Abstract
World human population is increasing with an alarming rate; and a variety of new types of health issues are popping up. For instance, increase in number of drug-resistant bacteria is a cause of concern. Research on antibiotics and other microbial natural products is pivotal in the global fight against the growing problem of antibiotic resistance. It is necessary to find new antibiotics to tackle this problem. The use of therapeutic plant species in traditional medicine is as old as mankind; and currently, it is strongly believed that all types of plant species across the plant kingdom do harbour endophytic bacteria (EB). The natural therapeutic compounds produced by EB do have several potential applications in pharmaceutical industry. The EB derived natural products such as Ecomycins, Pseudomycins, Munumbicins and Xiamycins are antibacterial, antimycotic and antiplasmodial. Some of these natural products have been reported to possess even antiviral (including Human Immunodeficiency Virus (HIV)) properties. Therefore, to deal with increasing number of drug-resistant pathogens EB could serve as a potential source of novel antibiotics.
Keywords: Bioprospecting, ecomycins, human immunodeficiency virus, kakadumycins, medicinal plants, munumbicins, natural products, pseudomycins, traditional medicine, xiamycins
INTRODUCTION
Endophytes are micro-organisms (bacteria and fungi) that live inside the living plant tissues for at least part of their life without causing any apparent disease symptoms in the host.[1] Endophytes are treated as endosymbiont. To represent these types of micro-organisms, De Bary[2,3] coined the term endophyte. Endophytes have been reported in many important medicinal plants, weeds, and ornamental and fruit trees from wild and domesticated settings. Both endophytic bacteria and endophytic fungi can co-exist in a single host plant.[4] The population of endophytes is considered as a subset of the rhizospheric microbial population as reported by Germida et al.[5] Endophytes enter inside plants primarily through the roots and the aerial portions of plants, such as leaves, flowers, stems and cotyledons.[6] They are localized at the point of entry and can spread in the whole host plant body.[7] After entering the host, they reside within cells or the intercellular spaces or in the vascular (tissue) system.[8,9,10] After gaining residence in the plant tissues, the endophytes are known to produce a diverse range of natural products which could be consistent and successful source of drugs. Therefore, the natural products from endophytic microbes do have a great potential not only in pharmaceutical industry but agrochemical and biotechnology industries also.[11]
The natural products obtained from endophytic microbes are found to be antimicrobial, antiviral, anticancer, antioxidants, antidiabetic and immunosuppressant.[12,13,14,15,16,17,18,19] The fungal endophytes are known to produce these types of natural products. Nonetheless, poorly explored and underutilized EB are known to produce antibiotics in addition to other natural products. The EB appears to be a potential source of novel antibiotics. It is well known fact that until now the soil bacteria have been the source for most of the antibiotics. Now the EB seem to be a promising alternative potential source of novel antibiotics. This article aims to provide an overview in brief on bacterial endophytes as a potential source of novel antibiotics.
MAIN TYPES OF EB
In general, EB are divided into two main types namely, obligate and facultative.[20] Facultative endophytes are capable to survive in the soil, on the plant surface, inside the plants as well as on artificial nutrients[20] and endophytes which inhabit inside plant tissues throughout their lifespan are called as obligate endophytes.[21] Uncultivable EB are also reported from several plant species and plantlets from in vitro cultures. Facultative EB (cultivable) are widely distributed across the plant kingdom and can be isolated from various plant species to explore their potential for the production of commercially important natural products.
DISTRIBUTION OF EB
Several species of cultivable EB (including both gram-positive and gram-negative) have been isolated, identified and reported from a large diverse terrestrial and aquatic plants.[22,23,24] The EB do not get any benefit from their host plant other than residency[25] and nutrients. It is important to note that population density of EB remains very low in comparison to that of rhizospheric bacteria or plant pathogenic bacteria. A very comprehensive lists of EB isolated from a broad range of plant species are available in public domain.[7,26] In our laboratory, we have also isolated and identified more than 1280 isolates of cultivable EB from various plant species available in Malaysia (our unpublished data at http://www.ncbi.nlm.nih.gov/nuccore/?term=bhore%20sj.[27,28,29] The systematic primary analysis of isolates suggests that EB are widely distributed in monocotyledonous and dicotyledonous plant species.
ROLES OF EB
The endophytes are known to boost the growth and development of host plants in varied environmental and ecological conditions.[30,31,32] The EB are also known to increase host plants resistance to pathogens and to promote biological nitrogen fixation as stated by Bhore et al.[27] However, the ability of EB to produce various natural products is of prime importance in pharmaceutical industry.[33] To explore EB, a number of research teams worldwide are involved in screening of endophytes for various natural products (bioactive compounds). Several EB have been shown to produce natural products like phytohormones, low molecular weight compounds, enzymes, siderophores, and antibiotics.[24,27,34,35,36,37,38]
ENDOPHYTES AS A SOURCE OF ANTIBIOTICS
The human population is increasing with an alarming rate; ecosystems are deteriorating rapidly; and a variety of new types of health issues are popping up. For instance, increase in number of drug-resistant bacteria is a cause of concern. Research on antibiotics and other microbial natural products is pivotal in the global fight against the growing problem of antibiotic resistance. It is necessary to find new antibiotics to tackle this problem; and EB are one of the potential sources of novel antibiotics.
Natural products are metabolites from micro-organisms, plants and animals.[39] These natural products are important, because they have been the traditional sources of drugs. In many cases, natural products have served as sources of lead molecules, which yielded many synthetic drugs. The world's first billion-dollar anticancer-drug, paclitaxel (Taxol) is an outstanding example of a natural product from Yew tree, Taxus wallachiana.[40] In 1996, Strobel et al. reported that endophytic fungus (Pestalotiopsis microspora) found in Yew tree is also able to produce Taxol.[41] The classical immunosuppressive cyclosporine isolated from the endophytic fungus (Tolypocladium inflatum) further increased the importance and significance of endophytes.[19,42,43] Like endophytic fungi, EB also have amazing potential in producing novel natural products. In fact, researchers are working in that direction to explore EB for the novel and unique natural products of commercial importance. Many natural products produced by endophytes have proven to be antibacterial, antifungal, antidiabetic, antioxidants and immunosuppressives. Thus, endophytes are viewed as a great source of bioactive natural products. The majority of EB do produce different kinds of antibiotics; and in fact, the EB are one of the untapped potential sources of novel antibiotics. Ecomycins, Pseudomycins, Munumbicins, Kakadumycins are some examples of the novel antibiotics produced by EB.
ECOMYCINS
The Ecomycins represent a family of novel lipopeptides and are made up of some unusual amino acids including homoserine and β-hydroxy aspartic acid. The Endophytic bacterium, Pseudomonas viridiflava is known to produce Ecomycins.[44] This endophyte is one of the plant-associated fluorescent Pseudomonads; and it is known to exist in the tissues of many grass species. The identified and partially characterized three antifungal lipopeptides produced by P. viridiflava strain EB273 are called as Ecomycin A, B and C. Out of these three molecules, the Ecomycin A is similar to (amino acid composition) an already reported antibiotic syringotoxin.[45] Conversely, Ecomycin B and C represent a unique set of related lipopeptides in not possessing phenylalanine, lysine, arginine, ornithine or diaminobutyric acid, which are constituents of such compounds as the pseudomycins, syringomycins, syringostatins and syringotoxin.[45,46,47,48] Further, studies performed with some other strains of the same bacterium viz, P. viridiflava strain EB274 (California, USA) and EB227 (Israel) also produced antifungal lipopeptides whose masses are identical to those of Ecomycins B and C. These compounds were able to inhibit the human pathogens Cryptococcus neoformans and Candida albicans as reported by Harrison et al.[48]
PSEUDOMYCINS
The Pseudomycins represent a group of peptide antifungal compounds isolated from liquid cultures of Pseudomonas syringae, a plant-associated bacterium. These antifungal peptide are also lipopeptides containing non traditional aminoacids like L-chlorothreonine, L-hydroxy aspartic acid and both D-and L-diaminobutyric acid. The P. syringae is a member of the Pseudomonadaceae family of Proteobacteria Phylum. Pseudomycin A, the predominant peptide in a family of four, pseudomycins A-D, has impressive activity against the human pathogen, Candida albicans.[48] Pseudomycins A-C contain hydroxyaspartic acid, serine, arginine, lysine and diaminobutyric acid. Pseudomycin D, on the other hand, has a molecular mass of 2401Da and is more complex than pseudomycins A-C. However, they are found to be different from the PReviously described antimycotics from P. syringae, including syringomycin, syringotoxin and syringostatins. They are effective against a variety of human and plant pathogenic fungi including C. albicans and C. neoformans.[48]
MUNUMBICINS
The Munumbicins represent a novel group of 4 bioactive substances. Munumbicins A, B, C and D are newly described antibiotics with a wide spectrum of activity against plant pathogenic fungi and bacteria, and a Plasmodium species. These compounds were obtained from a bacterium that has been deposited as Streptomyces NRRL 30562 in the mediPeoria USDA National Laboratory. It is an endophytic bacterium found in the Snake vine [Kennedia nigriscans], a medicinal plant native to the northern territory of Australia. The munumbicins act against Gram-positive bacteria such as Bacillus anthracis, Streptococcus pneumoniae, Enterococcus faecalis and Staphylococcus aureus.[49] Along with that, the methicillin-resistant strain of S.aureus (MRSA, ATCC 33591) and a vancomycin-resistant strain of E. faecalis (VREF, ATCC 51299) are two of the Gram-positive munumbicin-sensitive bacterial strains that are commonly drug-resistant. The munumbin B is effective against multiple-drug-resistant (MDR) Mycobacterium tuberculosis, an acid-fast bacterium. However, the most peculiar fact is that only the MDR strain of M. tuberculosis was sensitive to munumbin B whereas the drug-susceptible strain of this organism was less sensitive. The munumbicins C and D are of a special interest because in addition to being effective against Gram positive and negative bacteria, they are effective against the malarial parasite Plasmodium falciparum, the most pathogenic Plasmodium causing malaria. The munumbicin D was reported as more powerful than chloroquine, the gold-standard antimalarial drug.[50] Furthermore, munumbicins B, C and D did not cause any detectable lysis of human red blood cells. But these compounds were not effective against human pathogenic fungi. The munumbicins E-4 and E-5 were isolated from Streptomyces NRRL 30562. They are found to be effective against both gram-positive and gram-negative bacteria. The antimalarial activity of munumbicins E-4 and E-5 is reported to be double than that of chloroquine.[51]
KAKADUMYCINS
These are peptide antibiotics produced (in culture) by endophytic bacterium Streptomyces (NRRL30566) from a fern-leaved Grevillea tree [Grevillea pteridifolia, Synonym: Grevillea chrysodendron R.Br.] native to the northern territory of Australia.[52] Kakadumycin A is chemically related to echinomycin, another Streptomyces derived quinoxaline antibiotic, a potential anticancer drug.[53,54] Kakadumycin A, has antibacterial activity similar to Munumbicins; and it is also effective against P. falciparum.
XIAMYCINS
The Xiamycins represent one of the indolosesquiterpenes isolated from prokaryotes. They are novel pentacyclicindolosesquiterpene, named as Xiamycin-A and its methyl ester-2 obtained from Streptomyces sp. strain GT2002/1503, an endophyte from the mangrove plant, Bruguiera gymnorrhiza.[55] Interestingly, Xiamycin-A exhibits selective anti-HIV activity.[55] Three new indolosesquiterpenes, Xiamycin B (1b), Indosespene (2), and Sespenine (3), along with the known Xiamycin A (1a) from the culture broth of Streptomyces sp. strain HKI0595, a bacterial endophyte of the widespread mangrove tree, Kandelia candel has been reported by Ding et al.[56] Their research findings suggest that these Xiamycins do have moderate to strong antimicrobial activities against several bacteria, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis.
OTHER ANTIBIOTICS
From various tissues of medicinal plants, 78 EB has been reported by Jalgaonwala et al.[57] From these isolates, 15 EB do possess antifungal activity. They have also reported the strong antifungal activity of the bacterial isolates HB3 from rhizomes of Curcuma longa, KB4 from roots of Pinus glabra, and NB6 from stem of Eucalyptus globulus.[57]
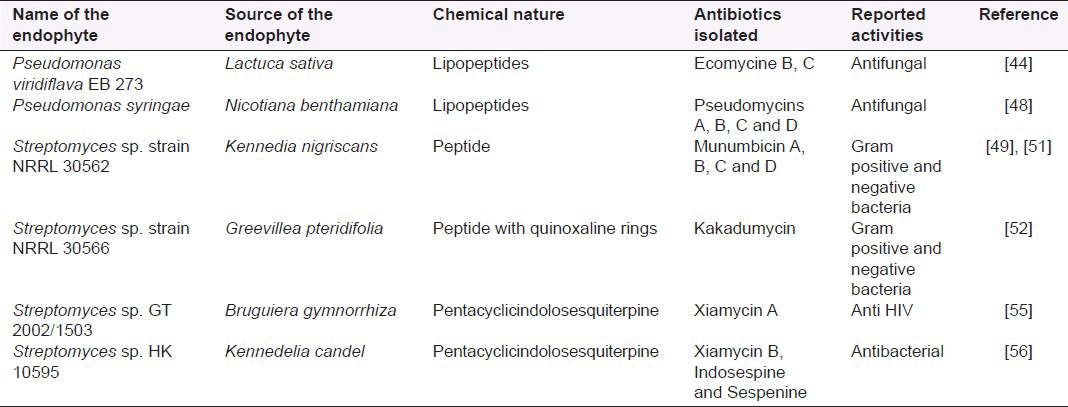
The ability of EB to produce novel antibiotics and antimicrobial natural products has greatly fascinated scientist; and due to proven reported activities [Table 1], EB are viewed as a promising potential source of novel antibiotics as well as a source of anticancer, antioxidant and immunosuppressive agents.
Table 1.
Novel antibiotics produced by endophytic bacteria and their reported activities
PERSPECTIVES
Endophytes are micro-organisms that are abundantly present in rainforest plants which occupy 7% of earth's land surface.[58] Due to huge diversity and untapped potential of EB, they appear as an alternate source of bioactive natural products with potential applications in pharmaceutical industry. Hence, EB have caught the attention of the pharmaceutical industry. However, EB as a source of new antibiotics against susceptible and resistant forms of micro-organisms is the most important and promising.
The development of resistance in microbes (especially pathogens) to most of the habitually used antibiotics keeps the search for new and novel antibiotics perpetual. For instance, some strains of Mycobacterium have developed resistance to many of the first line anti-tubercular drugs and a few second line anti-tubercular drugs leading to MDR and extensive drug resistant (XDR) Tubercle bacilli (TB). Therefore, the search for new drugs to deal with these resistant forms of TB has gained global attention. Much attention is being paid to the alternate sources of antibacterial agents against these resistant forms. Munumbicin B, an antibiotic from the endophytic bacterium is reported to be effective against MDR-TB. Further clinical trials with this compound may pave the way for an anti-MDR-TB drug.
Malaria (a protozoal infection) is very common in tropical countries. The gold standard anti-malarial agent chloroquin is associated with many fatal side-effects, for instance hemolysis. An agent (bioactive natural product/its derivative) devoid of these side-effects may be an added advantage in the treatment of malaria. Antibiotics like Munumbicin and Kakadumycins obtained from EB are reported to be effective against P. falcipuram, one of the causative organisms of malaria. Fixing these as the lead molecules, the search for anti-plasmodial drug, may yield an agent without the side-effects.
The MRSA and VREF represent the common resistant strains associated with hospital acquired infections. Presently few therapeutic options are available for the management of VREF[59] and MRSA.[60] Two of the antibiotics viz, Munumbicin and Xiamycins obtained from EB are proved to be effective against these resistant forms. Hence, future studies with such antibiotics in these infections may provide wider therapeutic options.
The fascinating fact is that some of these antibiotics (Ecomycins and Pseudomycins) are antifungal, especially against Candida albicans and Cryptococcus neoformans; whereas Xiamycins are anti-HIV in nature. It is well-known that the opportunist fungal infections in HIV patients are visceral candidiasis and cryptococcosis.[61] Thus a combination of Ecomycins and Xiamycins or Pseudomycins and Xiamycins may be additive and effective in HIV patients. However, the limitations of these combinations such as drug interactions and toxicity profile must be stringently monitored for the safety reasons. The elimination of these limitations may lead to invention of a new antiviral formulation. The EB which have multiple roles against many human pathogens may serve either an alternative or complimentary source of novel antibiotics in the future.
The International Union for Conservation of Nature and Natural Resources (IUCN) data suggest that there are 297,326 species of plants (including Monocotyledons, Dicotyledons, Gymnosperms, Ferns and allies, Mosses, Green Algae and Red Algae) [Source: http://www.factmonster.com/ipka/A0934288.html]; and a very little number of species are studied for their endophytes. The EB do exist in virtually every plant on the earth.[33] Roots, nodules, stems, petioles, leaves, and flower tissues from each plant species of Monocotyledons (59,300 species), Dicotyledons (199,350 species), and Gymnosperms (980 species) can be used to isolate EB. Two to more than 16 species of EB can be isolated from different tissues of each plant species collected from relatively small area [our unpublished research data]. A large number of medicinal plants from all the countries are not studied for their endophytes; and we strongly believe that plant species with antimicrobial properties are likely to have endophytes that can produce novel antibiotics.
To sum up, the EB do have a huge potential in bioprospecting; and in the future, these EB are going to serve as one of the potential sources of novel antibiotics. For this reason, the current scenario warrants the expansive research to explore untapped, underutilized and neglected EB. However, an effective and efficient cross-talk amongst chemists, ethnobotanists, microbiologists, molecular biologists, pharmacists, taxonomists and toxicologists is essential in exploring EB for novel antibiotics and other natural products.
Footnotes
Source of Support: Ministry of Agriculture and Agro-Based Industry (MoA), Malaysia (Research grant number: 05-02-16-SF1001)
Conflict of Interest: None declared.
REFERENCES
- 1.Wilson D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos. 1995;73:274–6. [Google Scholar]
- 2.De Bary HA. Leipzig: Verlag von Wilhelm Engelmann; 1866. Hofmeister's handbook of physiological botany. [Google Scholar]
- 3.De Bary HA. Leipzig: Verlag von Wilhelm Engelmann; 1884. Vergleichende morphologie und biologie der pilze mycetozoen und bacterien. [Google Scholar]
- 4.Ting AS, Mah SW, Tee CS. PRevalence of endophytes antagonistic towards Fusarium oxysporum f. sp. cubense race 4 in various plants. Am-Eurasian J Sustain Agric. 2009;3:399–406. Available from: http://www.cabdirect.org/abstracts/20103364656.html;jsessionid=F8685DA7C9CD2A7C61AE772FF6007AA9 . [Google Scholar]
- 5.Germida JJ, Siciliano SD, Freitas JR, Seib AM. Diversity of root-associated bacteria associated with field-grown canola (Brassica napus L.) and wheat (Triticum aestivum L.) FEMS Microbiol Ecol. 1998;26:43–50. [Google Scholar]
- 6.Kobayashy DY, Palumbo JD. Bacterial endophytes and their effects on plants and uses in agriculture. In: Bacon CW, White JF, editors. Microbial endophytes. New York: Marcel Dekker Inc; 2000. pp. 199–233. [Google Scholar]
- 7.Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43:895–914. [Google Scholar]
- 8.Jacobs MJ, Bugbee WM, Gabrielson DA. Enumeration, location, and characterization of endophytic bacteria within sugar beet roots. Can J Bot. 1985;63:1262–5. [Google Scholar]
- 9.Patriquin DG, Dobereiner J. Light microscopy observations of tetrazolium-reducing bacteria in the endorhizosphere of maize and other grasses in Brazil. Can J Microbiol. 1978;24:734–42. doi: 10.1139/m78-122. [DOI] [PubMed] [Google Scholar]
- 10.Bell CR, Dickie GA, Harvey WL, Chan JW. Endophytic bacteria in grapevine. Can J Microbiol. 1995;41:46–53. [Google Scholar]
- 11.Findlay JA, Bethelezi S, Li G, Sevek M. Insect toxins from an endophyte fungus from wintergreen. J Nat Prod. 1997;60:1214–5. [Google Scholar]
- 12.Walsh TA. Inhibitors of β-glucan synthesis. In: Sutcliffe JA, Georgopapadakou NH, editors. Emerging targets in antibacterial and antifungal chemotherapy. London: Chapman and Hall; 1992. pp. 349–73. [Google Scholar]
- 13.Zou WX, Meng JC, Lu H, Chen GX, Shi GX, Zhang TY, et al. Metabolites of Colletotrichumgloeosporioides, an endophytic fungus in Artemisia mongolica. J Nat Prod. 2000;63:1529–30. doi: 10.1021/np000204t. [DOI] [PubMed] [Google Scholar]
- 14.Guo B, Dai JR, Ng S, Huang Y, Leong C, Ong W, et al. Cytonic acids A and B: Novel tridepside inhibitors of hCMV protease from the endophytic fungus Cytonaema species. J Nat Prod. 2000;63:602–4. doi: 10.1021/np990467r. [DOI] [PubMed] [Google Scholar]
- 15.Suffness M. Florida: CRC Press; 1995. Taxol, science and applications. [Google Scholar]
- 16.Harper JK, Arif AM, Ford JE, Strobel GA, Porco J A, Jr, Tomer PD, et al. A 1, 3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron. 2003;59:2471–6. [Google Scholar]
- 17.Strobel G, Ford E, Worapong J, Harper JK, Arif AM, Grant DM, et al. Ispoestacin, An isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry. 2002;60:179–83. doi: 10.1016/s0031-9422(02)00062-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang BG, Salituro D, Szalkowski Z, Li Z, Zhang Y, Royo I, et al. Discovery of small molecule insulin mimetic with antidiabetic activity in mice. Science. 1999;284:974–81. doi: 10.1126/science.284.5416.974. [DOI] [PubMed] [Google Scholar]
- 19.Borel JF, Kis ZL. The discovery and development of cyclosporine. Transplant Proc. 1991;23:1867–74. [PubMed] [Google Scholar]
- 20.Baldani JI, Baldani VL, Goi S, Dobereiner J. Recent advances in BNF with non-legume plants. Soil Biol Biochem. 1997;29:911–22. [Google Scholar]
- 21.Stoltzfus JR, de Bruijin FJ. Evaluating diazotrophy, diversity, and endophytic colonization ability of bacteria isolated from surface-sterilized rice. In: Ladha JK, Reddy PM, editors. The quest for nitrogen fixation in rice. Los Baños, Philippines: IRRI; 2000. pp. 63–91. [Google Scholar]
- 22.Sturz AV, Christie BR. Endophytic bacteria of red clover as agents of allelopathic clover-maize syndromes. Soil Biol Biochem. 1996;28:583–8. [Google Scholar]
- 23.Sturz AV, Christie BR, Nowak J. Bacterial endophytes: Potential role in developing sustainable systems of crop production. CRC Crit Rev Plant Sci. 2000;19:1–30. [Google Scholar]
- 24.Bhore SJ, Sathisha G. Screening of endophytic colonizing bacteria for cytokinin-like compounds: Crude cell-free broth of endophytic colonizing bacteria is unsuitable in cucumber cotyledon bioassay. World J Agric Sci. 2010;6:345–52. [Google Scholar]
- 25.Kado CI. Plant pathogenic bacteria. In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer K, editors. The prokaryotes. New York: Springer-Verlag; 1992. pp. 659–74. [Google Scholar]
- 26.Lodewyckx C, Vangronsveld J, Porteous F, Moore ERB, Taghavi S, Mezgeay M, et al. Endophytic bacteria and their potential applications. CRC Crit Rev Plant Sci. 2002;21:583–606. [Google Scholar]
- 27.Bhore SJ, Nithya R, Loh CY. Screening of endophytic bacteria isolated from leaves of SambungNyawa [Gynura procumbens (Lour.) Merr.] forcytokinin-like compounds. Bioinformation. 2010;5:191–7. doi: 10.6026/97320630005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhore SJ, Tiong OK. Bacterial endophytes of therapeutically important strobilanthescrispa (L.) Bremek and Vernonia amygdalina Del. JPBMS. 2012;14:1–3. Available from: http://www.jpbms.info . [Google Scholar]
- 29.Bhore SJ, Tan YY, Komathi V, Weber JF. Bacterial endophytes in purple coraltree (Erythrinafusca Lour.) and their screening for cytokinins. JPBMS. 2012;14:1–4. Available from: http://www.jpbms.info . [Google Scholar]
- 30.Feng Y, Shen D, Song W. Rice endophyte Pantoeaagglomerans YS19 promotes host plant growth and affects allocations of host photosynthates. J Appl Microbiol. 2006;100:938–45. doi: 10.1111/j.1365-2672.2006.02843.x. [DOI] [PubMed] [Google Scholar]
- 31.Long HH, Schmidt DD, Baldwin IT. Native bacterial endophytes promote host growth in a species-specific manner: Phytohormone manipulations do not result in common growth responses. PLoS One. 2008;3:e2702. doi: 10.1371/journal.pone.0002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudeja SS, Giri R, Saini R, Suneja-Madan P, Kothe E. Interaction of endophytic microbes with legumes. J Basic Microbiol. 2012;52:248–60. doi: 10.1002/jobm.201100063. [DOI] [PubMed] [Google Scholar]
- 33.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson CB, Pasternak JJ, Glick BR. Partial purification and characterization of 1-amino-cyclopropane-1-carboxylate deaminase from the plant growth promoting rhizobacterium Pseudomonas putida GR 12-2. Can J Microbiol. 1994;40:1019–25. [Google Scholar]
- 35.Frommel MI, Nowak J, Lazarovits G. Growth enhancement and developmental modifications of in vitro grown potato (Solanum tuberosum sp. tuberosum) as affected by a nonfluorescent Pseudomonas sp. Plant Physiol. 1991;96:928–36. doi: 10.1104/pp.96.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glick BR, Penrose DM, Li J. A model for the lowering of plant ethylene concentrations by plant growth promoting bacteria. J Theor Biol. 1998;190:63–8. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- 37.Bangera MG, Thomashow LS. Characterization of a genomic locus required for synthesis of the antibiotic 2, 4-diacetylphloroglucinol by the biological control agent Pseudomonas fluorescens Q2-87. Mol Plant Microbe Interact. 1996;9:83–90. doi: 10.1094/mpmi-9-0083. [DOI] [PubMed] [Google Scholar]
- 38.O’sullivan DJ, O’ Gara F. Traits of fluorescent Pseudomonas sp. involved in suppression of plant root pathogens. Microbiol Rev. 1992;56:662–76. doi: 10.1128/mr.56.4.662-676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker D, Mocek U, Garr C. Natural products vs. combinatorials: A case study. In: Wrigley SA, Hayes MA, Thomas R, Chrystal EJT, Nicholson N, editors. New leads for pharmaceutical and agrochemical industries. Cambridge, United Kingdom: The royal society of chemistry; 2000. pp. 66–72. [Google Scholar]
- 40.Wani MC, Taylor HL, Wall ME, Goggon P, McPhail AT. Plant antitumor agents VI. The isolation and structure of Taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–7. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 41.Strobel GA, Yang X, Sears J, Kramer R, Sidhu RS, Hess WM. Taxol from Pestalotiopsismicrospora, an endophytic fungus of Taxus wallichiana. Microbiology. 1996;142:435–440. doi: 10.1099/13500872-142-2-435. [DOI] [PubMed] [Google Scholar]
- 42.Strobel GA, Stierle A, Stierle D, Hess WM. Taxomyces andreanae a proposed new taxon for a bulbilliferous hyphomycete associated with Pacific yew. Mycotaxon. 1993;47:71–8. Available from: http://www.cabdirect.org/abstracts/19932339243.html . [Google Scholar]
- 43.Toofanee SB, Dulymamode R. Fungal endophytes associated with Cordemoyaintegrifolia. Fungal Divers. 2002;11:169–75. [Google Scholar]
- 44.Miller CM, Miller RV, Garton-Kenny D, Redgrave B, Sears J, Condron MM, et al. Ecomycins, unique antimycotics from Pseudomonas viridiflava. J Appl Microbiol. 1998;84:937–44. doi: 10.1046/j.1365-2672.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- 45.Ballio A, Bossa F, Collina A, Gallo M, Iacobellis NS, Paci M, et al. Structure of syringotoxin, a bioactive metabolite of Pseudomonas syringae pv. syringae. FEBS Lett. 1990;269:377–80. doi: 10.1016/0014-5793(90)81197-v. [DOI] [PubMed] [Google Scholar]
- 46.Segre A, Bachmann RC, Ballio A, Bossa F, Grgurina I, Iacobellis NS, et al. The structure of syringomycins A1, E and G. FEBS Lett. 1989;255:27–31. doi: 10.1016/0014-5793(89)81054-3. [DOI] [PubMed] [Google Scholar]
- 47.Isogai A, Fukuchi N, Yamashita S, Suyama K, Suzuki A. Structures of syringostatins A and B, novel phytotoxins produced by Pseudomonas syringae pv. Syringae from lilac blights. Tetrahedron Lett. 1990;31:695–8. [Google Scholar]
- 48.Harrison LH, Teplow DB, Rinaldi M, Strobel G. Pseudomycins, a family of novel peptides from Pseudomonas syringae possessing broad-spectrum antifungal activity. J Gen Microbiol. 1991;137:2857–65. doi: 10.1099/00221287-137-12-2857. [DOI] [PubMed] [Google Scholar]
- 49.Castillo UF, Strobel GA, Ford EJ, Hess WM, Porter H, Jensen JB, et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedianigriscans. Microbiology. 2002;148:2675–85. doi: 10.1099/00221287-148-9-2675. [DOI] [PubMed] [Google Scholar]
- 50.Obianime AW, Aprioku JS. Comparative study of artesunate, ACTs and their combinants on the hormonal parameters of the male Guinea-pig. Niger J Physiol Sci. 2009;24:101–6. [PubMed] [Google Scholar]
- 51.Castillo UF, Strobel GA, Mullenberg K, Condron MM, Teplow DB, Folgiano V, et al. Munumbicins E-4 and E-5: Novel broad-spectrumantibiotics from Streptomyces NRRL3052. FEMS Microbiol Lett. 2006;255:296–300. doi: 10.1111/j.1574-6968.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 52.Castillo U, Harper JK, Strobel GA, Sears J, Alesi K, Ford E, et al. Kakadumycins, novel antibiotics from Streptomyces sp. NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol Lett. 2003;224:183–90. doi: 10.1016/S0378-1097(03)00426-9. [DOI] [PubMed] [Google Scholar]
- 53.Katagiri K, Yoshida T, Sato K. Quinoxaline antibiotics. In: Corcoran JW, Hahn FE, editors. Antibiotics Mechanism of Action of Antimicrobial and Antitumour Agents. Heidelberg: Springer Verlag; 1975. pp. 234–51. [Google Scholar]
- 54.Waring MJ, Wakelin LP. Echinomycin: Abifunctional intercalating antibiotic. Nature. 1974;252:653–7. doi: 10.1038/252653a0. [DOI] [PubMed] [Google Scholar]
- 55.Ding L, Münch J, Goerls H, Maier A, Fiebig HH, Lin WH, et al. Xiamycin, a pentacyclicindolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg Med Chem Lett. 2010;20:6685–7. doi: 10.1016/j.bmcl.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Ding L, Armin M, Heinz-Herbert F, Wen-Han L, Christian H. A family of multi-cyclic indolosesquiterpenes from a bacterial endophyte. Org Biomol Chem. 2011;9:4029–31. doi: 10.1039/c1ob05283g. [DOI] [PubMed] [Google Scholar]
- 57.Jalgaonwala RE, Mohite BV, Mahajan RT. Evaluation of endophytes for their antimicrobial activity from indigenous medicinal plants belonging to north Maharashtra region India. IJPBR. 2010;5:136–41. Available from: http://www.kejapub.com/ijpbr . [Google Scholar]
- 58.Strobel G. Harnessing endophytes for industrial microbiology. Curr Opin Microbiol. 2006;9:240–4. doi: 10.1016/j.mib.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Kauffman CA. Therapeutic and PReventative options for the management of vancomycin- resistant enterococcal infections. J Antimicrob Chemother. 2003;51:23–30. doi: 10.1093/jac/dkg273. [DOI] [PubMed] [Google Scholar]
- 60.Dancer SJ, Robb A, Crawford A, Morrison D. Oral streptogramins in the management of patients with methicillin-resistant Staphylococcus aureus (MRSA) infections. J Antimicrob Chemother. 2003;51:731–5. doi: 10.1093/jac/dkg143. [DOI] [PubMed] [Google Scholar]
- 61.Marques SA, Robles AM, Tortorano AM, Tuculet MA, Negroni R, Mendes RP. Mycoses associated with AIDS in the Third World. Med Mycol. 2000;38:269–79. [PubMed] [Google Scholar]


