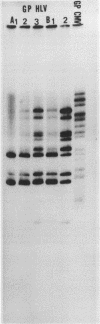
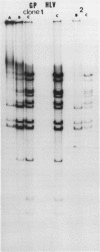
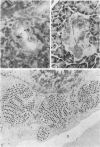
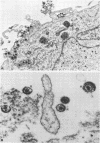
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. K., Jeppesen T. Virus-like particles in guinea pig oogonia and oocytes. J Natl Cancer Inst. 1972 Nov;49(5):1403–1410. [PubMed] [Google Scholar]
- Bhatt P. N., Percy D. H., Craft J. L., Jonas A. M. Isolation and characterization of a herpeslike (Hsiung-Kaplow) virus from guinea pigs. J Infect Dis. 1971 Feb;123(2):178–189. doi: 10.1093/infdis/123.2.178. [DOI] [PubMed] [Google Scholar]
- Bia F. J., Hastings K., Hsiung G. D. Cytomegalovirus infection in guinea pigs. III. Persistent viruria, blood transmission, and viral interference. J Infect Dis. 1979 Dec;140(6):914–920. doi: 10.1093/infdis/140.6.914. [DOI] [PubMed] [Google Scholar]
- Black V. H. Virus particles in primordial germ cells of fetal guinea pigs. J Natl Cancer Inst. 1974 Feb;52(2):545–551. doi: 10.1093/jnci/52.2.545. [DOI] [PubMed] [Google Scholar]
- Booss J., Hsiung G. D. Herpes-like virus of the guinea pig: propagation in brain tissue of guinea pigs and mice. J Infect Dis. 1971 Mar;123(3):284–291. doi: 10.1093/infdis/123.3.284. [DOI] [PubMed] [Google Scholar]
- Chang P. W., Hsiung G. D. Experimental infection of parainfluenza virus type 5 in mice, hamsters and monkeys. J Immunol. 1965 Oct;95(4):591–601. [PubMed] [Google Scholar]
- Choi Y. C., Hsiung G. D. Cytomegalovirus infection in guinea pigs. II. Transplacental and horizontal transmission. J Infect Dis. 1978 Aug;138(2):197–202. doi: 10.1093/infdis/138.2.197. [DOI] [PubMed] [Google Scholar]
- Choi Y. C., Swack N. S., Hsiung G. D. Effect of heparin on cytomegalovirus replication. Proc Soc Exp Biol Med. 1978 Apr;157(4):569–571. doi: 10.3181/00379727-157-40098. [DOI] [PubMed] [Google Scholar]
- Connor W. S., Johnson K. P. Cytomegalovirus infection in weanling guinea pigs. J Infect Dis. 1976 Nov;134(5):442–449. doi: 10.1093/infdis/134.5.442. [DOI] [PubMed] [Google Scholar]
- Craft J. L., Hilding D. A. Virus-like particles in the spiral ganglion of the guinea pig cochlea. Science. 1968 Dec 27;162(3861):1485–1487. doi: 10.1126/science.162.3861.1485. [DOI] [PubMed] [Google Scholar]
- Cuendet J., Bonifas V. Mode (nouveau) d'enveloppement d'un Herpesvirus. Pathol Microbiol (Basel) 1973;39(1):32–34. [PubMed] [Google Scholar]
- Dahlberg J. E., Perk K., Dalton A. J. Virus-like particles induced in guinea pig cells by 5-bromo-2'-deoxyuridine are morphologically similar to murine B-type virus. Nature. 1974 Jun 28;249(460):828–830. doi: 10.1038/249828a0. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P. Expression of endogenous retroviral genes in leukemic guinea pig cells. J Virol. 1977 Aug;23(2):263–271. doi: 10.1128/jvi.23.2.263-271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P., Lofgren J. Induction of endogenous guinea pig retrovirus by 5-bromodeoxyuridine: amplification of virus-specific RNA. J Virol. 1978 Jun;26(3):603–614. doi: 10.1128/jvi.26.3.603-614.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioşi P., Georgescu L. Degeneration of cytomegalovirus-infected cells in lymph nodes of naturally infected guinea-pigs. Rev Roum Morphol Physiol. 1974;20(2):91–94. [PubMed] [Google Scholar]
- ENDERS A. C. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF THE TROPHOBLAST IN SEVERAL HEMOCHORIAL PLACENTAS. Am J Anat. 1965 Jan;116:29–67. doi: 10.1002/aja.1001160103. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., ACHONG B. G., BARR Y. M. VIRUS PARTICLES IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Lancet. 1964 Mar 28;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Feldman D. G., Gross L. Electron microscopic study of the guinea pig leukemia virus. Cancer Res. 1970 Nov;30(11):2702–2711. [PubMed] [Google Scholar]
- Fong C. K., Bia F., Hsiung G. D., Madore P., Chang P. W. Ultrastructural development of guinea pig cytomegalovirus in cultured guinea pig embryo cells. J Gen Virol. 1979 Jan;42(1):127–140. doi: 10.1099/0022-1317-42-1-127. [DOI] [PubMed] [Google Scholar]
- Fong C. K., Bia F., Hsiung G. D. Ultrastructural development and persistence of guinea pig cytomegalovirus in duet cells of guinea pig submaxillary gland. Arch Virol. 1980;64(2):97–108. doi: 10.1007/BF01318013. [DOI] [PubMed] [Google Scholar]
- Fong C. K., Hsiung G. D. In vitro transformation of hamster embryo cells by a guinea pig herpes-like virus. Proc Soc Exp Biol Med. 1973 Dec;144(3):974–978. doi: 10.3181/00379727-144-37723. [DOI] [PubMed] [Google Scholar]
- Fong C. K., Hsiung G. D. Morphogenic studies of herpesvirus and oncornavirus from leukemic and normal guinea pigs. Fed Proc. 1977 Aug;36(9):2320–2327. [PubMed] [Google Scholar]
- Fong C. K., Hsiung G. D. Oncornavirus of guinea pigs. 1. Morphology and distribution in normal and leukemic guinea pig cells. Virology. 1976 Apr;70(2):385–398. doi: 10.1016/0042-6822(76)90280-4. [DOI] [PubMed] [Google Scholar]
- Fong C. K., Hsiung G. D. The interaction between herpesvirus and oncornavirus of guineapigs: in vitro and in vivo studies. IARC Sci Publ. 1978;(24 Pt 2):981–989. [PubMed] [Google Scholar]
- Griffith B. P., Hsiung G. D. Cytomegalovirus infection in guinea pigs. IV. Maternal infection at different stages of gestation. J Infect Dis. 1980 Jun;141(6):787–793. doi: 10.1093/infdis/141.6.787. [DOI] [PubMed] [Google Scholar]
- HARTLEY J. W., ROWE W. P., HUEBNER R. J. Serial propagation of the guinea pig salivary gland virus in tissue culture. Proc Soc Exp Biol Med. 1957 Nov;96(2):281–285. doi: 10.3181/00379727-96-23455. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D., CHANG P. W., CUADRADO R. R., ISACSON P. STUDIES OF PARAINFLUENZA VIRUSES. 3. ANTIBODY RESPONSES OF DIFFERENT ANIMAL SPECIES AFTER IMMUNIZATION. J Immunol. 1965 Jan;94:67–73. [PubMed] [Google Scholar]
- Hampton E. G., Bruce M., Jackson F. L. Virus-like particles in a fibrovascular growth in guinea pigs. J Gen Virol. 1968 Jan;2(1):205–206. doi: 10.1099/0022-1317-2-1-205. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D. Activation of guinea pig C-type virus in cultured spleen cells by 5-bromo-2'-deoxyuridine. J Natl Cancer Inst. 1972 Aug;49(2):567–570. [PubMed] [Google Scholar]
- Hsiung G. D., Choi Y. C., Bia F. Cytomegalovirus infection in guinea pigs. I. Viremia during acute primary and chronic persistent infection. J Infect Dis. 1978 Aug;138(2):191–196. doi: 10.1093/infdis/138.2.191. [DOI] [PubMed] [Google Scholar]
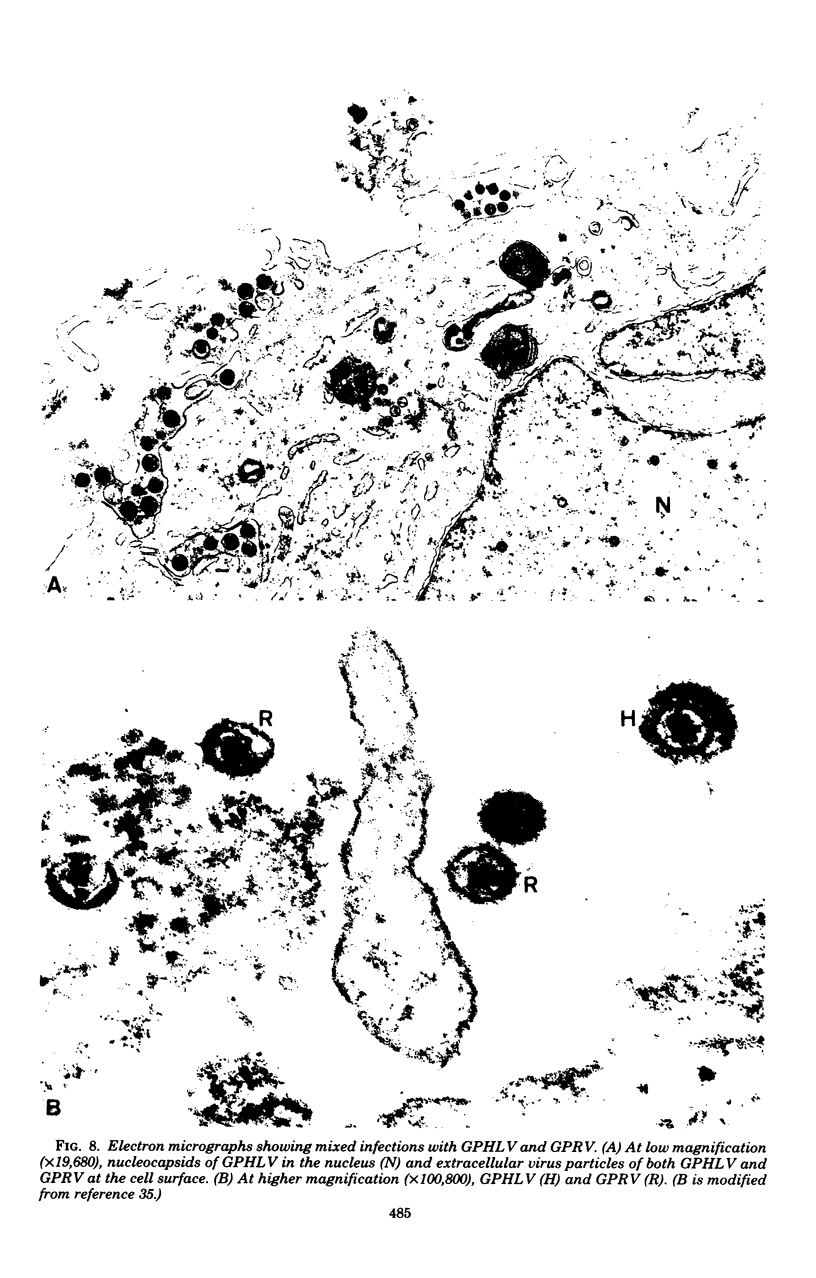
- Hsiung G. D., Fong C. K. Dual infection with endogenous herpes- and C-type viruses in cultured cells derived from normal and leukemic guinea pigs. Proc Soc Exp Biol Med. 1974 Dec;147(3):635–639. doi: 10.3181/00379727-147-38404. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Fong C. K., Evans C. H. Prevalence of endogenous oncornavirus in guinea pigs. Intervirology. 1974;3(5-6):319–331. doi: 10.1159/000149769. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Fong C. R., Lam K. M. Guinea pig leukocytes: in vivo and in vitro infection with a herpes-like virus. J Immunol. 1971 Jan;106(6):1686–1689. [PubMed] [Google Scholar]
- Hsiung G. D., Kaplow L. S., Booss J. Herpesvirus infection of guinea pigs. I. Isolation, characterization and pathogenicity. Am J Epidemiol. 1971 Apr;93(4):298–307. doi: 10.1093/oxfordjournals.aje.a121261. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D., Kaplow L. S. Herpeslike virus isolated from spontaneously degenerated tissue culture derived from leukemia-susceptible guinea pigs. J Virol. 1969 Mar;3(3):355–357. doi: 10.1128/jvi.3.3.355-357.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D. Latent virus infections in primate tissues with special reference to simian viruses. Bacteriol Rev. 1968 Sep;32(3):185–205. doi: 10.1128/br.32.3.185-205.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D., McTighe A. H. An animal model for DNA-RNA virus interaction based upon virological and histological findings. Cancer Res. 1976 Feb;36(2 Pt 2):674–677. [PubMed] [Google Scholar]
- Hsiung G. D. Parainfluenza-5 virus. Infection of man and animal. Prog Med Virol. 1972;14:241–274. [PubMed] [Google Scholar]
- Hsiung G. D., Tenser R. B., Fong C. K. Comparison of guinea pig cytomegalovirus and guinea pig herpes-like virus: growth characteristics and antigentic relationship. Infect Immun. 1976 Mar;13(3):926–933. doi: 10.1128/iai.13.3.926-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D. Virological studies of guinea pig leukemia: an overview with reference to herpesvirus and oncornavirus. Fed Proc. 1977 Aug;36(9):2285–2289. [PubMed] [Google Scholar]
- Hsiung G. Natural history of herpes and C-type virus infections and their possible relation to viral oncogenesis. An animal model. Prog Med Virol. 1975;21:58–71. [PubMed] [Google Scholar]
- Huang A. S., Palma E. L., Hewlett N., Roizman B. Pseudotype formation between enveloped RNA and DNA viruses. Nature. 1974 Dec 20;252(5485):743–745. doi: 10.1038/252743a0. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Connor W. S. Guinea pig cytomegalovirus: transplacental transmission. Brief report. Arch Virol. 1979;59(3):263–267. doi: 10.1007/BF01317422. [DOI] [PubMed] [Google Scholar]
- Kumar M. L., Nankervis G. A. Experimental congenital infection with cytomegalovirus: a guinea pig model. J Infect Dis. 1978 Nov;138(5):650–654. doi: 10.1093/infdis/138.5.650. [DOI] [PubMed] [Google Scholar]
- Lam K. M., Hsiung G. D. Guinea pig herpes-like virus infections. II. Antibody response and virus persistence in mice and rabbits. Infect Immun. 1973 Mar;7(3):432–437. doi: 10.1128/iai.7.3.432-437.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K. M., Hsiung G. D. Herpesvirus infection of guinea pigs. II. Transplacental transmission. Am J Epidemiol. 1971 Apr;93(4):308–313. doi: 10.1093/oxfordjournals.aje.a121262. [DOI] [PubMed] [Google Scholar]
- Ma B. I., Swartzendruber D. C., Murphy W. H. Detection of virus-like particles in germinal centers of normal guinea pigs. Proc Soc Exp Biol Med. 1969 Feb;130(2):586–590. doi: 10.3181/00379727-130-33613. [DOI] [PubMed] [Google Scholar]
- Michalides R., Schlom J., Dahlberg J., Perk K. Biochemical properties of the bromodeoxyuridine-induced guinea pig virus. J Virol. 1975 Oct;16(4):1039–1050. doi: 10.1128/jvi.16.4.1039-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski F. J., Fong C. K., Hsiung G. D., Schneider R. D. Induction of tumors by a guinea pig herpesvirus-transformed hamster cell line. J Natl Cancer Inst. 1976 Jun;56(6):1165–1170. doi: 10.1093/jnci/56.6.1165. [DOI] [PubMed] [Google Scholar]
- Middelkamp J. N., Patrizi G., Reed C. A. Light and electron microscopic studies of the guinea pig cytomegalovirus. J Ultrastruct Res. 1967 Apr;18(1):85–101. doi: 10.1016/s0022-5320(67)80233-8. [DOI] [PubMed] [Google Scholar]
- Murray P. R., Nayak D. P. Characterization of bromodeoxyuridine-induced endogenous guinea pig virus. J Virol. 1974 Sep;14(3):679–688. doi: 10.1128/jvi.14.3.679-688.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel E., Banfield W., Burstein S., Tousimis A. J. Virus particles associated with strain 2 guinea pig leukemia (L2C/N-B). J Natl Cancer Inst. 1967 Jun;38(6):979–981. [PubMed] [Google Scholar]
- Nayak D. P. Endogenous guinea pig virus: equability of virus-specific DNA in normal, leukemic, and virus-producing cells. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1164–1168. doi: 10.1073/pnas.71.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P. Isolation and characterization of a herpesvirus from leukemic guinea pigs. J Virol. 1971 Oct;8(4):579–588. doi: 10.1128/jvi.8.4.579-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Murray P. R. Induction of type C viruses in cultured guinea pig cells. J Virol. 1973 Jul;12(1):177–187. doi: 10.1128/jvi.12.1.177-187.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opler S. R. Observations on a new virus associated with guinea pig leukemia: preliminary note. J Natl Cancer Inst. 1967 May;38(5):797–800. [PubMed] [Google Scholar]
- Putman D. L., Rhim J. S. Characterization of bromodeoxyuridine-induced guinea pig type B retravirus. Fed Proc. 1977 Aug;36(9):2316–2319. [PubMed] [Google Scholar]
- Rhim J. S., Duh F. G., Cho H. Y., Wuu K. D., Vernon M. L. Brief communication: Activation by 5-bromo-2'-deoxyuridine of particles resembling guinea-pig leukemia virus from guinea-pig nonproducer cells. J Natl Cancer Inst. 1973 Oct;51(4):1327–1331. doi: 10.1093/jnci/51.4.1327. [DOI] [PubMed] [Google Scholar]
- Rhim J. S. Malignant transformation of rat embryo cells by a herpesvirus isolated from L2C guinea pig leukemia. Virology. 1977 Oct 1;82(1):100–110. doi: 10.1016/0042-6822(77)90036-8. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Wuu K. D., Ro H. S., Vernon M. L., Huebner R. J. Induction of guinea pig leukemia-like virus from cultured guinea pigs cells. Proc Soc Exp Biol Med. 1974 Oct;147(1):323–330. doi: 10.3181/00379727-147-38335. [DOI] [PubMed] [Google Scholar]
- SCHULTZ E. W., HABEL K. SA virus; a new member of the myxovirus group. J Immunol. 1959 Mar;82(3):274–278. [PubMed] [Google Scholar]
- Schlom J., Michalides R., Perk K., Pearson J., Dahlberg J. Biochemical properties of the B-type retravirus of guinea pigs and an agent in the plasma of guinea pigs with L2C leukemia. Fed Proc. 1977 Aug;36(9):2310–2315. [PubMed] [Google Scholar]
- Tenser R. B., Hsiung G. D. Comparison of guinea pig cytomegalovirus and guinea pig herpes-like virus: pathogenesis and persistence in experimentally infected animals. Infect Immun. 1976 Mar;13(3):934–940. doi: 10.1128/iai.13.3.934-940.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Hsiung G. D. Oncornavirus particles in neurons of guinea pig trigeminal ganglion. J Neuropathol Exp Neurol. 1978 Sep;37(5):508–517. doi: 10.1097/00005072-197809000-00006. [DOI] [PubMed] [Google Scholar]
- VONEULER L., KANTOR F. S., HSIUNG G. D. STUDIES OF PARAINFLUENZA VIRUSES. I. CLINICAL, PATHOLOGICAL AND VIROLOGICAL OBSERVATIONS. Yale J Biol Med. 1963 Jun;35:523–533. [PMC free article] [PubMed] [Google Scholar]
- Závada J. Pseudotypes of vesicular stomatitis virus with the coat of murine leukaemia and of avian myeloblastosis viruses. J Gen Virol. 1972 Jun;15(3):183–191. doi: 10.1099/0022-1317-15-3-183. [DOI] [PubMed] [Google Scholar]