Abstract
The wide diversity of plant secondary metabolites is largely used for the production of various pharmaceutical compounds. In vitro cell tissue or organ culture has been employed as a possible alternative to produce such industrial compounds. Tissue culture techniques provide continuous, reliable, and renewable source of valuable plant pharmaceuticals and might be used for the large-scale culture of the plant cells from which these secondary metabolites can be extracted. Alkaloids are one of the most important secondary metabolites known to play a vital role in various pharmaceutical applications leading to an increased commercial importance in recent years. The tissue culture techniques may be utilized to improve their production of alkaloids via somaclonal variations and genetic transformations. The focus of this review is toward the application of different tissue culture methods/techniques employed for the in vitro production of alkaloids with a systematic approach to improve their production.
Keywords: Alkaloids, in vitro cultures, plant tissue culture, secondary metabolites
INTRODUCTION
Plants have been an important source of medicines/life-saving drugs since thousands of years for the majority of the world's population.[1] They have been used as medicinal products in two ways: (1) As traditional medicines or in formulations and (2) they are prepared and dispensed by traditional medical practitioners.[2] Approximately, one quarter of prescribed drugs contains plant extracts or active ingredients obtained from or modeled on plant substances; for example, most popular analgesic aspirin was originally derived from species of Salix and Spiraea and some of the most valuable anti-cancer agents such as paclitaxel and vinblastine are derived solely from plant sources.[3]
As the demand for medicinal plants is growing at a very fast pace, consequently some of them are increasingly being threatened even in their natural habitats.[4] Therefore, in search for alternatives to production of desirable medicinal compounds from plants, cell culture technologies were introduced as a possible tool for studying and producing plant secondary metabolites[5] as in vitro regeneration holds tremendous potential for the production of high-quality plant-based medicines.
An alkaloid-producing plant cultured in vitro retained the capacity to synthesize alkaloids identical to that of an intact plant.[6] Callus culture facilitated the optimization of alkaloid production, whereas media composition was effective for the callus induction so as to enhance the alkaloid production and conservation of threatened genotype.[7]
Factors and approaches affecting the production of alkaloids using plant tissue culture
Number of factors [Figure 1] and approaches can affect the production of plant-specific bioactive metabolites resulting in accumulation of significant amounts of alkaloids in cultured cells resulting in maximization of the production with high yields suitable for commercial exploitation. The efforts have been focused on isolating the biosynthetic activities of cultured cells achieved by various techniques as cited below.
Figure 1.
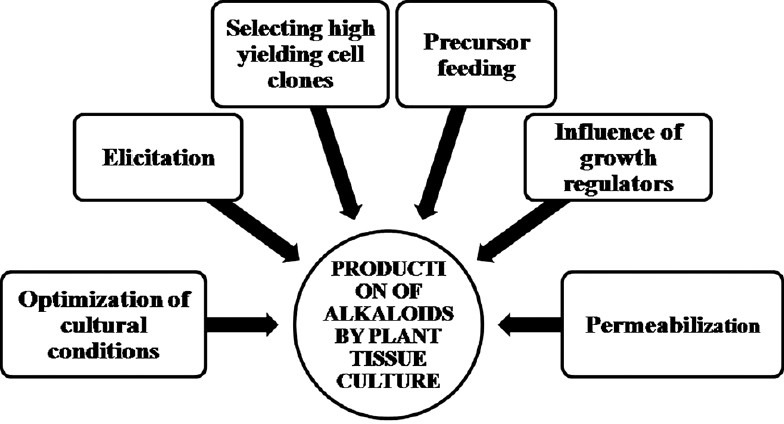
Factors affecting in vitro production of alkaloids
Optimization of cultural conditions
Manipulation of physical aspects and nutritional elements in a culture is the most fundamental approach for optimization of culture productivity.
Biosynthesis of ergot alkaloids (EA) such as clavine ergot alkaloids and quinoline alkaloids (QA) such as quinocitrinines got enhanced in the medium enriched with various carbon and nitrogen sources in the presence of iron, copper, and zinc, whereas supplementation of mannitol and sucrose increased the biosynthesis of EA and QA, respectively.[8] Optimization of nitrate, ammonium, phosphate ions, and sucrose concentration increased the production of galanthamine in Leucojum aestivum shoot culture.[9]
Selection of high metabolite producing strains
Cell cloning methods provide a promising way for selecting cell lines and yielding the increased levels of products; for example, in cell aggregates of Coptis japonica, selection of cell lines increased the growth to about 6-fold in 3 weeks and the highest amount of alkaloid produced was 1.2 g/L of the medium as well as the strain was found to be very stable, producing a high level of berberine even after 27 generations.[10]
Precursor feeding
Attempts to induce or increase the yield of the final product by supplying precursors or intermediate compounds are found to be effective in many cases. The amino acids added to cell suspension culture enhanced the production of tropane and indole alkaloids[11] as well as supplementation of precursors, organic compounds and multiple feedings of loganin increased the biosynthesis followed by production of indole alkaloids in transformed roots of Catharanthus roseus.[12]
Influence of growth regulators
The effects of auxins and cytokinins on shoot multiplication in various medicinal plants are reported; for example, benzylaminopurine (BA) at higher concentration (1-5 ppm) stimulated the development of the axillary meristems and shoot tips in Atropa belladonna.[13] The content of vasicine was found to increase in the leaf and petiolar callus cultures of Adhatoda zeylanicum employing Murashige and Skoog (MS) medium supplemented with 10.7 μM of Naphthalene acetic acid (NAA), 4.4 μM [BA] and 2.3 μM kinetin [Kin], NAA, 4.5 μM 2,4 Di-chlorophenoxy acetic acid [2,4-D], respectively.[14]
Elicitation
Elicitors are signals triggering the formation of secondary metabolites and classified as biotic and abiotic, both of them being used to stimulate the formation of secondary metabolites, thereby reducing the time to attain their high concentrations;[15] for example, incorporation of yeast extract and salicylic acid in the nutrient medium resulted in the elicitation of hyoscyamine and scopolamine content in the in vitro root cultures of Datura metel to the levels comparable to the intact parent plant.[16] The production of indole alkaloids enhanced in catharanthus cultures by employing amino acid, casein hydrolysate supplementations along with irradiation treatments.[17] The rise in KNO3 concentration up to 35 mM increased the tropane alkaloidal content up to 3-20 times with improved ratio of hyoscyamine/scopolamine.[18] In vitro grown tissues (non-regenerative callus, regenerative callus, and microshoot-derived leaves) of Solanum nigrum cultured under salinity stress (0-150 mM) were found to yield maximum of solasodine with 150 mM of sodium chloride.[19]
Permeabilization
Since, the alkaloids may be highly toxic for plant cells and permeabilization might be a factor enhancing the alkaloid production. The permeability effect of tween-80 was studied on the production of tropane alkaloids from transformed roots of Datura innoxia. The permeabilization with tween modulated the tropane alkaloid accumulation by the release of alkaloid into the medium and the restoration of hyoscyamine content.[20] The immobilized callus cultures of Tinospora cordifolia were subjected to cell permeabilization with chitosan that showed a 10-fold increase in production of arabinogalactans as compared to respective controls devoid of resin and chitosan.[21]
Use of cell suspension cultures
Cell suspension culture systems can be utilized for large-scale culturing of plant cells with additional advantages [Figure 2], from which alkaloids could be extracted and thus ultimately providing a continuous and reliable source of bioactive natural products.[5] Screening, selection, and medium optimization of suspended cells resulted into a 20-30-fold increase in alkaloid concentrations in the case of high strain-producing cultures.[22]
Figure 2.
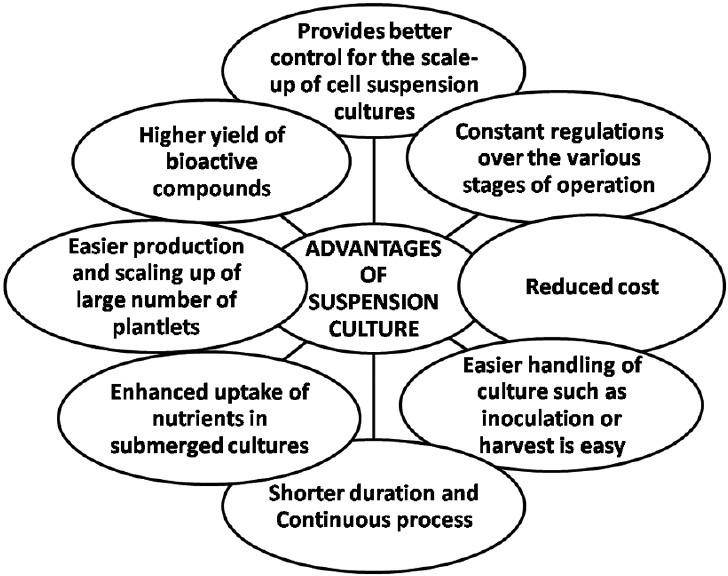
Advantages of cell suspension culture for production of alkaloids
The fast growing suspension cultures of Vernonia cinerea developed at the different combinations of BA and NAA for the production of alkaloids with maximum of biomass in 20 days and alkaloid content up to 1.15 mg/L.[23]
The significant amounts of sanguinarine were produced in cell suspension cultures of Papaver somniferum using bioreactors.[24] A bench top bioreactor allowing continuous extraction of secondary metabolites was designed for Catharanthus roseus and Santalum album plant cell suspensions, yielding various secondary metabolites.[25] Similarly, production of tropane alkaloids, scopolamine and hyoscyamine, has been achieved from the adventitious roots of Scopolia parviflora by employing bubble column bioreactor followed by elicitation using Staphylococcus aureus results in their enhanced production.[26]
In vitro regeneration micropropagation
In vitro propagation of plants holds tremendous potential for the production of high-quality plant-based medicines and the same can be achieved through micropropagation[27] Plant regeneration from shoot, stem, and meristems has yielded encouraging results in Catharanthus roseus, Cinchona ledgeriana, Rehmannia glutinosa, and Rauwolfia serpentina.[28,29] Propagation through axillary shoot cultures of Ranunculus asiaticus L. was practiced using in vitro conditions for the accelerated propagation of selected indexed genotypes to supply more performing plant materials of a superior genotype.[30]
Callus-mediated organogenesis
The induction of callus growth, followed by differentiation and organogenesis is accomplished by the differential application of growth regulators along with the controlled conditions in the culture medium. A rapid in vitro propagation system leading to the formation of shoot from callus, roots, and plantlets was developed from Schizanthus hookeri. Ten alkaloids ranging from simple pyrollidine derivatives to tropane esters derived from angelic acid, tiglic acid, seneciocic acid, and methyl mesaconic acid were obtained from in vitro regenerated plantlets.[31]
Somatic embryogenesis
Efficient development and germination of somatic embryos are pre-requisites for the plantlet production and are achievable on MS medium even without the addition of growth regulators.[32] Techniques for the development of somatic embryogenesis of Veratrum californicum were developed. The in vitro developed plantlets were found to contain steroidal alkaloids, cyclopamine and veratramine, at the various steps of somatic embryogenesis.[33]
Metabolic engineering
Metabolic engineering plays an important role in production of drugs which are difficult to synthesize and are operated at whole cell level; for example, strain development for the production of artemisinin and benzylisoquinoline alkaloids.[34] Tropane alkaloids such as scopolamine and their transformation products have been biosynthesized successfully.[35] Escherichia coli has also been used successfully for the production of l-valine, an important drug precursor.[34]
Genetic transformations
The stable introduction of foreign genetic information into the plants represents one of the significant developments in recent advances of plant biotechnology including high volume production of pharmaceuticals[36] and opened the new avenues for the production of several biologically active natural compounds such as artemisinin, paclitaxel, scopolamine, etc.[37] Derivatives of plant pathogens Agrobacterium tumefaciens and Agrobacterium rhizogenes have been proved to be efficient and highly versatile vehicles for the production of genes into the plant genome resulting in the transfer and integration of genes of the plasmids from the bacteria into the plant DNA, transformed neoplastic tissues, crown galls, and hairy roots.[38]
An efficient protocol has been developed for the root cultures of transgenic opium poppy (Papaver somniferum L.) and California poppy (Eschscholzia californica Cham.) using A. rhizogenes.[39] Similarly, solasodine production was found to be regulated by A. rhizogenes induced transformed roots of Solanum aviculare.[40] In another instance, transformed roots of Artemesia annua yielded a sesquiterpene named artemisinin.[41]
A binary vector system in conjunction with the use of the Ri plasmid had been widely used for the integration of foreign genes into medicinal plants. The shooty teratomas of Catharanthus roseus epicotyls and stem nodal explants exhibited a 10-fold increased production of vincristine as compared to untransformed control cultures.[42]
Biotransformation using immobilized cell cultures
Immobilization or entrapment of the cells may produce a microenvironment that resembles the organized tissue in the intact cells causing differentiation and production of secondary metabolites.[43] Increased biotransformation yields of capsaicin and dihydrocapsaicin, major pungent principles of chilli pepper were obtained when immobilized placental tissues of Capsicum frutescens were fed with intermediate metabolites of the capsaicinoid pathway.[44]
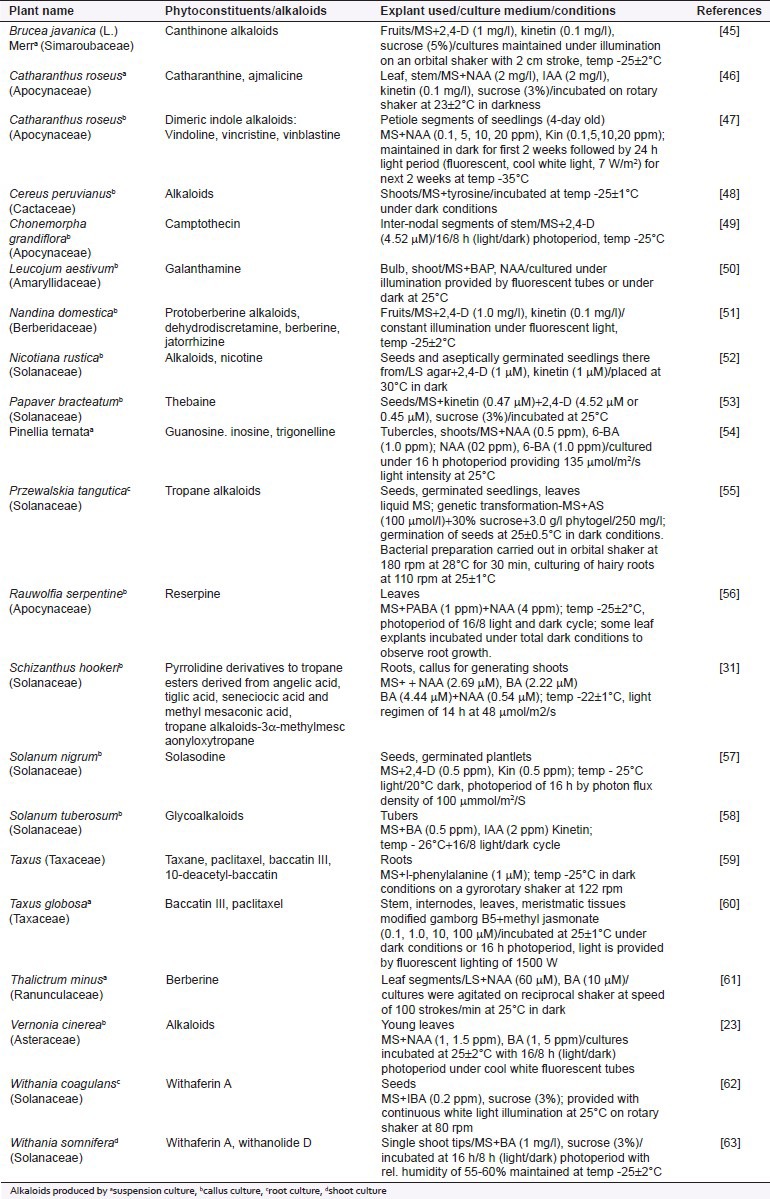
Some of the examples for the in vitro production of different nucleus of alkaloids by adopting the various tissue culture techniques have been cited in Table 1.
Table 1.
Alkaloids produced by in vitro culture techniques
Challenges faced by tissue culture technique
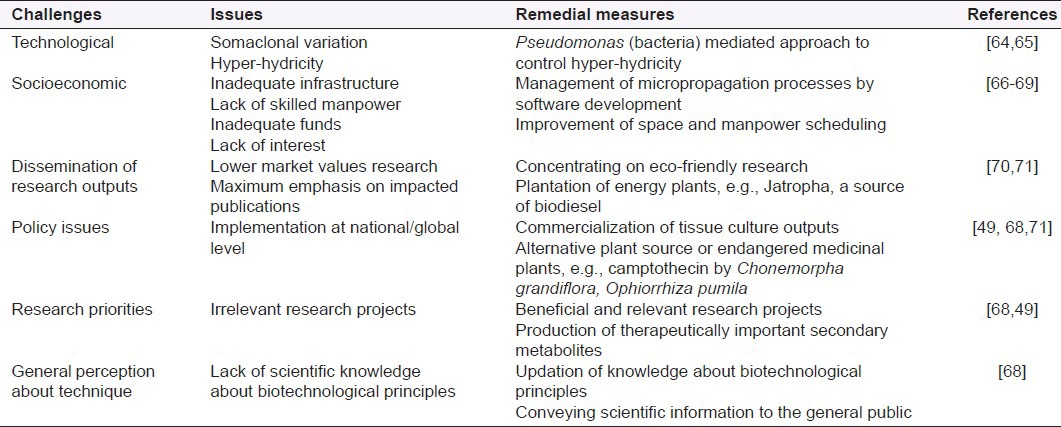
Challenges related to technological factors, socioeconomic factors, public perception of biotechnology, policy issues, and aspects related to research priorities and dissemination of research outputs are among the bottle necks affecting the productivity of plant tissue culture techniques and they have been summarized in Table 2.
Table 2.
Challenges faced by plant tissue culture techniques
FUTURE PROSPECTS
Plant tissue culture has remained an art because of the unique culture conditions required for each medicinal plant. To accommodate a genotype or species that has not been manipulated in culture PReviously, one must adapt an established protocol or create a new one bearing in mind about the efficiency imperatives. Plant tissue culture provides valuable tool for synthesizing same range of chemicals as that of natural plant as well as novel compounds are also synthesized via biotransformations.[68] The ability of A. rhizogenes to induce hairy roots in a range of medicinal plants has been exploited as a source of root-derived pharmaceutical compounds.[72] Over the past two decades, the concept of plant-based production of high volume-based pharmaceutical proteins such as vaccines and antibodies has received growing research interest and offered critical advantages over traditional bacterial and mammalian cell-based systems.[73] The production of recombinant proteins using either whole plant[74] or in vitro plant cell cultures commonly known as molecular framing is emerging as promising prospects for the pharmaceutical industry. Similarly, metabolomics and dereplication of medicinal plants and their cultures may lead to development of new drug molecules from tissue culture techniques since several times it produces novel molecules unrelated to its parent plant.[75]
CONCLUSION
This review gives an insight to the different aspects of tissue culture for the production of alkaloids under in vitro conditions. The review gives an insight to the various factors that may affect their production along with the suitable examples. An update has been provided to the different approaches utilized for increasing the production of alkaloids. Different challenges posed to tissue culture techniques at various stages were discussed along with their respective remedial measures. At the end, future perspective has been framed in lieu of their advanced applications to utilize them at industrial and commercial scales.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tripathi L, Tripathi JN. Role of biotechnology in medicinal plants. Trop J Pharm Res. 2003;2:243–53. [Google Scholar]
- 2.Jha S, Badypadhyay M, Chaudhuri KN, Ghosh S, Ghosh B. Biotechnological approaches for the production of forskolin, withanolides, colchicine and tylophorine. Plant Genet Resour. 2005;3:101–15. [Google Scholar]
- 3.Roberts MF. Medicinal products through plant biotechnology. In: Robins RJ, Rhodes JC, editors. Manipulating secondary metabolism in culture. Cambridge: University Press; 1988. pp. 201–6. [Google Scholar]
- 4.Muthukumar B, Arochiasamy DI, Natarajan E. Direct organogenesis in Datura metel L. from in vitro and in vivo nodal explants. Ind J Biotechnol. 2004;3:449–51. [Google Scholar]
- 5.Vanisree M, Lee CY, Lo SF, Nalawade SM, Lin CY, Tsay HS. Studies on the production of some important secondary metabolites from medicinal plants by tissue culture. Bot Bull Acad Sin. 2004;45:1–22. [Google Scholar]
- 6.Yoshimatsu K, Shimomura K. Efficient shoot formation on internodal segments and alkaloid formation in the regenerates of Cephalis ipecacuanha. Plant Cell Rep. 1991;9:567–70. doi: 10.1007/BF00232333. [DOI] [PubMed] [Google Scholar]
- 7.Salma U, Rahman MS, Islam S, Haque N, Jubair TA, Haque AK, et al. The influence of different hormone concentration and combination on callus induction and regeneration of Rauwolfia serpentina L. Benth. Pak J Biol Sci. 2008;11:1638–41. doi: 10.3923/pjbs.2008.1638.1641. [DOI] [PubMed] [Google Scholar]
- 8.Koslovskiĭ AG, Zhelifonova VP, Antipova TV, Zelenkova NF. The influence of medium composition on alkaloid biosynthesis by Penicillium citrinum. Prikl Biokhim Mikrobiol. 2010;46:572–6. [PubMed] [Google Scholar]
- 9.Georgiev V, Berkov S, Georgiev M, Burrus M, Codina C, Bastida J, et al. Optimized nutrient medium for galanthamine production in Leucojum aestivum L. in vitro shoot system. Z Naturforsch C. 2009;64:219–24. doi: 10.1515/znc-2009-3-412. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y, Sato F. Production of berberine in the cultured cells of Coptis japonica. Phytochemistry. 1981;20:545–47. [Google Scholar]
- 11.Ellis BE, Towers GH. Biogenesis of rosmarinic acid in Mentha. Biochem J. 1970;118:291–7. doi: 10.1042/bj1180291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaviraj EN, Veerasham C. Effect of precursors and organic compounds on alkaloid production in transformed root cultures of Catharanthus roseus var. nirmal. Pharmaceut Biol. 2006;44:371–7. [Google Scholar]
- 13.Benjamin BD, Roja P, Heble MR, Chadha M. Multiple shoot cultures of Atropa belladonna effect of physicochemical factors on growth and alkaloid formation. J Plant Nutr. 1987;129:129–35. [Google Scholar]
- 14.Jayapaul K, Kavi Kishor PB, Reddy KJ. Production of pyrroquinzoline alkaloid from leaf and petiole derived callus cultures of Adhatoda zeylanica. In Vitro Cell Dev Biol Plant. 2005;41:682–5. [Google Scholar]
- 15.Eilert U. Elicitation: Methodology and aspects of application. In: Constable F, Vasil I, editors. Cell Culture and Somatic Cell Genetics of Plants. San Diego: Academic Press; 1987. pp. 153–96. [Google Scholar]
- 16.Ajungla L, Patil PP, Barmukh RB, Nikam TD. Influence of biotic and abiotic elicitors on accumulation of hyoscyamine and scopolamine in root cultures of Datura metel L. Indian J Biotechnol. 2009;8:317–22. [Google Scholar]
- 17.Ahmed FA, Abdel-Fateh OM, Kobeasy MT, Ahmed OK. Factors affecting growth and indole alkaloid content of catharanthus calli (Catharanthus roseus L.) amino acids, casein hydrolysate and irradiation. Arab J Biotechnol. 2000;3:61–70. [Google Scholar]
- 18.Chashmi NA, Sharifi M, Karimi F, Rahnama H. Differential production of tropane alkaloids in hairy roots and in vitro cultured two accessions of Atropa belladonna L. under nitrate treatments. Z Naturforsch C. 2010;65:373–9. doi: 10.1515/znc-2010-5-609. [DOI] [PubMed] [Google Scholar]
- 19.Bhat MA, Ahmad S, Aslam J, Mujib A, Mahmooduzzfar Salinity stress enhances production of solasodine in Solanum nigrum L. Chem Pharm Bull (Tokyo) 2008;56:17–21. doi: 10.1248/cpb.56.17. [DOI] [PubMed] [Google Scholar]
- 20.Boitel Conti M, Laberche JC, Lanoue A, Ducrocq C, Sangwan NB. Influence of feeding precursors on tropane alkaloid production during an abiotic stress in Datura innoxia transformed roots. Plant Cell Tissue Organ Cult. 2000;60:131–7. [Google Scholar]
- 21.Roja G, Bhangale AS, Juvekar AR, Eapen S, D’souza SF. Enhanced production of the polysaccharide arabinogalactan using immobilized cultures of Tinospora cordifolia by elicitation and in situ adsorption. Biotechnol Prog. 2005;21:1688–91. doi: 10.1021/bp050188w. [DOI] [PubMed] [Google Scholar]
- 22.Zhong JJ. Biochemical engineering of the production of plant-specific secondary metabolites by cell suspension cultures. Adv Biochem Eng Biotechnol. 2001;72:1–26. doi: 10.1007/3-540-45302-4_1. [DOI] [PubMed] [Google Scholar]
- 23.Maheshwari P, Songara B, Kumar S, Jain P, Srivastava K, Kumar A. Alkaloid production in Vernonia cinerea: Callus, cell suspension and root cultures. Biotechnol J. 2007;2:1026–32. doi: 10.1002/biot.200700033. [DOI] [PubMed] [Google Scholar]
- 24.Park JM, Yoon SY. Production of sanguinarine by suspension cultures of Papaver somniferum in bioreactors. J Ferm Bioeng. 1992;74:292–6. [Google Scholar]
- 25.Valluri JV. Bioreactor production of secondary metabolites from cell cultures of periwinkle and sandal wood. Methods Mol Biol. 2009;547:325–35. doi: 10.1007/978-1-60327-287-2_26. [DOI] [PubMed] [Google Scholar]
- 26.Min JY, Jung HY, Kang SM, Kim YD, Kang YM, Park DJ, et al. Production of tropane alkaloids by small-scale bubble column bioreactor cultures of Scopolia parviflora adventitious roots. Bioresour Technol. 2007;98:1748–53. doi: 10.1016/j.biortech.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Murch SJ, Krishna Raj S, Saxena PK. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in-vitro regenerated St. John's wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000;19:698–704. doi: 10.1007/s002990000206. [DOI] [PubMed] [Google Scholar]
- 28.Paek KY, Yu KJ, Park SI, Sung NS, Park CH. Micropropagation of Rehmannia glutinosa as medicinal plant by shoot tip and root segment culture. Acta Hortic. 1995;390:113–20. [Google Scholar]
- 29.Roy SK, Hossain MZ, Islam MS. Mass propagation of Rauvolfia serpentina by in vitro shoot tip culture. Plant Tissue Cult. 1994;4:69–75. [Google Scholar]
- 30.Beruto M. In vitro propagation through axillary shoot culture of Ranunculus asiaticus L. Methods Mol Biol. 2010;589:29–37. doi: 10.1007/978-1-60327-114-1_4. [DOI] [PubMed] [Google Scholar]
- 31.Jordan M, Humam M, Bieri S, Christen P, Poblete E, Muñoz O. In vitro shoot and root organogenesis, plant regeneration and production of tropane alkaloids in some species of Schizanthus. Phytochemistry. 2006;67:570–8. doi: 10.1016/j.phytochem.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Ma H, Guo F, Luo X. Effects of thidiazuron on somatic embryogenesis of Coptis japonica. Plant Cell Tissue Organ Cult. 1994;36:73–9. [Google Scholar]
- 33.Ma R, Ritala A, Oksman-Caldentey KM, Rischer H. Development of in vitro techniques for the important medicinal plant Veratrum californicum. Planta Med. 2006;72:1142–8. doi: 10.1055/s-2006-946697. [DOI] [PubMed] [Google Scholar]
- 34.Lee SY, Kim HU, Park JH, Park JM, Kim TY. Metabolic engineering of microorganisms: General strategies and drug production. Drug Discov Today. 2009;14:78–88. doi: 10.1016/j.drudis.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Palazón J, Navarro-Ocaña A, Hernandez-Vazquez L, Mirjalili MH. Application of metabolic engineering to the production of scopolamine. Molecules. 2008;13:1722–42. doi: 10.3390/molecules13081722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen G, Wright MS. Recent advances in the transformation of plants. Trends Plant Sci. 1999;4:226–31. doi: 10.1016/s1360-1385(99)01412-0. [DOI] [PubMed] [Google Scholar]
- 37.Sheludko YV. Recent advances in plant biotechnology and genetic engineering for production of secondary metabolites. Tsitol Genet. 2010;44:65–75. [PubMed] [Google Scholar]
- 38.Saito K. Genetic engineering in tissue culture of medicinal plants. Plant Tissue Cult Lett. 1993;10:1–8. [Google Scholar]
- 39.Park SU, Facchini PJ. Agrobacterium rhizogenes-mediated transformation of opium poppy, Papaver somniferum L., and California poppy, Eschscholzia californica cham., root cultures. J Exp Bot. 2000;51:1005–16. doi: 10.1093/jexbot/51.347.1005. [DOI] [PubMed] [Google Scholar]
- 40.Argôlo AC, Charlwood BV, Pletsch M. The regulation of solasodine production by Agrobacterium rhizogenes-transformed roots of Solanum aviculare. Planta Med. 2000;66:448–51. doi: 10.1055/s-2000-8580. [DOI] [PubMed] [Google Scholar]
- 41.Souret FF, Weathers PJ, Wobbe KK. The mevalonate-independent pathway is expressed in transformed roots of Artemisia annua and regulated by light and culture age. In Vitro Cell Dev Biol Plant. 2002;38:581–8. [Google Scholar]
- 42.Begum F, Nageswara Rao SS, Rao K, Prameela Devi Y, Giri A, Giri CC. Increased vincristine production from Agrobacterium tumefaciens C58 induced shooty teratomas of Catharanthus roseus G. Don. Nat Prod Res. 2009;23:973–81. doi: 10.1080/14786410802131153. [DOI] [PubMed] [Google Scholar]
- 43.Williams PD, Mavituna F. Immobilized plant cells. In: Fowler MW, Warren GS, Moo-Young M, editors. Plant Biotechnol. Oxford: Pergamon; 1992. pp. 1–7. [Google Scholar]
- 44.Johnson TS, Ravishankar GA. Precursor transformation in immobilized placental tissues of Capsicum fructescens mill: II. Influence of feeding intermediates of capsacinoid pathway in the combination with L-valine on capsaicin and dihydrocapsaicin accumulation. J Plant Physiol. 1998;153:240–3. [Google Scholar]
- 45.Liu KC, Yang SH, Roberts MF, Philipson JD. Production of canthin-6-one alkaloids by cell suspension cultures of Brucea javanica (L.) Merr. Plant Cell Rep. 1990;9:261–3. doi: 10.1007/BF00232297. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, Zhu W, Hu Q. Enhanced catharanthine production in Catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enzyme Microb Technol. 2001;28:673–81. doi: 10.1016/s0141-0229(01)00306-4. [DOI] [PubMed] [Google Scholar]
- 47.Ataei-Azimi A, Hashemloian BD, Ebrahimzadeh H, Majd A. High in vitro production of anti-canceric indole alkaloids from periwinkle (Catharanthus roseus) tissue culture. Afr J Biotechnol. 2008;7:2834–9. [Google Scholar]
- 48.de Oliveira AJ, Machado MF. Alkaloid production by callus tissue cultures of Cereus peruvianus (Cactaceae) Appl Biochem Biotechnol. 2003;104:149–55. doi: 10.1385/abab:104:2:149. [DOI] [PubMed] [Google Scholar]
- 49.Kulkarni AV, Patwardhan AA, Lele U, Malpathak NP. Production of camptothecin in cultures of Chonemorpha grandiflora. Pharmacognosy Res. 2010;2:296–9. doi: 10.4103/0974-8490.72327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanilova MI, Molle ED, Yanev SG. Galanthamine production by Leucojum aestivum cultures in vitro. Alkaloids Chem Biol. 2010;68:167–270. doi: 10.1016/s1099-4831(10)06805-7. [DOI] [PubMed] [Google Scholar]
- 51.Ikuta A, Itokawa H. Alkaloids of tissue cultures of Nandina domestica. Phytochemistry. 1988;27:2143–5. [Google Scholar]
- 52.Tabata M, Hiraoka N. Variation of alkaloid production in Nicotiana rustica callus cultures. Physiol Plant. 1976;38:19–23. [Google Scholar]
- 53.Day KB, Draper J, Smith H. Plant regeneration and the baine content of plants derived from callus culture of Papaver bracteatum. Plant Cell Rep. 1986;5:471–4. doi: 10.1007/BF00269645. [DOI] [PubMed] [Google Scholar]
- 54.Yong-Hong L, Zong-Suo L, Dong-Feng Y, Wen-Ting L. Suspension culture establishment and alkaloid analysis of tubercules of Pinellia ternate. J Northwest A and F Univ. 2010;37:168–74. [Google Scholar]
- 55.Lan X, Quan H. Hairy root culture of Przewalskia tangutica for the enhanced production of pharmaceuticals tropane alkaloids. Res J Med Plant. 2010;4:1477–81. [Google Scholar]
- 56.Pandey VP, Cherian E, Patani G. Effect of growth regulators and culture conditions on direct root induction of Rauwolfia serpentine L. Benth by leaf explants. Trop J Pharm Res. 2010;9:27–34. [Google Scholar]
- 57.Sutkovic J, Ler D, Ragab M, Gawwad A. In vitro production of solasodine alkaloid in Solanum nigrum under salinity stress. J Phytol. 2011;3:43–9. [Google Scholar]
- 58.Al-Ashaal HA. Regeneration in vitro glycoalkaloids production and evaluation of bioactivity of callus methanolic extract of Solanum tuberosum L. Fitoterapia. 2010;81:600–6. doi: 10.1016/j.fitote.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Syklowska-Baranek K, Pietrosiuk A, Kokoszka A, Furmanowa M. Enhancement of taxane production in the hairy root culture of Taxusxmedia var. Hicksii. J Plant Physiol. 2009;166:1950–4. doi: 10.1016/j.jplph.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Barradas-Dermitz DM, Hayward-Jones PM, Mata-Rosas M, Palmeros-Sánchez B, Platas-Barradas OB, Velásquez-Toledo RF. Taxus globose S. cell lines: Initiation selection and characterization in terms of growth, and of baccatin III and paclitaxel production. Biocell. 2010;34:1–6. [PubMed] [Google Scholar]
- 61.Nakagawa K, Fukui H, Tabata M. Hormonal regulation of berberine production in cell suspension cultures of Thalictrum minus. Plant Cell Rep. 1986;5:69–71. doi: 10.1007/BF00269722. [DOI] [PubMed] [Google Scholar]
- 62.Abouzid SF, Bassuony AA, Nasib A, Khan S, Qureshi J, Choudhary MI. Withaferin A production by root cultures of Withania coagulans. Int J Appl Res Nat Prod. 2010;3:23–7. [Google Scholar]
- 63.Ray S, Jha S. Production of withaferin A in shoot cultures of Withania somnifera. Planta Med. 2001;67:432–6. doi: 10.1055/s-2001-15811. [DOI] [PubMed] [Google Scholar]
- 64.Bairu MW, Aremu AO, Van SJ. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regulat. 2011;63:147–73. [Google Scholar]
- 65.Shetty K, Carpenter TL, Curtis OF, Potter TL. Production of hyperhydricity in tissue cultures of oregano (Origanum vulgare) by extracellular polysaccharide isolated from Pseudomonas species. South Afr J Bot. 1996;120:175–83. [Google Scholar]
- 66.Fennell CW, Van Staden J. Biotechnology of South African bulbs. South Afr J Bot. 2004;70:37–46. [Google Scholar]
- 67.Posuris A. Review of funding environments for biotechnology in South Africa. 2008. [Last accessed on 2011 Jun 21]. Available from: http://www.nacinnovaion.biz/wp-content/uploads/the funding environment of South African biotechnology .
- 68.Moyo M, Bairu MW, Amoo SO, Van Staden J. Plant biotechnology in South Africa: Micropropagation research endeavours, prospects and challenges. South Afr J Bot. 2011;77:996–1011. [Google Scholar]
- 69.Cloete TE, Nel LH, Theron J. Biotechnology in South Africa. Trends Biotechnol. 2006;24:557–62. doi: 10.1016/j.tibtech.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Ceasar SA, Ignacimuthu S. Applications of biotechnology and biochemical engineering for the improvement of Jatropha and Biodiesel: A review. Renew Sustain Energy Rev. 2011;15:1576–85. [Google Scholar]
- 71.Saito K, Sudo H, Yamazaki M, Koseki NM, Kitajima M, Takayama H. Feasible production of camptothecin by hairy root culture of Ophiorrhiza pumila. Plant Cell Rep. 2001;20:267–71. [Google Scholar]
- 72.Shanks JV, Morgan J. Plant ‘hairy root’ culture. Curr Opin Biotechnol. 1999;10:151–5. doi: 10.1016/s0958-1669(99)80026-3. [DOI] [PubMed] [Google Scholar]
- 73.Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnol J. 2010;8:620–37. doi: 10.1111/j.1467-7652.2010.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu J, Ge X, Dolan MC. Towards high-yield production of pharmaceutical proteins with plant suspension cultures. Biotechnol Adv. 2011;29:278–99. doi: 10.1016/j.biotechadv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Bakshi V. In vitro cultures of Morus alba for enhancing production of phytoestrogens. PhD desertation, University of North Texas. 2009. [Last accessed on 2011 June 21]. Available from: http://digital.library.unt.edu/ark:/67531/ metadc12078/m1/1/high_res_d/dissertation.pdf .


