severity. One-way analysis of covariance was used to adjust for the influence of confounding variables. Pearson correlation and stepwise multiple linear regression were used to determine the independent associations of baseline characteristics and OSAS severity on exercise capacity and HRQoL. Statistical significance for all analyses was set at P < 0.05.
Results
Subject baseline characteristics
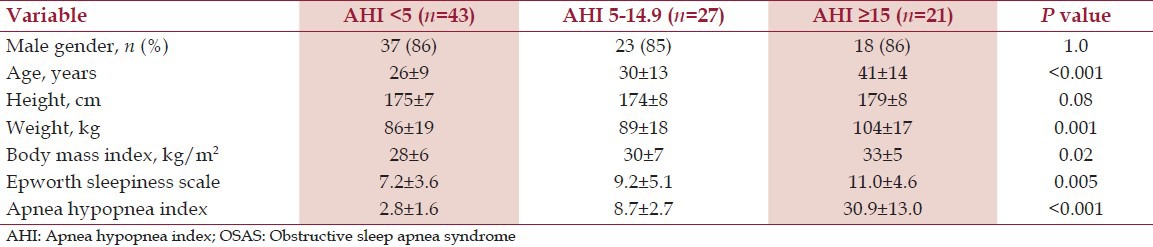
Descriptive characteristics of the three groups are displayed in Table 1. Greater OSAS severity was associated with higher age (P < 0.001), body weight (P = 0.001), and BMI (P = 0.02).
Table 1.
Baseline subject characteristics by OSAS severity
Exercise data
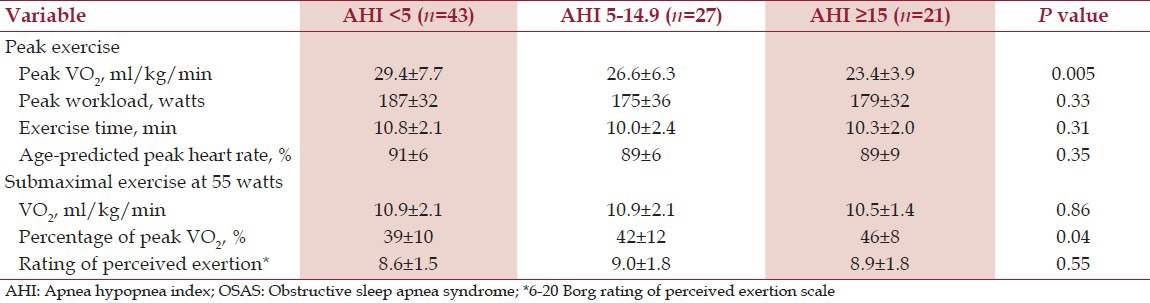
Table 2 presents respiratory metabolic data from the maximal ramp cycle ergometer test. Peak RER and RPE from the cycle ergometer test were similar in both groups and the mean values (RER > 1.10, RPE > 16.5), suggesting maximal effort by participants. Greater OSAS severity was associated with lower VO2pk and a higher percentage of VO2pk during submaximal exercise, but not with peak workload, exercise duration, age-predicted peak heart rate, submaximal VO2, and submaximal RPE. However, when effects of age and body weight were controlled statistically, no differences in VO2pk or percentage of VO2pk by OSAS severity were detected. Specifically, adjusted mean VO2pk in ml/kg/min was 27.0, 26.3, and 28.4 and adjusted mean percentage of VO2pk at the standardized 55 watt workload was 41%, 42%, and 41% for the no, mild, and moderate/severe OSAS groups, respectively.
Table 2.
Peak and submaximal exercise data by OSAS severity
HRQoL
Table 3 presents data from the SF-36 HRQoL questionnaire. Greater OSAS severity was significantly associated with lower PCS (physical functioning), but not MCS (mental functioning), scores. After controlling for age and body weight, no differences in PCS were detected. Specifically, adjusted mean PCS values were 53, 53, and 51 for no, mild, and moderate/severe OSAS groups, respectively. For reference, the mean and standard deviation for PCS and MCS in population-based studies is 50 ± 10, with higher values representative of higher HRQoL.
Table 3.
Health-related quality of life by OSAS severity

Correlation and linear regression
The relationship between cardiorespiratory fitness and HRQoL with demographics and OSAS severity is presented in Table 4. For each of the selected cardiorespiratory fitness and HRQoL parameters, age, height, body weight, and/or BMI yielded the highest correlations. AHI and ESS were weakly related to all cardiorespiratory fitness and HRQoL parameters (all R2 ≤ 15%).
Table 4.
Relationship of cardiorespiratory fitness and health-related quality of life with demographics and OSAS severity (n=91)
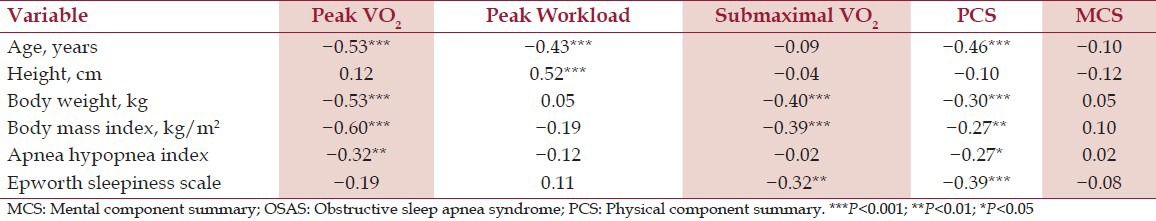
In multiple regression analysis, BMI and age explained 53% of the variance in peak VO2, height, age, and PCS explained 44% of the variance in peak workload, and body weight explained 17% of the variance in submaximal VO2. Age, body weight, and gender explained 30% of the variance in PCS. No variables were significantly associated with MCS. Neither AHI nor ESS was independently associated with any cardiorespiratory fitness or HRQoL parameter in multiple regression analysis.
Discussion
Subjects with OSAS may have difficulty with regular aerobic exercise participation because of two hallmark symptoms – excessive daytime sleepiness and chronic fatigue.[9] There is a need for research that objectively examines patterns and modulating factors that affect physical activity in OSAS patients. Published data on this topic has relied primarily on self-report.[9] The current study represents the first to evaluate subjective and objective measures of exercise capacity in OSAS patients. We have demonstrated that OSAS severity, as quantified by AHI classifications widely used in clinical settings, has no independent association with exercise capacity and HRQoL.
The contribution of excessive daytime sleepiness as a potential barrier to exercise in patients with OSAS merits special consideration. Vgontzas et al.[17] suggested that fatigue and excessive daytime sleepiness may be intimately related to health risks fundamentally associated with obesity such as insulin resistance, psychological distress or very low physical activity levels. Aguillard et al.[8] concluded that fatigue may be linked to reduced physical fitness levels in OSAS subjects. We demonstrated that neither AHI nor ESS directly influence exercise capacity or HRQoL and that factors such as age and body weight explain more of the variance in these outcomes.
The benefits of regular exercise participation in OSAS are well-documented, including improvement in quality of life and mood, reduced levels of anger, depression, bodily pain, and total mood disturbances, and increased participation in social activities.[18,19] Exercise has also been shown to improve insulin resistance and reduce central adiposity.[7,20,21] Hong and Dimesdale,[9] reported that physical activity was significantly correlated with subjective well-being and was a significant predictor of fatigue, vitality, and vigor in OSAS subjects. Lopes et al.[22] found that subjects with moderate OSAS (AHI score 5-15) who exercised regularly (> 2 h/week) had higher HRQoL scores than non-sleepy and non-active OSAS groups. A randomized controlled trial by Sengul et al.[23] showed significant improvements in the Vitality and Mental Health domains of the SF-36 and reduced AHI scores in the exercise group, which followed an aerobic exercise program 3 times a week for 12 weeks compared to the control group, which had SF-36 and AHI scores that remained unchanged. Quan et al.[20] showed that a minimum of 3 h a week of vigorous exercise can reduce the risk of developing OSAS and others,[21] similarly have reported that 3-6 h of exercise per week is associated with less severe OSAS. These studies, along with current results showing OSAS subjects had comparable physical capabilities to healthy control subjects, suggest that encouragement of regular physical activity may be a viable option to improve OSAS symptoms and quality of life.[7] Overall, improving physical fitness may be important for alleviating symptoms of OSAS and lowering cardiovascular risk. We have shown that OSAS severity has no deleterious effect on cardiorespiratory fitness and HRQoL.
A limitation of this study was that depression was not evaluated in this group of subjects, which is a known influencer of exercise capacity. An additional limitation of the study was the relatively young and healthy subject population. Therefore, these results may not be generalizable to older OSAS patients. Lastly, it is plausible that OSAS severity has an influence on exercise tolerance or HRQoL with a magnitude that was undetectable with the sample size in this study. Even if this were the case, the effect would be of such small magnitude as to have little practical significance. Our study was however, strengthened by initial screening to include only those subjects with objective measures of OSAS severity who were free from known or diagnosed comorbidities or prescriptive medications that might confound interpretation of the effects of OSAS.
In conclusion, OSAS severity is not associated with exercise tolerance or HRQoL and should not be considered a barrier to exercise participation. Regular physical exercise could be beneficial to OSAS patients in alleviating symptoms, improving quality of life, and reducing chronic disease risk.
Footnotes
Source of Support: This research was supported through a grant from ResMed Sleep Disordered Breathing Foundation, Poway, CA
Conflict of Interest: None declared.
References
- 1.Shah N, Roux F, Mohsenin V. Improving health-related quality of life in patients with obstructive sleep apnea: What are the available options? Treat Respir Med. 2006;5:235–44. doi: 10.2165/00151829-200605040-00002. [DOI] [PubMed] [Google Scholar]
- 2.Yang EH, Hla KM, McHorney CA, Havighurst T, Badr MS, Weber S. Sleep apnea and quality of life. Sleep. 2000;23:535–41. [PubMed] [Google Scholar]
- 3.Reishtein JL. Obstructive sleep apnea: A risk factor for cardiovascular disease. J Cardiovasc Nurs. 2011;26:106–16. doi: 10.1097/JCN.0b013e3181e3d724. [DOI] [PubMed] [Google Scholar]
- 4.Gordon P, Sanders MH. Sleep. 7: Positive airway pressure therapy for obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2005;60:68–75. doi: 10.1136/thx.2003.007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindberg E, Berne C, Elmasry A, Hedner J, Janson C. CPAP treatment of a population-based sample: What are the benefits and the treatment compliance? Sleep Med. 2006;7:553–60. doi: 10.1016/j.sleep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. 2000;4:387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- 7.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–9. [PubMed] [Google Scholar]
- 8.Aguillard RN, Riedel BW, Lichstein KL, Grieve FG, Johnson CT, Noe SL. Daytime functioning in obstructive sleep apnea patients: Exercise tolerance, subjective fatigue, and sleepiness. Appl Psychophysiol Biofeedback. 1998;23:207–17. doi: 10.1023/a:1022257514209. [DOI] [PubMed] [Google Scholar]
- 9.Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc. 2003;35:1088–92. doi: 10.1249/01.MSS.0000074566.94791.24. [DOI] [PubMed] [Google Scholar]
- 10.Himashree G, Banerjee PK, Selvamurthy W. Sleep and performance: Recent trends. Indian J Physiol Pharmacol. 2002;46:6–24. [PubMed] [Google Scholar]
- 11.Aron A, Zedalis D, Gregg JM, Gwazdauskas FC, Herbert WG. Potential clinical use of cardiopulmonary exercise testing in obstructive sleep apnea hypopnea syndrome. Int J Cardiol. 2009;132:176–86. doi: 10.1016/j.ijcard.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Kaleth AS, Chittenden TW, Hawkins BJ, Hargens TA, Guill SG, Zedalis D, et al. Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med. 2007;8:160–8. doi: 10.1016/j.sleep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J, Jr, et al. Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 14.Ng SS, Chan TO, To KW, Ngai J, Tung A, Ko FW, et al. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS) Respirology. 2010;15:336–42. doi: 10.1111/j.1440-1843.2009.01697.x. [DOI] [PubMed] [Google Scholar]
- 15.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 16.Hays R, Sherbourne C, Mazel R. Durham, N.C: Duke University Press; 1992. User's manual for the medical outcomes study (MOS) core measures of health-related quality of life. [Google Scholar]
- 17.Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: The role of the stress system and cytokines. Ann N Y Acad Sci. 2006;1083:329–44. doi: 10.1196/annals.1367.023. [DOI] [PubMed] [Google Scholar]
- 18.Stewart KJ, Turner KL, Bacher AC, DeRegis JR, Sung J, Tayback M, et al. Are fitness, activity, and fatness associated with health-related quality of life and mood in older persons? J Cardiopulm Rehabil. 2003;23:115–21. doi: 10.1097/00008483-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Drewnowski A, Evans WJ. Nutrition, physical activity, and quality of life in older adults: Summary. J Gerontol A Biol Sci Med Sci. 2001;56:89–94. doi: 10.1093/gerona/56.suppl_2.89. [DOI] [PubMed] [Google Scholar]
- 20.Quan SF, O’Connor GT, Quan JS, Redline S, Resnick HE, Shahar E, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11:149–57. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 21.Peppard PE, Young T. Exercise and sleep-disordered breathing: An association independent of body habitus. Sleep. 2004;27:480–4. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 22.Lopes C, Esteves AM, Bittencourt LR, Tufik S, Mello MT. Relationship between the quality of life and the severity of obstructive sleep apnea syndrome. Braz J Med Biol Res. 2008;41:908–13. doi: 10.1590/s0100-879x2008005000036. [DOI] [PubMed] [Google Scholar]
- 23.Sengul YS, Ozalevli S, Oztura I, Itil O, Baklan B. The effect of exercise on obstructive sleep apnea: A randomized and controlled trial. Sleep Breathing. 2009;15:49–56. doi: 10.1007/s11325-009-0311-1. [DOI] [PubMed] [Google Scholar]


