Abstract
Saffron is the most valuable medicinal food product because of its importance in Iran's agricultural economy. The dried stigmas of the plant Crocus sativus (Iridaceae) are processing to produce saffron as a well-known spice which has some other importance in pharmaceutics, cosmetics, perfumery, and textile dye-producing industries. Recently, reports about the pharmacological activity of this plant increase its importance in the world. The world's annual saffron production is estimated around 300 tons per year (Iran produces 76% of total) and also saffron is considered to be the most expensive spice in the world; hence, there are efforts for its artificial production or defraud. Therefore, the quality conservation of saffron needs to certify in the international trade market following international ISO or the Food and Drug Administration (FDA) criteria and standards. In this paper, the recent (or sometimes less documented) reports on phytochemistry, pharmacology, and standard methods for quality evaluation of saffron, as a medicinal food spice, from field cultivation to market are reviewed.
Keywords: Crocus sativus, Iridaceae, phytochemistry, saffron, standardization
INTRODUCTION
Crocus sativus L. is a perennial spicy herb (Iridaceae family) and well known as Red Gold in producer countries. This plant is the most expensive cultivated herb in the world.[1] The origin of the word saffron is the French term Safran, which was derived from the Latin word safranum and comes from the Arabic word as far that means “yellow.”[2,3,4] But this word is different from the ancient Persian word as Karkum which was used by the people living around Zagros Mountains. It has been documented that saffron was used as a food or spicy plant product for culinary purposes in Achamenian Imperial court.[5] The underground parts of the plant, corms or bulbs, can be used to produce new plant as this plant has no seed propagation. The outstanding feature of the colored flowers of saffron is three stigmas (25-30 mm long), drooped over the petals. The flower has also three yellow stamens, which do not contain the active compounds and usually are not collected. Each bulb produces one to seven flowers. It seems that the cultivated species has originated as a natural hybrid so that it has been selected for its long stigmas and maintained ever since. The flower of C. sativa is a light purple, but it is the thread-like reddish-colored stigma of the flower that is valued both as a spice and as a natural colorant. It takes about 36,000 flowers to yield just 1 pound of stigmas.[6,7,8] Over 200,000 dried stigmas (obtained from about 70,000 flowers) yield 500 g of pure saffron (not contaminated with safflower) which cost as much as $30 per ounce in the American market.[9]
Traditional usage
Saffron has been used in folk medicine and Ayurvedic health system as a sedative, expectorant, anti-asthma, emmenagogue, and adaptogenic agent. Saffron was used in various opioid preparations for pain relief (16-19th centuries).[10] The Complete German Commission E Monographs has not approved this plant for use in cramps or asthma.[11]
Pharmacological activities
There are clinical trials conducted evaluating the efficacy of saffron in mild-to-moderate depression. The studies reported that saffron was more effective than placebo and at least equivalent to therapeutic doses of imipramine and fluoxetine. No significant differences were found in adverse effects in any of the studies.[12,13] As a dietary supplement, saffron extracts may PRevent retinal damage in rats and have a role in the treatment of ischemic retinopathy and age-related macular degeneration.[14] Anti-nociceptive and anti-inflammatory activities were reported from stigmas and petals of saffron.[15] Literature review showed that a decrease in cholesterol and triglyceride levels and reduction in vascular damage were observed when hyper-lipidemic rabbits were treated by crocetin. Hypoxia at the vascular wall was also reduced.[16] However, in another study, 400 mg/day (for 7 days) of saffron showed no effect on the lipid profile of the healthy volunteers.[17] In addition, an anti-oxidant effect was observed in human platelets together with the inhibition of lipid peroxidation.[18] There is a report that reviewed the potential role of saffron extracts in cancer therapy.[19] However, saffron appears to be a selective cytotoxic plant but its mechanism is not clearly determined.
Besides the above-mentioned activity, improvement of ethanol-impaired memory of mice, effects on learning behavior and neuronal cell death, and management of psoriasis were reported from C. sativus.[19,20,21] Saffron is generally not toxic when ingested in culinary amounts, but a lethal dose at 20 g and an abortifacient dose at 10 g have been indicated in the literature.[10] Adverse reactions such as rhino-conjunctivitis, bronchial asthma, cutaneous pruritus, and a case report of anaphylaxis have been existed.[10,22]
Phytochemistry
Saffron is a noteworthy medicinal and spicy plant because of its powerful odor and intensive natural yellow color. Phytochemical researches have revealed that the color is mainly due to the degraded carotenoid compounds, crocin (1) and crocetin (2) the flavor comes from the carotenoid oxidation products, mainly safranal (3) and the bitter comes from glucoside picrocrocin (4) Pfander and Schurtenberger (1982) suggested a proposal for biogenesis of the color and odor active compounds which may be derived by bio-oxidative cleavage of zeaxanthin (5)[23] The major principle of C. sativus is safranal, a carboxaldehyde volatile compound (70% of total volatile constituents), which is easily formed by de-glucosylation of picrocrocin.[24] Alongside the above compounds, anthocyanins isolated from the blue flowers of C. sieberi and identified as 3,5-β-diglucosides of delphinidin (6) and petunidin (7) and from C. chrysanthus and identified as 3-β-rutinosides (8,9)[25] Furthermore, anthocyanins [Figure 1] have been isolated from the blue perianth segments of C. antalyensis and identified as delphinidin 3-O-(β-d-glucopyranoside)-5-O-(6-O-malonyl-β-d-glucopyranoside (10) and petudin 3,7-di-O-(β-d-glucopyranoside (11) 3,7-di-O-β-d-glucoside of delphinidin (12) 3,5-di-O-β-d-glucosides of delphinidin (13) and petunidin (14).[26]
Figure 1.
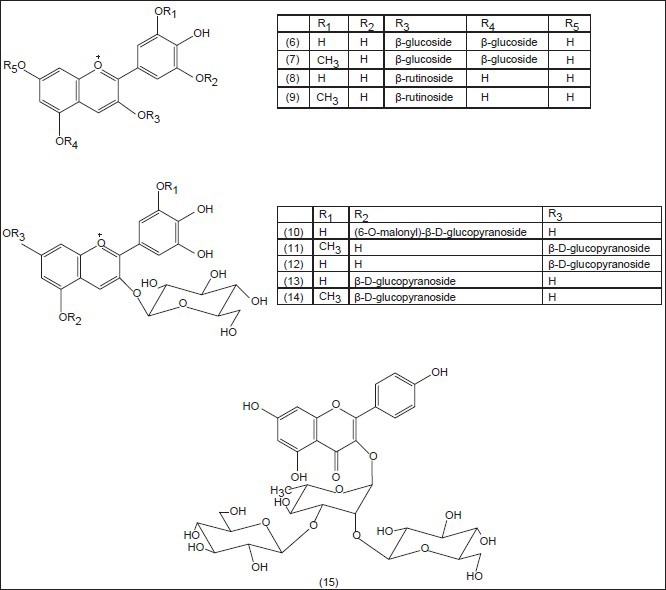
Structures of the isolated anthocyanins from the genus Crocus
Besides anthocyanins, flavonol glycosides have been isolated and identified from the flower extracts of C. speciosus and C. antalyensis. Among them, there was a new flavonol glycoside identified as kaempferol 3-O-alpha-(2,3-di-O-beta-d-glucopyranosyl) rhamnopyranoside (15).[27] Figure 2 shows the main chemical structures of the isolated compounds from the genus Crocus till now.
Figure 2.
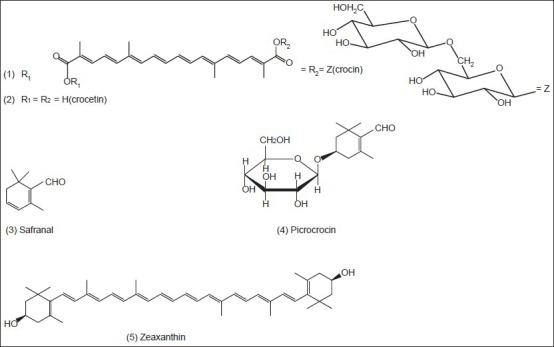
The main chemical structures of the isolated compounds from Crocus sativus
Natural habitat and the land under cultivation
C. sativus is the most valuable medicinal food plant, as the source of saffron, which has a great exporting importance in Iran (South Khorasan) and India's (Jammu and Kashmir) agricultural economies. Iran comprises a land area of 1.64 million km2 and lies in the northern of the temperate zone;[28] only 12% of the total land area is under cultivation. The North-East Khorasan Razavi province exported 57 tons of saffron in 2010. In addition to Iran, as one of the most important area for cultivation, West of Asia and Mediterranean countries (from 10°W to 80°E and 30°N to 50°S), which have cold winter and warm summer especially with less humidity, are the appropriate areas for cultivation of saffron. Spain, Italy, Greek, India, Morocco, and Azerbaijan are the best examples. In Iran, Khorasan Province is the biggest producer followed by Fars Province and the land under cultivation is estimated more than 47,000 Hectares. So far, the production of saffron has been increased from 17 tons in 1973 to 138 tons in 2001.[29] The price of this expensive medicinal spicy food inside Iran is related to the exporting and world price. It is assumed that 80% of the collected products are exported with an exclusive share of more than 65% of the total world saffron production.[8,29]
Standards and criteria
Appropriate season for collection
Collection of saffron flowers and isolation of stigmas are not easy and started just after the appearance of the first flower in the field because the flowers remain only for 3-4 days. If the flowers remain under warm weather and sunlight or wind, then the quality of color and odor will be changed and decreased. The appropriate season for collection is changed and related to the first watering. This season in Khorasan province is started around October until November. Flowering period is usually elongated for 15-25 days and the best time for collation is before sunrise.[2,29]
Appropriate method of collection
Collection of flowers has been carried out by hands of the workers. Plastic or wood baskets are used for moving to PRevent any mechanical damage or pollution. Flowers have to be opened in the same day of harvesting by hands. If the flowers are opened before collection, then the stigmas may crash or mix with petals and the quality will decrease.[2,30]
Drying methods
Collected stigmas of saffron must be dried in order to store for a long time. Drying method mainly affects the quality and cost of the product. In traditional method, stigmas have been grouped inside baskets, which contained fine holes, and then hung from the ceiling of a room with appropriate temperature. When the color of stigmas changed to dark red and they crashed between two fingers, the drying process comes to an end. This method takes a long time (around 1 week) and for this reason, the producers sometimes put the saffron under sunlight which makes the product more darker.[31] In the last few years, this system is gradually losing ground and electric ovens are employed.
Recently, advanced method of drying has been used, in which stigmas put on the sterile silk net (150-200 g) with a 2-3 cm layer in the appropriate distance to oven (50-60°C). By using this method, the humidity of products will be equal to the standard 13%. Fast drying, high quality, and less affected by air pollution and mold are other advantages.[31] Literature showed that saffron samples from the countries such as UK, Iran, and India were compared with values obtained from ISO/TS 332-1 as chemical requirements for saffron samples. The results revealed that moisture content values of samples from the three countries were close to the standard value; however, the values of Indian saffron were slightly lower than others. This could be explained using different drying procedures in different countries as well as climate differences.[32]
International standards of the plant material
Saffron as a medicinal food plant material must follow the international standards. The important chemical characteristics of dried saffron on the basis of ISO 3632-1 are indicated in Table 1.
Table 1.
The important chemical characteristics of dried saffron on the basis of ISO 3632-1
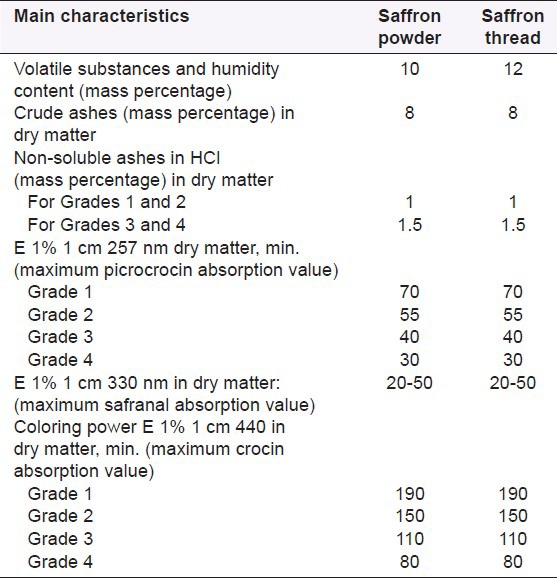
One of the main parameters is measurement of coloring power through crocin, picrocrocin, and safranal, which defined four different saffron qualities (1-4) using ultraviolet-visible (UV-Vis) spectrophotometry. The 2003 issue contains important amendments, questioned by the enterprises of the sector, especially regarding detection of adulterations. The four quality categories for saffron threads or powder were reduced to three, determined by coloring power at 440 nm (190, 150, and 100). The category IV of the former ISO was annulated, given the fact that a product with more than 80 units of coloring power may not be considered as saffron. The new ISO specification has unified the criteria such as “maximum non-soluble ashes content” for the three categories and annulated the specifications regarding nitrogen and cellulose.[33]
The food and drug administration criteria
Based on the FDA rules,[30] saffron approved as a natural food dressing and flavoring without limitation in culinary purposes; hence, the product must follow the below criteria:
Stigmas must be yellow and the foreign organic compounds not more than 10%.
The volatiles and humidity must not more than 14% when the saffron dried at 100°C.
Maximum total ash must not more than 1% and the amount of soluble ash not more than 1%.
Microbial pollutions
Crocus flowers are blooming on the surface of the soil and hence the organic fertilizers and compost are the main sources of microorganisms. Microbial flora of saffron usually contains aerobic spore-forming bacteria such as Bacillus and Fungi and rarely E. coli, Lactobacillus, Micrococcus, Staphylococcus, and Streptococcus. Sterilization process by heating is not recommended because it influences on the color, taste, and odor of the product. As found in the literature, gamma, microwave, and ultraviolet (UV) radiations together with fumigation by ethylene oxide should be more useful,[29] whereas the recent report shows that a decrease in glycosides and an increase in aglycon content have been observed in irradiated samples. The possibility of degradation of pigments during gamma irradiation is considered.[34]
Adulterants
Mixing of saffron with materials such as beet, pomegranate fibers, and red-dyed silk fibers are occasionally observed for decreasing the cost of saffron. The yellow stamens of saffron have been mixed with the saffron stigma or powder to increase the product mass. Sometimes the flowers of other plants, particularly Carthamus tinctorius, or safflower, Calendula officinalis, or marigold, arnica and tinted grasses are fraudulently mixed with the genuine stigmas. It is reported that adulterants have been loaded with calcium sulfate and attached to saccharine or glucose which yielded on incineration 40% of ash. Turmeric, paprika, and other substances have still been combined with saffron powder.[29,35]
The common mislabeling of turmeric (Curcuma longa) as “Indian saffron,” “American saffron,” or “Mexican saffron,” also borders fraud, so should be wary of packets listing above because neither of them are from C. sativus. Addition of artificial colorants is the most common way of adulteration with the aim of misleading the consumer for improving the appearance of the dried stigmas or even other extraneous materials to give rise to the coloring strength of the aqueous extract. When saffron is used for therapeutic purposes, adulterations make it completely useless or even harmful.[35,36]
Methods for purity determination
There are various methods used to detect the adulterants in saffron products. When the saffron product is disperse in water, it immediately changes to a characteristic form that is simply distinguished from Crocus stamens, florets, safflower, marigold, or arnica. In addition, true saffron should easily dissolve in aqueous phase and spread the special odor of saffron. If the water extract of true saffron is dried and a rod (dipped in sulfuric acid) is drawn across the surface, a blue color appears and turns purple or reddish-brown. Other detection methods include color reactions, microscopic study, thin layer chromatography (TLC), and high-performance liquid chromatography (HPLC). HPLC is considered the most effective one.[35,37]
Most of the countries have prohibited the use of synthetic dyes as food coloring agents. Detection of artificial colors, such as naphthol yellow, tartrazine, quinoline yellow, sunset yellow, in saffron is nowadays carried out by UV-Vis spectrometry, when crocetin is precipitated.[38] The least amount of each color which may be detectable is related to the chemical structure. This procedure may be used alternatively or in combination with HPLC procedures.[35,38]
Alonso and co-authors were reported a colorimetric reflection method on saffron samples.[39] In this method, the chromatic parameters of saffron samples were defined by CIE system (the International Commission on Illumination), as * (brightness), a* (redness-greenness), and b* (yellowness-blueness), and correlated well with their coloring power. This method is a very useful technique for quality assessment of saffron. Moreover, there is a Hunter Lab system which is similar to CIE system for color measurement of food products.[40] Literature review indicated that the atmospheric chemical ionization-mass spectrometry (APCI-MS) technique, used for quantitative analysis, is a simple, sensitive, and reliable method for volatile compound analysis.[41] Fourier transform near infrared (FT-NIR) spectroscopy analysis of saffron has been introduced for the first time in saffron. This method analyzes fast and did not require any sample treatment.[42]
Packaging and storage
The most important factors that cause decomposition or reduce the quality are as follows: Humidity of product and relative air humidity, temperature of the surroundings, direct sunlight, oxygen, and quality of packages.[43] It is clear that the lower the temperature and humidity, the higher the quality. Packaging with polystyrene covered by cellophane MXXT Grade (Polyvinylidene Chloride Coated) can decrease the time of storage. Color resistance in relative humidity of 5-23% is higher and mainly decrease in 75%. Steryl glycosides of saffron are strongly influenced by high temperature, humidity, and oxygen and then decreased in the product because of the β-glucosidase activation.[31,44]
CONCLUSION
C. sativus, Cuminum cyminum and Brassica napus are of important medicinal food plants growing widely in Iran and also cultivated for their nutritional purposes and economic significance.[45,46] The plant C. sativus is mostly distributed in Irano-Touranian region and west of Asia with low annual rainfall, cool winters, and hot summers. Nowadays, saffron is cultivated in Iran and a few countries with old civilization.[47] Saffron is the most expensive spice in the world and its high price is due to the labor cultivation, harvesting, and handling. The restrictions of production and high demand of consumption resulted in the high price of saffron. The recent reports about pharmacological activity and medicinal properties of saffron make it the object of frequent adulteration and frauds, and the object of various phytochemical and biotechnological researches. Furthermore, the certification of the origin and quality of saffron as a medicinal food led to the frequent usages of chemical and molecular techniques. Determining of chemical composition of saffron is another effort for PReventing the saffron adulteration. The HPLC analytical procedures are sensitive, reproducible, and allow obtaining the sufficient amounts of the saffron components for further analytical assessments. In addition, APCI-MS technique, FT-NIR spectroscopy analysis, and UV-Vis spectrometry are mainly used for qualitative and quantitative analyses. The relative air humidity, temperature of the surroundings, direct sunlight, and quality of packages are the final parameters that impact on the quality of the product.
Footnotes
Source of Support: The research was supported by MPRC, Tehran University of Medical Sciences
Conflict of Interest: The authors declare that there is no conflict of interest.
REFERENCES
- 1.Saeidnia S. Future position of crocus satives as a valuable medicinal herb in phytotherapy. Pharmacognosy J. 2012;4:71. [Google Scholar]
- 2.Evans WC. 14th ed. London: WB Saunders Company Ltd; 1997. Trease and Evans’ Pharmacognosy; p. 438. [Google Scholar]
- 3.Harper D. Online Etymology Dictionary. 2001. [Last accessed on 2006 Jan 10]. Availabe from: www.etymonline.com/index.php?search=saffron .
- 4.Mozaffarian V. Tehran: Farhang Moaser Publisher; 1996. A dictionary of iranian plant names; p. 165. [Google Scholar]
- 5.Abrishami MH. Mashhad, Iran: Astan Ghods Razavi Publication; 1997. Iranian saffron. Historic, cultural and agronomic prospects; pp. 1–10. [Google Scholar]
- 6.Leffingwell JC. saffron. Leffingwell Reports. 2002;2:1–6. [Google Scholar]
- 7.Rechinger KH. Graz, Austria: Academische Druck-U-Verganstalt; 1975. Flora Iranica, Iridaceae; pp. 1–79. [Google Scholar]
- 8.Wani BA, Hamza AKR, Mohiddin FA. Saffron: A repository of medicinal properties. J Med Plant Res. 2011;5:2131–5. [Google Scholar]
- 9.USDA N. The PLANTS database. 2009. [Last accessed on 2009 Sep 17]. Availabe from: http://plants.usda.gov/java .
- 10.Schmidt M, Betti G, Hensel A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien Med Wochenschr. 2007;157:315–9. doi: 10.1007/s10354-007-0428-4. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal M. Boston: American Botanical Council; 1998. The complete german commission E monographs. [Google Scholar]
- 12.Sarris J. Herbal medicines in the treatment of psychiatric disorders: A systematic review. Phytother Res. 2007;21:703–6. doi: 10.1002/ptr.2187. [DOI] [PubMed] [Google Scholar]
- 13.Thachil AF, Mohan R, Bhugra D. The evidence base of complementary and alternative therapies in depression. J Affect Disord. 2007;97:23–35. doi: 10.1016/j.jad.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Maccarone R, Di Marco S, Bisti S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Invest Ophthalmol Vis Sci. 2008;49:1254–61. doi: 10.1167/iovs.07-0438. [DOI] [PubMed] [Google Scholar]
- 15.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gainer JL, Jones JR. The use of crocetin in experimental atherosclerosis. Experientia. 1975;31:548–9. doi: 10.1007/BF01932451. [DOI] [PubMed] [Google Scholar]
- 17.Modaghegh MH, Shahabian M, Esmaeili HA, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032–7. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee S, Poduval TB, Tilak JC, Devasagayam TP. A modified, economic, sensitive method for measuring total antioxidant capacities of human plasma and natural compounds using Indian saffron (Crocus sativus) Clin Chim Acta. 2005;352:155–63. doi: 10.1016/j.cccn.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoPRevention trials. Cancer Detect PRev. 2004;28:426–32. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res. 2000;14:149–52. doi: 10.1002/(sici)1099-1573(200005)14:3<149::aid-ptr665>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Brown AC, Hairfield M, Richards DG, McMillin DL, Mein EA, Nelson CD. Medical nutrition therapy as a potential complementary treatment for psoriasis: Five case reports. Altern Med Rev. 2004;9:297–307. [PubMed] [Google Scholar]
- 22.Wüthrich B, Schmid-Grendelmeyer P, Lundberg M. Anaphylaxis to saffron. Allergy. 1997;52:476–7. doi: 10.1111/j.1398-9995.1997.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 23.Pfander H, Rychener M. Separation of crocetinglycosyl esters by high-performance liquid chromatography. J Chromatogr. 1982;234:443–7. [Google Scholar]
- 24.Himeno H, Sano K. Synthesis of crocin, picrocrocin and safranal by saffron stigma-like structures proliferated in vitro. J Agric Biol Chem. 1987;51:2395–400. [Google Scholar]
- 25.Norbaek R, Kondo T. Anthocyanins from flowers of Crocus (Iridaceae) Phytochemistry. 1998;47:861–4. [Google Scholar]
- 26.Norbaek R, Kondo T. Further anthocyanins from flowers of Crocus antalyensis (Iridaceae) Phytochemistry. 1999;50:325–8. doi: 10.1016/s0031-9422(99)00109-0. [DOI] [PubMed] [Google Scholar]
- 27.Nørbaek R, Kondo T. Flavonol glycosides from flowers of Crocus speciosus and C. antalyensis. Phytochemistry. 1999;51:1113–9. doi: 10.1016/s0031-9422(99)00109-0. [DOI] [PubMed] [Google Scholar]
- 28.Saeidnia S, Gohari AR. Germany: (LAP) Lambert Academic Publishing; 2012. Pharmacognsy and molecular pharmacognosy in practice. [Google Scholar]
- 29.Kafi M. Mashhad: Ferdowsi University Publication; 2002. Saffron: Production technology and manufacture. [Google Scholar]
- 30.Hemmati Kakhki A. Optimization of effective parameters on production of food color from Saffron petals. Agr Sci Tech. 2001;15:13–20. [Google Scholar]
- 31.Dadkhah MR, Ehtesham M, Fekrat H. Tehran: Shahr Ashoob Publication; 2003. Iranian saffron an unknown jewel; pp. 1–20. [Google Scholar]
- 32.Yadollahi A, Shojaei ZA, Farahnaky A. Study of colouring, aromatic strength and bitterness of Saffron (Crucos sativus L.) cultivated in the UK. Acta Hortic (ISHS) 2007;739:455–61. [Google Scholar]
- 33.Switzerland: ISO; 2003. ISO/TS-Technical Specification. Crocus sativus L. Saffron; pp. 3236–1. [Google Scholar]
- 34.Rastkari N, Razzaghi N, Afarin L, Alemi R, Ahmadkhaniha R. Effect of gammairradiationon chemical properties of Saffron pigments. Acta Hortic (ISHS) 2007;739:451–3. [Google Scholar]
- 35.Hagh-Nazari S, Keifi N. Saffron and various fraud mannersin its production and trades. Acta Hortic (ISHS) 2007;739:411–6. [Google Scholar]
- 36.Organic Food Items and Food Adulteration FAQs. 2010. [Last accessed on 2010 Sep 25]. Available from: http://www.food adulteration info.com/?q=node/4 and page=5 and sort=desc and order=Adulterant .
- 37.Hint for detection of food adulteration. 2006. [Last accessed on 2006 Oct 11]. Availabe from: http://www.women excel.com/cuisine/foodadulteration.htm .
- 38.Zalacain A, Ordoudi SA, Blázquez I, Díaz-Plaza EM, Carmona M, Tsimidou MZ, et al. Screening method for the detection of artificial colours in Saffron using derivative UV-Vis spectrometry after precipitation of crocetin. Food Addit Contam. 2005;22:607–15. doi: 10.1080/02652030500150051. [DOI] [PubMed] [Google Scholar]
- 39.Alonso GL, Sanchez-Fernandz MA, Seaz JR, Zalacian A, Salinas MR. Evaluation of the colour of spanish saffron using tristimuluscolorimetry. Italian J Food Sci. 2003;15:249–58. [Google Scholar]
- 40.Ferreira VL, Fernandz V, Yotsuyanagi K. The color of chicken and pork meat loaf with added cured bovine blood as evaluated by RAB, Hunter Lab L-Asterisk A-Asterisk B-Asterisk and XYZ-CIE systems. Revista espanola de ciencia y tecnologia de alimentos, Ferreira. 1994;34:311–2. [Google Scholar]
- 41.Taylor AJ, Linforth RS. Direct mass spectrometry of complex volatile and non-volatile flavour mixtures. Int J Mass Spect. 2003;223:179–91. [Google Scholar]
- 42.Zalacaín A, Díaz-Plaza EM, Blázquez I, Carmona M, Alonso GL. FT-NIR spectrometry approach for determining saffron origin. Acta Hortic. 2003;650:22–5. [Google Scholar]
- 43.Hadjiakhoondi A, Saeidnia S, Gohari AR. Tehran: Tehran University of Medicinal Sciences; 2005. Rational phytotherapy. [Google Scholar]
- 44.Morimoto S, Umezaki Y, Shoyama Y, Saito H, Nishi K, Irino N. Post-harvest degradation of carotenoid glucose esters in saffron. Planta Med. 1994;60:438–40. doi: 10.1055/s-2006-959527. [DOI] [PubMed] [Google Scholar]
- 45.Saeidnia S, Gohari AR. Importance of Brassica napus as a medicinal food plant. J Med Plant Res. 2012;6:2700–3. [Google Scholar]
- 46.Gohari AR, Saeidnia S. A review on phytochemistry of Cuminum cyminum seeds and its standards from field to market. Pharmacognosy J. 2011;3:1–5. [Google Scholar]
- 47.Sepaskhah AR, Kamgar-Haghighi AA. Saffron irrigation regime. Int J Plant Prod. 2009;3:1–16. [Google Scholar]


