Abstract
Medicinal plants are used by traditional practitioners to treat several ailments. Ethnomedicinal studies on Trema orientalis Linn. Blume (Ulmaceae) have shown that it is used in the treatment of diabetes mellitus, respiratory diseases, oliguria, and malaria. This article is aimed at providing comprehensive information on the medicinal uses, biology, phytochemical constituents, and pharmacological data available on T. orientalis. This has been done to explore its therapeutic potential for future research opportunities. This review was compiled with information obtained from databases such as Medline, Elsevier, Springer, Science Direct, Pubmed, Google Scholar, and a library search for articles published in peer-reviewed journals. Compounds present in the plant include tannins, saponins, flavanoids, triterpenes, phytosterols, and several constituents of xanthones. Some pharmacological research done on the plant has focused on, hypoglycemic activity, analgesic, anti-inflammatory activities, anti-plasmodial activity, diuretic activity, laxativity effect, anti-convulsant activity, anti-helmintic activity, anti-sickling effect, anti-oxidant, and anti-bacterial activity. This compilation strongly supports the view that T. orientalis has beneficial therapeutic properties, and indicates its potential as an effective herbal remedy for several diseases. The promising results from several research works could be further substantiated by clinical trials.
Keywords: Phytoconstituents, Trema orientalis Linn. Blume, Ulmaceae
BACKGROUND
Trema orientalis is an evergreen tree which belongs to the family Ulmaceae. It has been used extensively in various ways. T. orientalis is the same as Celtis orientalis Linn., Celtis guineensis Schum. and Thonn., Trema bracteolate Hochst Blume, Sponia orientalis Linn. Decne, and Trema guineensis (Schum. and Thonn.) Ficalho. Aside its uses in paper production and in the manufacturing of poles, it has been used for medicinal purposes including the treatment of respiratory, inflammatory, and helminthic diseases. Almost every part of the plant is used as medicine in various parts of Africa.[1,2] The generic name Trema is derived from a Greek word which means perforation or hole and alludes to pitted seeds of the tree, whereas the specific name orientalis is derived from the Latin word “orientalis” meaning eastern. The plant has common names such as pigeon wood, hop out, charcoal tree, Indian charcoal tree, Indian nettle tree, and gunpowder tree.
Medicinal plants are a major source of compounds of therapeutic value, and contain different phytochemical compounds resulting in numerous pharmacological activities. According to a World Health Organization report, about 70-95% of the developing world populations rely on non-conventional medicinal sources, especially plants, for their primary healthcare.[3,4] The aim of this review is to provide comprehensive information on the botanical description, traditional uses, phytochemical constituents, and pharmacological activities available on the T. orientalis plant. This review was compiled with information obtained from search engines such as Medline, Elsevier, Springer, Science Direct, Pubmed, Google Scholar, and a library search for articles published in peer-reviewed journals. This was done with the view of exploring its therapeutic potential for future research opportunities.
ECOLOGICAL AND GEOGRAPHICAL DISTRIBUTION
T. orientalis is widely distributed all over the world in countries such as Ghana, Senegal, Sierra Leone, Niger, Cote d’Ivoire, Angola, Australia, Bangladesh, Brunei, Cambodia, Cameroon, Central African Republic, Chad, China, Democratic Republic of Congo, Ethiopia, India, Indonesia, Japan, Kenya, Laos, Madagascar, Malaysia, Mali, Myanmar, Nepal, Nigeria, Philippines, Saudi Arabia, South Africa, Sudan, Tanzania, Uganda, Vietnam, Zambia and Zimbabwe.[5] It is among the first few plants that establish on flood-damaged river banks. It can grow on a wide range of soils from heavy clay to light sand.[6]
Description of the plant
T. orientalis is a shrub or small to medium size tree and its height varies depending on the location and climatic conditions. It can grow up to 18 m high in forest regions, and up to 1.5 m tall in the savannah. The slender branchlets are covered with white velvety hairs.[6] It has an extensive root system that enables it to survive long periods of drought.
The leaves are simple, alternate and stipulate although the stipules drop early and usually three-nerved from the base. The leaf base is frequently unequal. Leaves taper from the base to the apex and vary from 2 to 20 cm long, and 1.2 to 7.2 cm wide.[7] Leaf margins are finely serrated, whereas the young leaves are rough and hairy, occasionally becoming smooth when old.[8] Photos of the leaves are shown in Figures 1a and 1b.
Figure 1a.

Trema orientalis leaves
Figure 1b.

Underside of a single leaf of Trema orientalis
The flowers are small, inconspicuous, and greenish, carried in short dense bunches. They are usually unisexual, occasionally they are bisexual. Flowers appear irregularly from late February to April.[8] The fruits are small, round, and dark purple or green drupes that become black when ripe, and they are carried on very short stalks.[7] The onset of fruit ripening varies with the locality, but in most places, occurs from December to May. Birds are very fond of the fruit and disperse the fleshy drupes.[9]
Ethnomedicinal uses
The plant is used in various parts of Africa for medicinal purposes. The young leaves are eaten as spinach by the Zulus in South Africa, who also use the roots and stem bark as traditional medicine.[8] The fruit, leaves, bark, stem, twig and seeds are extensively used in traditional medicine.[10]
The root
The root of T. orientalis plants is used in folk medicine for treatment of trauma, blood stasis, hematuria and bleeding of intestines and stomach.[11]
The stem
The stem bark decoctions are used as vermifuge and anti-dysenteries.[12] The stems and twigs infusion are used to treat fever and toothache.[6] Both stem bark and leaf decoction of the T. orientalis are used to treat malaria, manage pain in tired muscles and aching bones as well as venereal disease.[13,14,15] Both, the stem bark and leaf decoctions are used as a gargle, inhalation, drink, vapor bath for relieve of toothache.[5]
The leaf
The leaf of T. orientalis is mixed with leaves of Bidens pilosa, Citrus aurantifolia, and peels of unripe pineapple. It is boiled and the decoction used in the management of jaundice.[16] The leaves macerated in lemon juice are used as remedy for bronchitis, pneumonia and pleurisy.[16,17] The leaves macerated in lemon juice are used as remedy for cough.[1] The leaves decoction of T. orientalis plants is also used as an anti-helminthic medicine for roundworms and hookworms in West Africa, East Africa, and some parts of Central Africa and Madagascar.[18]
The fruits and flowers
The fruits and flowers are used to prepare infusion that are administered to children as a therapy for bronchitis, pneumonia and pleurisy.[16,17]
MAJOR PHYTOCHEMICAL CONSTITUENTS
The leaves of T. orientalis contain tannins, saponins, flavanoids, triterpenoid (simiarenol, simiarenone, trematol).[1] Octacosanoic acid, 1-octacosanyl acetate, simiarenone, simiarenol, episimiarenol, and a new triterpene alcohol, trematol has been isolated from stem bark.[19] Other studies have also reported the presence of methylswertianin, decussatin, glycosides of decussatin, sweroside, scopoletin, (-)-epicatechin, lupeol, p-hydroxybenzoic acid, adian-5-en-3-one, 2α, 3ß-dihydroxyurs-12-en-28-oic acid in the stem bark.[20,21,22,23] The stem bark also contains (-)-ampelopsin F, (-)-epicatechin, (+)-catechin, (+)-syringaresinol, N-(trans-p-coumaroyl) tyramine, N-(trans-p-coumaroyl) octopamin, trans-4-hydroxycinnamic acid, and 3,5-dimethoxy-4-hydroxyphenyl-1-O-ß-D-glucoside.[24] Chemical investigation of extracts from root bark of this plant has led to the isolation of compounds such as hexacosanoic acid, 3-O-ß-glucopyranosyl-ß-sitosterol, simiarenone, 3,4-dihydroxybenzoic acid, 2α, 3α, 23-trihydroxyurs-12-en-28-oic acid and ß-sitosterol.[20,21,25,26] Structures of some compounds are shown in Figure 1c.
Figure 1c.
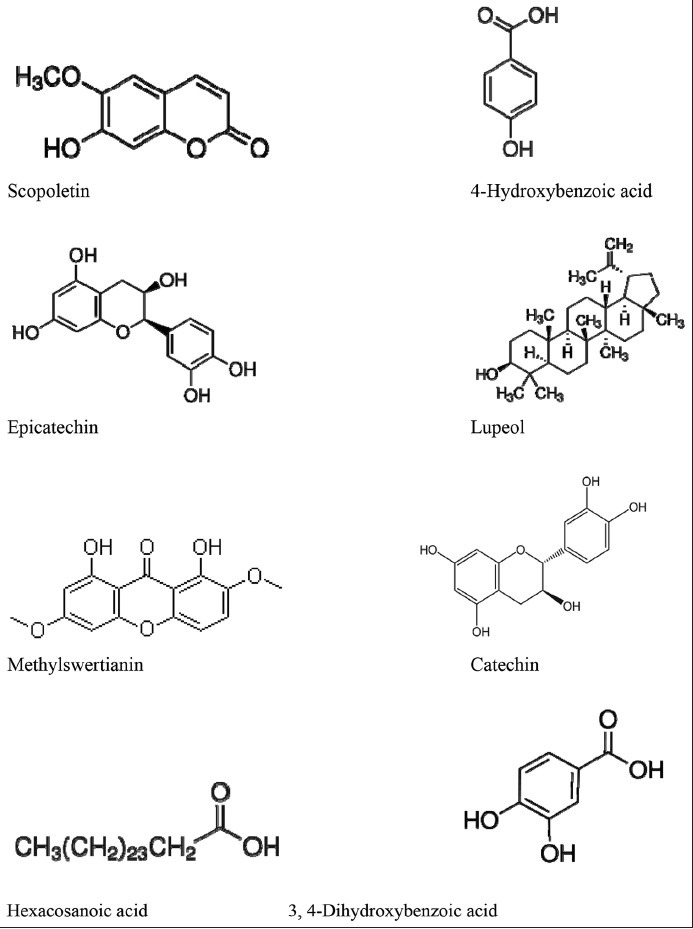
Structures of some biological compounds isolated from different parts of Trema orientalis plant
Pharmacological activities
Researchers have reported the different pharmacological effects of T. orientalis in various test models. The aerial parts, flowers, bark, and seeds of T. orientalis exhibit various pharmacological activities including laxativity, hypoglycemic, anti-pyretic, analgesic, anti-microbial properties, anti-convulsant activity, and anti-plasmodial.[27,28,29,30,31] These effects may be mainly due to the fact that it contains important biologically active compounds as shown in Figure 1c of this review. This section of the article focuses on the main pharmacological properties of T. orientalis.
Laxativity effect
Laxatives also known as purgatives are foods, compounds, or drugs taken to induce bowel movements or to loosen the stool, most often taken to treat constipation. Sufficiently high doses of laxatives cause diarrhoea. Aqueous extract of T. orientalis was found to induce the stimulation of rabbit duodenum contractility and therefore can be used as a laxative due to its stimulating effects on duodenal contractility.[28] An experiment to determine, the effects of the aqueous extract tested against the catalytic activity of the acetylcholinesterase extracted from the duodenal muscle, showed that aqueous extract of T. orientalis exerts cholinimimetic and anti-cholinesterasic effects, and therefore can be used as laxative in traditional medicine practice.[28]
Hypoglycaemic activity
Diabetes mellitus is a metabolic disease in which there are persistent high glucose levels in the blood, either because the body does not produce insulin at all or produces but it is insufficient because receptors are insensitive to insulin produced. This high blood glucose levels produces symptoms of polyuria, polydipsia, and polyphagia among others. Aqueous stem bark extract of T. orientalis was reported to have hypoglycemic effects in induced diabetic rats by mechanism different from that of sulfonylurea agents.[29] This study provides pharmacological evidence that the use of this plant in herbal medicinal practice can be of importance in diabetic patients.
Anti-plasmodial activity
Plasmodium falciparum is a protozoan parasite, one of the species of Plasmodium that cause malaria in humans. It is transmitted by the female Anopheles mosquito. P. falciparum is the most dangerous of these infections as P. falciparum malaria has the highest rates of complications and mortality. It is more PRevalent in sub-Saharan Africa than in other regions of the world; in most African countries, more than 75% of cases are due to P. falciparum.[32] Hexane extract of stem bark of T. orientalis has anti-plasmodial activity against P. falciparum.[28] Also, the bark and leaf decoctions has been reported to have anti-plasmodial properties.[5]
Diuretic activity
The increased excretion of water from the body is a conditional metabolic process known as diuresis. There are several forms, although each class does occur in a distinct way. This may also help in getting rid of other water soluble wastes or toxins from the body. T. orientalis contains polyphenols which have potassium retention ability, and thus may be responsible for diuresis.[33]
Anti-convulsant activity
A convulsion is a medical condition where body muscles contract and relax rapidly and repeatedly, resulting in an uncontrolled shaking of the body. Convulsion is often a symptom of an epileptic seizure, thus the term is sometimes used as a synonym for seizure. However, not all epileptic seizures lead to convulsions, and not all convulsions are caused by epileptic seizures. Methanolic and aqueous extracts of T. orientalis leaves has anti-convulsant activity against pentylenetetrazole-induced seizures and maximum electroshock-induced seizures in mice.[31] This indicates that the plant has a good potential for the management of convulsion.
Analgesic and anti-arthritic activities
Pain is an unpleasant sensation often caused by intense or damaging stimuli and it is a response to malfunction of the body that occurs in association with every ailments. The leave extracts of T. orientalis have significant ability to reduce pain in mice in acetic acid induced writhing test, and in rats by the hot plate model.[33] The leaves extract have also been shown to have anti-arthritic effects in acute and chronic models in mice.[34]
Anti-bacterial activity
According to Chowdhury and Islam (2004), crude methanol and aqueous root extracts of T. orientalis both showed activity against Gram-negative bacteria. Though both extracts showed anti-bacterial activity against gram-positive bacteria and i.e., Gram-negative bacteria, aqueous extract showed higher anti-microbial activities than methanol extract.[35,36] The results obtained provide support for the use of this plant in traditional medicine.
Anti-helmintic activity
T. orientalis has been reported to have anti-helmintic properties against intestinal worms.[36] Also, according to Diehl et al. (2004), the leaf and root extract of T. orientalis showed 100% mortality rate on Haemonchus contortus larvae species.[37]
Anti-sickling effect
Anthocyanins extracts from T. orientalis have been found to possess anti-sickling activity, as treated sickle erythrocyte it reverted to the normal and classical biconcave form of red blood cells, with a radius value of 3.5 ± 0.2 μm similar to that of normal erythrocytes values.[38]
Anti-oxidant activity
Anti-oxidants are substances that protect cells against the effects of free radicals. Free radicals are molecules produced through physiological reactions, or by environmental exposures like tobacco smoke and radiation. Free radicals can damage cells, and may play a role in heart disease, cancer, and other diseases.[39] In a study by Salprima et al. (2011), methanol extracts of the leaves of T. orientalis showed potential free-radical scavenging activity whereas aqueous extract showed very little free-radical scavenging activity. The methanol fraction extracted from T. orientalis leaves has been shown to exhibit an anti-radical activity that is almost similar to that of ascorbic acid.[40]
CONCLUSION
T. orientalis has emerged as a good source of phytomedicine. This compilation strongly supports the view that T. orientalis has beneficial therapeutic properties, and indicates that it has potential as an effective herbal remedy for several diseases. There is also the need to search for individual compounds responsible for these biological effects, and study their mechanism of actions and pharmacokinetics in detail. These promising results should be further substantiated by clinical trials.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Accra, Ghana: The Adventist Press; 1992. Ghana Herbal Pharmacopeia; pp. 141–43. [Google Scholar]
- 2.Yanes CV. Germination of a pioneer tree from Equatorial Africa. Turrialba. 2007;27:301–2. [Google Scholar]
- 3.The World Medicines Situation. WHO/EMP/MIE/2011.2.3. 2011. [Last accessed on 2011 Nov 29]. Available from: http://www.who.int/medicines/areas/policy/world_medicines_situation/WMS_ch18_wTraditionalMed.pdf .
- 4.Harsha VH, Hebbar SS, Shripathi V, Hegde GR. Ethnomedicobotany of Uttara Kannada District in Karnataka, India: Plants in treatment of skin diseases. J Ethnopharmacol. 2003;84:37–40. doi: 10.1016/s0378-8741(02)00261-1. [DOI] [PubMed] [Google Scholar]
- 5.Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A. Agroforestree database: A tree reference and selection guide version 4.0, 2009. [Last accessed on 2009 Feb 18]. Available from: http://www.worldagroforestry.org/af/treedb .
- 6.Smith CA. Pretoria: Department of Agricultural Technical Services; 1966. Common names of South African plants. Memoirs of the Botanical Survey of South Africa 35. [Google Scholar]
- 7.Natural resources and the human environment for food and agriculture in Africa, FAO Environment and Energy Paper No. 6. :88. [Google Scholar]
- 8.Coates PK. 1st ed. Cape Town, Johannesburg: C. Struik Publishers CapeTown, Johannesberg; 1977. Trees of Southern Africa. [Google Scholar]
- 9.Malan C, Notten A. Kirstenbosch National Botanical Garden. 2005. [Last accessed on 2011 Jul 16]. Available from: http://www.plantzafrica.com .
- 10.Iwu MM. Boca Raton, Florida: CRC Press Inc; 1993. Handbook of African Medicinal Plants; pp. 251–2. [Google Scholar]
- 11.Hines DA, Eckman K. Ottawa Ontario Canada: Cultural Survival Canada and Development Services Foundation of Tanzania; 1993. Indigenous multipurpose trees for Tanzania: Uses and economic benefits to the people; pp. 26–27. [Google Scholar]
- 12.Githens TS. African Handbooks. Vol. 8. United States: University of Pennsylvania Press, Lancaster Press, Lancaster; 1948. Drug plants of Africa; p. 125. [Google Scholar]
- 13.Ayensu ES. Medicinal plants of West Africa. Nordic J Bot. 1978;4:1–3. [Google Scholar]
- 14.Rasoanaivo P, Petitjean A, Ratsimamanga-Urverg S, Rakoto-Ratsimamanga A. Medicinal plants used to treat malaria in Madagascar. J Ethnopharmacol. 1992;37:117–27. doi: 10.1016/0378-8741(92)90070-8. [DOI] [PubMed] [Google Scholar]
- 15.Bhat RB, Ttejere EO, Oladipo VI. Ethnobotanical studies from Central Nigeria. Econ Bot. 1990;44:382–90. [Google Scholar]
- 16.Katende AB. Useful trees and shrubs for Uganda. Identification, Propagation and Management for Agricultural and Pastoral Communities. Regional Soil Conservation Unit (RSCU), Swedish International Development Authority (SIDA); 1995 [Google Scholar]
- 17.Watt JM, Breyer-Brandwijk MG. 2nd ed. London: Livingstone; 1962. The Medicinal and Poisonous Plants of Southern and Eastern Africa; pp. 982–7. [Google Scholar]
- 18.Akendengue J. Entheogenic drugs, their plant sources and history. J Ethnopharmacol. 1992;37:165. [Google Scholar]
- 19.Rastogi RP, Mehrotra BN. Vol. 2. New Delhi, India: Central Drug Research Institute, Lucknow Publications and Information Directorate; 1993. Compendium of Indian Medicinal Plants; pp. 169–70. [Google Scholar]
- 20.Tchamo DN, Dijoux-Franca MG, Mariotte AM, Tsamo E, Daskiewicz JB, Bayet C, et al. Prenylated xanthones as potential P-glycoprotein modulators. Bioorg Med Chem Lett. 2000;10:1343–5. doi: 10.1016/s0960-894x(00)00234-1. [DOI] [PubMed] [Google Scholar]
- 21.Ogunkoya L, Olubajo OO, Sondha DS. Simiarenone from Trema orientalis. Phytochemistry. 1973;12:732–3. [Google Scholar]
- 22.Ogunkoya L, Olubajo OO, Sondha DS. Triterpenoid alcohols from Trema orientalis. Phytochemistry. 1972;11:3093–4. [Google Scholar]
- 23.Ogunkoya L, Olubajo OO, Sondha DS. A new triterpenoid alcohol from Trema orientalis. Phytochemistry. 1977;16:1606–8. [Google Scholar]
- 24.Wen-Lung K, Yu-Ling H, Shr-Ting W, Ching-Li N, Bor-Jinn S, Chien-Chih C. Chemical constituents of Trema orientalis. J Chin Med. 2007;18:27–36. [Google Scholar]
- 25.Dijoux-Franca MG, Noungoué Tchamo D, Cherel B, Cussac M, Tsamo E, Mariotte AM. New dihydrophenanthrene and phenyldihydroisocoumarin constituents of Trema orientalis. J Nat Prod. 2001;64:832–5. doi: 10.1021/np000275s. [DOI] [PubMed] [Google Scholar]
- 26.Noungoue TD, Cartier G, Dijoux Franca MG, Tsamo AM. Xanthones and others constituents of Trema orientalis. Pharm Biol. 2001;39:202–5. [Google Scholar]
- 27.Abbiw DK. Kew, London: Intermediate Technology Publications and the Royal Botanic Gardens Kew, London; 1990. Useful Plants of Ghana; pp. 337–40. [Google Scholar]
- 28.Abiodun O, Gbotosho G, Ajaiyeoba E, Happi T, Falade M, Wittlin S, et al. In vitro antiplasmodial activity and toxicity assessment of some plants from Nigerian ethnomedicine. Pharm Biol. 2011;49:9–14. doi: 10.3109/13880209.2010.490224. [DOI] [PubMed] [Google Scholar]
- 29.Dimo T, Ngueguim FT, Kamtchouing P, Dongo E, Tan PV. Glucose lowering efficacy of the aqueous stem bark extract of Trema orientalis (Linn.) Blume in normal and streptozotocin diabetic rats. Pharmazie. 2006;61:233–6. [PubMed] [Google Scholar]
- 30.N’guessan K, Tiébré M-S, Aké-Assi E, Zirihi GN. Ethnobotanical study of plants used to treat arterial hypertension, in traditional medicine, by Abbey and Krobou populations of agboville (Côte-d’Ivoire) Eur J Sci Res. 2009;35:85–8. [Google Scholar]
- 31.Panchal HS, Master SM, Shah UD, Saluja AK, Dholwani KK. Anti-convulsion activity of leaf of Trema orientalis. Int J Pharm Res. 2010;2:53–5. [Google Scholar]
- 32.Perlmann P, Troye-Blomberg M. Malaria blood-stage infection and its control by the immune system. Folia Biol (Praha) 2000;46:210–8. [PubMed] [Google Scholar]
- 33.Uddin SN, Uddin KM, Ahmed F. Analgesic and antidiarrhoeal activities of Trema orientalis Linn.in mice. Orient Pharm Exp Med. 2008;8:187–91. [Google Scholar]
- 34.Barbera R, Trovato A, Rapisarda A, Ragusa S. Analgesic and antiinflammatory activity in acute and chronic conditions of Trema guineense (Schum. et Thonn.) ficalho and trema micrantha blume extracts in rodents. Phytother Res. 1992;6:146–8. [Google Scholar]
- 35.Chowdhury A, Islam MS. Antibacterial activity of Trema orientalis. Dhaka Univ. J. Pharm. Sci. 2004;3:115–7. [Google Scholar]
- 36.McGaw LJ, Jäger AK, van Staden J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J Ethnopharmacol. 2000;72:247–63. doi: 10.1016/s0378-8741(00)00269-5. [DOI] [PubMed] [Google Scholar]
- 37.Diehl MS, Atindehou KK, Téré H, Betschart B. Prospect for anthelminthic plants in the Ivory Coast using ethnobotanical criteria. J Ethnopharmacol. 2004;95:277–84. doi: 10.1016/j.jep.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Mpiana PT, Ngbolua KN, Mudogo V, Tshibangu DS, Atibu EK, Tshilanda DD, et al. Anti sickle erythrocytes haemolysis properties and inhibitory effect of anthocyanins extracts of Trema orientalis on the aggregation of human deoxyhemoglobin S in vitro. J Med Sci. 2011;11:129–37. [Google Scholar]
- 39.Baillie JK, Thompson AA, Irving JB, Bates MG, Sutherland AI, Macnee W, et al. Oral antioxidant supplementation does not PRevent acute mountain sickness: Double blind, randomized placebo-controlled trial. QJM. 2009;102:341–8. doi: 10.1093/qjmed/hcp026. [DOI] [PubMed] [Google Scholar]
- 40.Salprima YS, Eka A, Sri N, Syalfinaf M, Anggria MS, Fatan U. Iron chelating and antiradical activity of kayu manik leaves (Trema orientalis) Indo J Chem. 2011;11:196–9. [Google Scholar]


