Summary
Background and objectives
Although patients with ESRD have a higher fracture risk than the general population, there is conflicting evidence regarding fracture incidence in those with CKD. This study sought to determine the association between estimated GFR (eGFR) and fracture rates.
Design, setting, participants, & measurements
This study identified 1,815,943 community-dwelling adults who had at least one outpatient serum creatinine measurement between 2002 and 2008. Patients with eGFR <15 ml/min per 1.73 m2 and those who required dialysis were excluded. Incident fractures of the hip, wrist, and vertebrae were identified using diagnostic and procedure codes. Poisson regression was used to determine adjusted rates of each fracture type by eGFR, age, and sex.
Results
The median age of the cohort was 47 years (interquartile range, 24), and 7.1% had eGFR <60 ml/min per 1.73 m2. Over a median follow-up of 4.4 years, fracture rates increased with age at all sites. Within each age stratum, unadjusted rates increased with declining eGFR; however, adjusted rates were similar across eGFR categories. For example, among women aged 65–74 years, adjusted hip fracture rates were 3.41 per 1000 person-years (95% confidence interval, 2.30 to 4.53) and 4.58 per 1000 person-years (95% confidence interval, 0.02 to 9.14) in those with eGFR ≥90 and 15–29 ml/min per 1.73 m2, respectively. Similar results were observed for wrist and vertebral fractures.
Conclusions
In contrast to earlier studies, patients with eGFR<60 ml/min per 1.73 m2 do not appear to have increased rates of hip, wrist, and vertebral fractures independent of age and sex.
Introduction
Fractures are common with advancing age in the general population and result in excess morbidity, mortality, and healthcare costs (1). Among patients with dialysis-dependent ESRD, fractures occur approximately four times more frequently, and at an earlier age, than in the general population (2–5). The increased fracture risk in patients with ESRD has been associated with both risk factors for fracture common to the general population (advanced age, female sex, and lower body mass index) (3,5–7) as well as features specific to CKD (CKD–mineral and bone disorders) (2,8–10).
It is less clear whether individuals with nondialysis CKD have an increased risk of fractures. Although several abnormalities of mineral metabolism, including vitamin D deficiency, hyperphosphatemia, and secondary hyperparathyroidism, are common at earlier stages of CKD (11,12), prior studies have noted conflicting results with respect to an association between nondialysis CKD and fractures (13–16). Although some have identified increased fracture risk among those with lower estimated GFR (eGFR), existing studies vary with respect to included populations, definitions of fracture sites and mechanisms, determination and classification of eGFR, and consideration of potential effect modification by patient age and sex.
Given the adverse health consequences and economic burden associated with fractures (17,18), identifying whether earlier stages of CKD increase the risk for fractures would be important for designing future interventions. Therefore, we sought to determine the association between eGFR and rates of fractures of the hip, wrist, and vertebrae among adult residents of Alberta, Canada. We hypothesized that lower levels of eGFR would be independently associated with increased rates of fractures for these three sites.
Materials and Methods
Study Design and Population
We used a population-based cohort that included participants aged ≥18 years identified from the Alberta Kidney Disease Network, a provincial laboratory repository from Alberta, Canada (19). Eligible individuals had at least one outpatient serum creatinine measurement performed in the province of Alberta from May 1, 2002 to March 31, 2008 for seven of the nine geographically based provincial health regions, and from July 1, 2003 to March 31, 2008 and January 1, 2005 to March 31, 2008, respectively, for the other two regions. The index date was the date of each participant’s first outpatient creatinine measurement. Patients were excluded if, at baseline, they had ESRD requiring renal replacement therapy (dialysis or renal transplantation) or eGFR <15 ml/min per 1.73 m2.
Measurement of Kidney Function
Serum creatinine measurements were obtained from provincial laboratories, with measurements standardized across laboratories and traceable to an isotope dilution mass spectrometry reference standard as previously described (19). Consistent with prior studies by our group (20,21), the index serum creatinine measurement was used to estimate renal function (eGFR) according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. The CKD-EPI equation has been validated in populations with and without kidney disease and has been found to more accurately estimate renal function, particularly at higher GFR (22). Patients were categorized by level of eGFR as ≥90, 60–89, 45–59, 30–44, and 15–29 ml/min per 1.73 m2.
Measurement of Covariates
The study cohort was linked to administrative data files from the provincial health ministry (Alberta Health) to define demographic variables and comorbidities. Diabetes mellitus, hypertension, and history of a kidney stone episode in the 3 years before cohort entry were identified from physician claims and hospital discharge records based on validated algorithms (23–25). Other comorbidities defined in the Charlson comorbidity index were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision (ICD-10) coding algorithms applied to physician claims and hospitalization data (26). The presence of one or more diagnostic codes in any position up to 3 years before cohort entry was used for identification of comorbidities. The six-digit residential postal code for each participant was linked to the Canadian Census (2001 or 2006, the closest year before the index date) using the postal code conversion file to determine rural or urban location of residence and median household income quintile.
Proteinuria was measured by urine dipstick on outpatient random spot urine samples. Urine dipstick results were categorized as normal (negative), mild (trace or 1+), or heavy (≥2+) proteinuria. Proteinuria for patients without urine dipstick measurements was classified as “unmeasured.” All outpatient urine dipstick measurements in the 6-month periods before and after the index eGFR were used to estimate baseline proteinuria.
Outcomes
We considered three outcomes: hip, wrist, and vertebral fractures. We specified hip fracture as the primary outcome given its validity and widespread application in studies of fragility fractures, whereas we considered wrist and vertebral fractures as secondary outcomes. Validated case definitions for fracture at each site were applied to administrative data according to ICD-9-CM and ICD-10 coding algorithms of physician billing claims and/or hospitalization records, with or without inclusion of physician procedure codes for fracture repair or immobilization as previously described (Supplemental Table 1) (27). These fracture case definitions from administrative data have been validated against population-based clinically validated fractures. Because the objective of our study was to determine the association between eGFR and fracture rates, and due to the difficulty in determining fracture cause from administrative data, our primary outcome was fracture irrespective of the mechanism. In a sensitivity analysis, we excluded traumatic fractures from transport accidents based on ICD-10 codes (V01–V94) from the hospitalization file. Fractures were defined as incident using a 12-month washout period (no fracture of the same type during the preceding 12 months). Only the first fracture was considered for each patient.
Statistical Analyses
Baseline characteristics of the cohort by eGFR categories (≥90, 60–89, 45–59, 30–44, and 15–29 ml/min per 1.73 m2) were presented as medians and interquartile ranges for continuous variables and percentages for categorical variables. We hypothesized that lower levels of eGFR would be associated with fractures of the hip, wrist, and vertebrae. To test this, we used Poisson regression models to estimate the rates of hip, wrist, and vertebral fractures separately within each eGFR stratum. If the Poisson assumption that the outcome variance equals its mean was not met, a quasi-Poisson model was used. Given the well established variation in the rates of fracture for women and with increasing age, rates were stratified by age (<65, 65–74, 75–84, and ≥85 years) and sex. Rates were adjusted for sociodemographic characteristics, diabetes, hypertension, kidney stone episode, and Charlson comorbidities. We also tested for a U-shaped trend among each age and sex category for the three fracture sites by including a quadratic eGFR term in the models. Participants were followed from their index date (i.e., first available eGFR) until development of the outcome of interest or until study end on March 31, 2009. Patients were censored on the date of initiation of dialysis therapy or kidney transplantation, emigration outside of Alberta (identified from the Alberta Population Registry database), or death (identified from the Alberta Vital Statistics Registry). In a sensitivity analysis, we excluded traumatic fractures from transport accidents.
A significance level α of 0.05 was used on all statistical tests. Statistical analyses were conducted using R software (The R Project for Statistical Computing; www.r-project.org). Ethics approval for the study was obtained from the conjoint health research ethics board of the University of Calgary.
Results
Cohort Formation and Characteristics
We identified 1,823,430 patients who had at least one serum creatinine measurement during the study period. We excluded 3168 patients who required renal replacement therapy before study entry, and 1615 patients who had eGFR <15 ml/min per 1.73 m2. In addition, we excluded 1811 patients because they either died on their index date or had emigrated from Alberta and 893 patients who experienced a hip, wrist, or vertebral fracture on their index date. Therefore, the final study cohort included 1,815,943 adults (median follow-up of 4.4 years for each fracture outcome).
Baseline characteristics of the participants by eGFR category are listed in Table 1. The median age of the cohort was 47 years (interquartile range, 24), and 55.7% were women. A total of 128,957 patients (7.1%) had baseline eGFR <60 ml/min per 1.73 m2. Those with eGFR <60 ml/min per 1.73 m2 were older and had a higher prevalence of comorbidities and dipstick-positive proteinuria compared with those with normal renal function.
Table 1.
Baseline characteristics of study cohort by eGFR
| Characteristic | eGFR (ml/min per 1.73 m2) | ||||
|---|---|---|---|---|---|
| ≥90 | 60–89 | 45–59 | 30–44 | 15–29 | |
| Number of patients (%) | 1,005,777 (55.4) | 681,209 (37.5) | 88,165 (4.9) | 31,345 (1.7) | 9447 (0.5) |
| Age (yr), median (IQR) | 39 (19) | 55 (20) | 73 (16) | 79 (14) | 80 (15) |
| Female sex | 58.1 | 51.4 | 58.3 | 61.6 | 59.7 |
| Diabetes mellitus | 4.9 | 8.3 | 17.1 | 24.4 | 31.6 |
| Hypertension | 11.9 | 31.2 | 63.7 | 79.4 | 83.4 |
| Cerebrovascular disease | 0.9 | 2.8 | 8.3 | 13.0 | 15.9 |
| Peripheral vascular disease | 0.5 | 1.7 | 5.2 | 9.1 | 12.9 |
| Congestive heart failure | 0.6 | 2.6 | 11.0 | 22.0 | 33.9 |
| Myocardial infarction | 0.8 | 2.7 | 7.4 | 12.4 | 17.6 |
| COPD | 12.6 | 14.1 | 20.0 | 25.1 | 29.1 |
| Dementia | 0.2 | 1.5 | 5.7 | 9.8 | 12.4 |
| Mild liver disease | 0.9 | 0.8 | 1.0 | 1.4 | 1.9 |
| Moderate-severe liver disease | 0.1 | 0.1 | 0.2 | 0.4 | 0.6 |
| Rheumatic disease | 0.9 | 1.4 | 2.7 | 3.4 | 3.8 |
| Peptic ulcer disease | 1.8 | 2.2 | 3.7 | 4.7 | 5.9 |
| Episode of kidney stones | 1.0 | 1.1 | 1.4 | 1.6 | 1.9 |
| Any malignancy (except skin) | 2.4 | 5.0 | 9.5 | 11.9 | 12.8 |
| Metastatic solid tumor | 0.4 | 0.6 | 1.2 | 1.7 | 2.2 |
| Hemiplegia or paraplegia | 0.4 | 0.4 | 0.9 | 1.3 | 1.9 |
| HIV/AIDS | 0.1 | 0.03 | 0.03 | 0.02 | 0.02 |
| Location of residence | |||||
| Urban | 80.9 | 78.8 | 77.1 | 76.7 | 76.0 |
| Rural | 17.5 | 20.1 | 21.9 | 22.2 | 23.0 |
| Unknown | 1.6 | 1.1 | 1.0 | 1.1 | 1.1 |
| Income quintile | |||||
| 1 (lowest) | 19.2 | 15.6 | 18.2 | 19.8 | 21.1 |
| 2 | 18.4 | 17.1 | 18.4 | 19.6 | 19.9 |
| 3 | 17.9 | 18.3 | 18.9 | 18.9 | 18.5 |
| 4 | 19.9 | 20.9 | 18.9 | 17.9 | 16.1 |
| 5 (highest) | 19.0 | 22.3 | 19.1 | 16.5 | 16.7 |
| Unknown | 5.8 | 5.8 | 6.6 | 7.3 | 7.8 |
| Proteinuria | |||||
| Normal | 57.6 | 56.2 | 46.1 | 35.7 | 25.7 |
| Mild | 4.1 | 4.3 | 7.0 | 9.2 | 12.8 |
| Heavy | 0.5 | 0.7 | 2.0 | 4.4 | 9.7 |
| Unmeasured | 37.8 | 38.9 | 45.0 | 50.7 | 51.8 |
All results are expressed as percentages unless otherwise specified. eGFR, estimated GFR; IQR, interquartile range; COPD, chronic obstructive pulmonary disease.
Hip Fracture
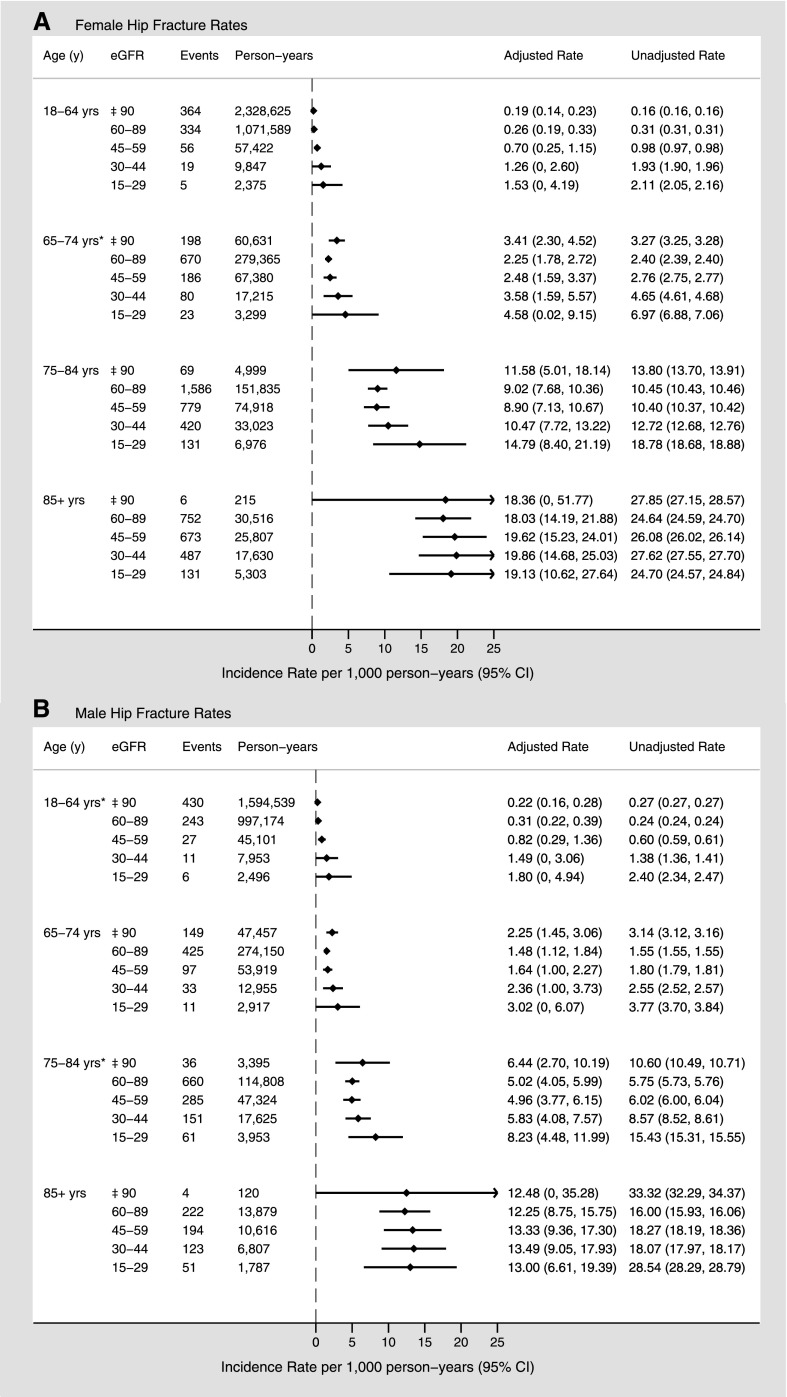
During the follow-up period, fracture of the hip occurred in 10,188 patients (0.6%). In women, the adjusted rates of hip fracture increased with increasing age for each eGFR stratum (Figure 1A). At eGFR 60–89 ml/min per 1.73 m2, adjusted rates of hip fracture (presented with 95% confidence intervals [95% CIs]) were approximately 70-fold higher for women aged ≥85 years compared with those aged 18–64 years (18.05 [95% CI, 14.2 to 21.9] versus 0.26 [95% CI, 0.19 to 0.33] per 1000 person-years, respectively). Within each age and sex stratum, unadjusted hip fracture rates increased slightly with declining eGFR. However, adjusted hip fracture rates were similar across eGFR categories, with a slight increase at lower eGFR levels, particularly for the 18–64 age stratum. Among women aged 65–74 years, the adjusted hip fracture rate (per 1000 person-years) was 2.25 (95% CI, 1.78 to 2.72) for eGFR 60–89 ml/min per 1.73 m2 and 4.58 (95% CI, 0.02 to 9.14) for eGFR 15–29 ml/min per 1.73 m2. Similar trends were evident for men (Figure 1B), although in general the rates were lower for each age and eGFR stratum compared with women. A statistically significant U-shaped trend (P<0.05) was observed for hip fracture rates only among women aged 65–74 years and men aged 18–64 and 75–85 years.
Figure 1.
Sex-specific hip fracture rates (per 1000 person-years) by age and eGFR. Overall fracture rates for women (A) and men (B). Adjusted for sociodemographic characteristics, diabetes, hypertension, Charlson comorbidities, and episode of kidney stones. *P<0.05 for U-shaped trend.
Wrist Fracture
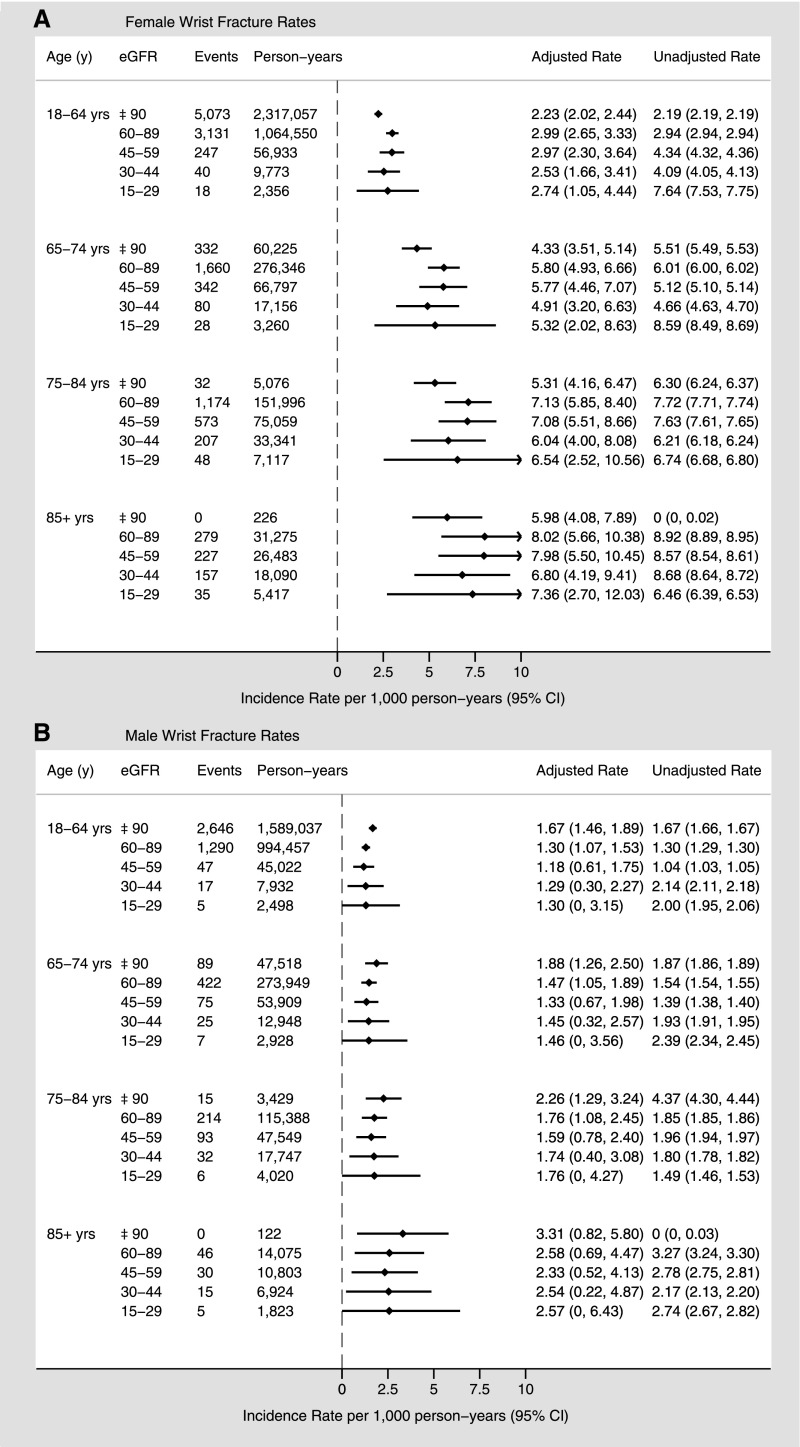
During follow-up, a total of 18,762 patients (1.0%) experienced a wrist fracture. A similar trend as seen in hip fractures was evident for wrist fractures, with increasing adjusted rates with advancing age. As for hip fractures, unadjusted wrist fracture rates increased slightly at lower eGFR, particularly in women in the younger age categories. Adjusted wrist fracture rates, however, were similar across eGFR categories within each age stratum (Figure 2). For women aged >85 years, adjusted rates of wrist fracture (per 1000 person-years) were 8.01 (95% CI, 5.65 to 10.37) and 7.36 (95% CI, 2.70 to 12.01) in those with eGFR 60–89 ml/min per 1.73 m2 and 15–29 ml/min per 1.73 m2, respectively. Men experienced a similar trend in wrist fracture rates, although the increase in rates with age was less pronounced and the overall rates were lower than for women in each age group.
Figure 2.
Sex-specific wrist fracture rates (per 1000 person-years) by age and eGFR. Overall fracture rates for women (A) and men (B). Adjusted for sociodemographic characteristics, diabetes, hypertension, Charlson comorbidities, and episode of kidney stones.
Vertebral Fracture
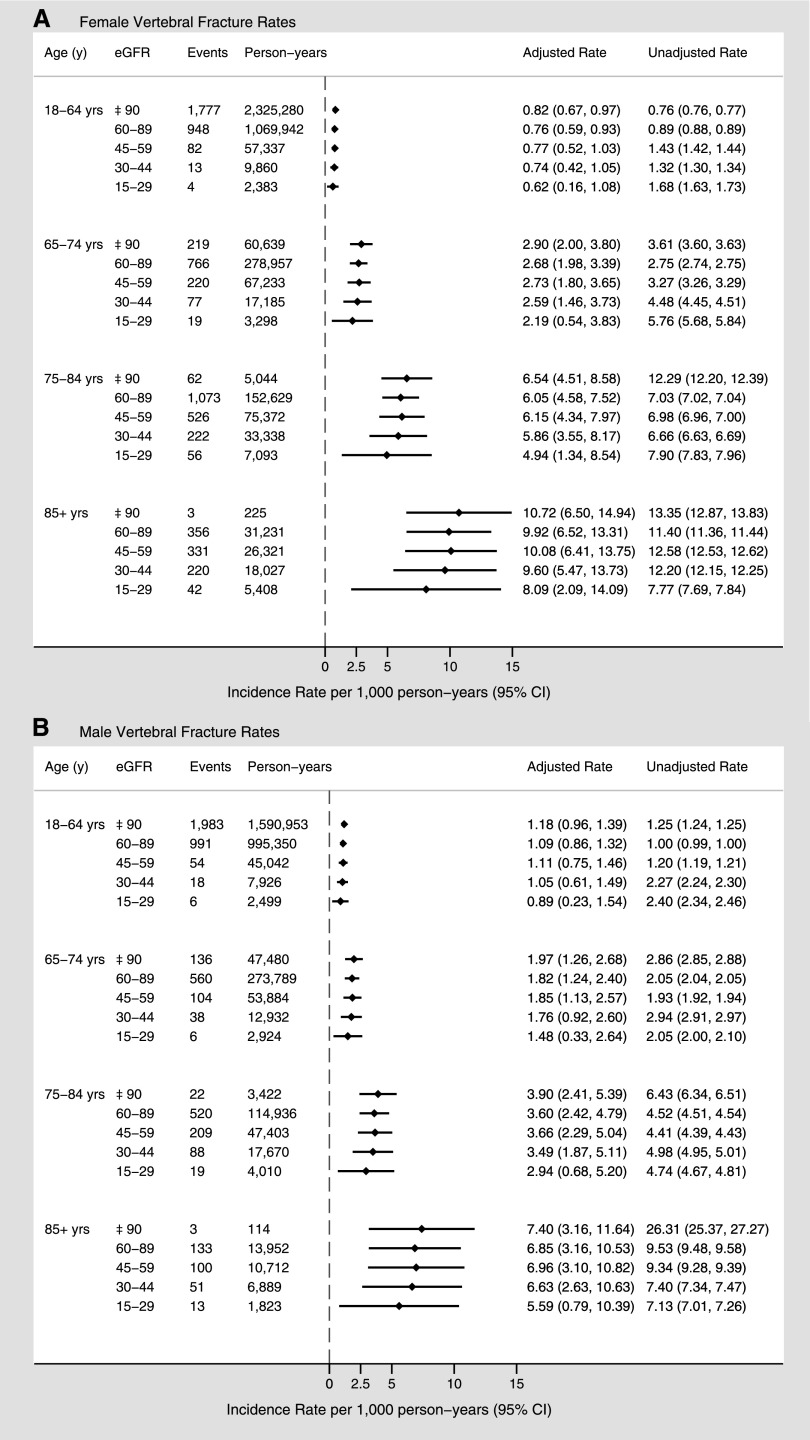
Overall, 12,070 patients (0.7%) experienced a vertebral fracture. The rates of vertebral fractures were higher in the higher age categories in both men and women, but again there was no apparent increase in unadjusted and adjusted rates across eGFR categories within each age group (Figure 3). In women, vertebral fracture rates ranged from 8.1 to 10.7 per 1000 person-years in the group aged >85 years, and 2.2–2.9 per 1000 person-years in the group aged 65–74 years. In general, the vertebral fracture rates were lower in men than in women of a similar age. For men in the same age categories, fracture rates ranged from 5.6 to 7.4 per 1000 person-years and from 1.5 to 2.0 per 1000 person-years, respectively.
Figure 3.
Sex-specific vertebral fracture rates (per 1000 person-years) by age and eGFR. Overall fracture rates for women (A) and men (B). Adjusted for sociodemographic characteristics, diabetes, hypertension, Charlson comorbidities, and episode of kidney stones.
Overall Fracture Rates
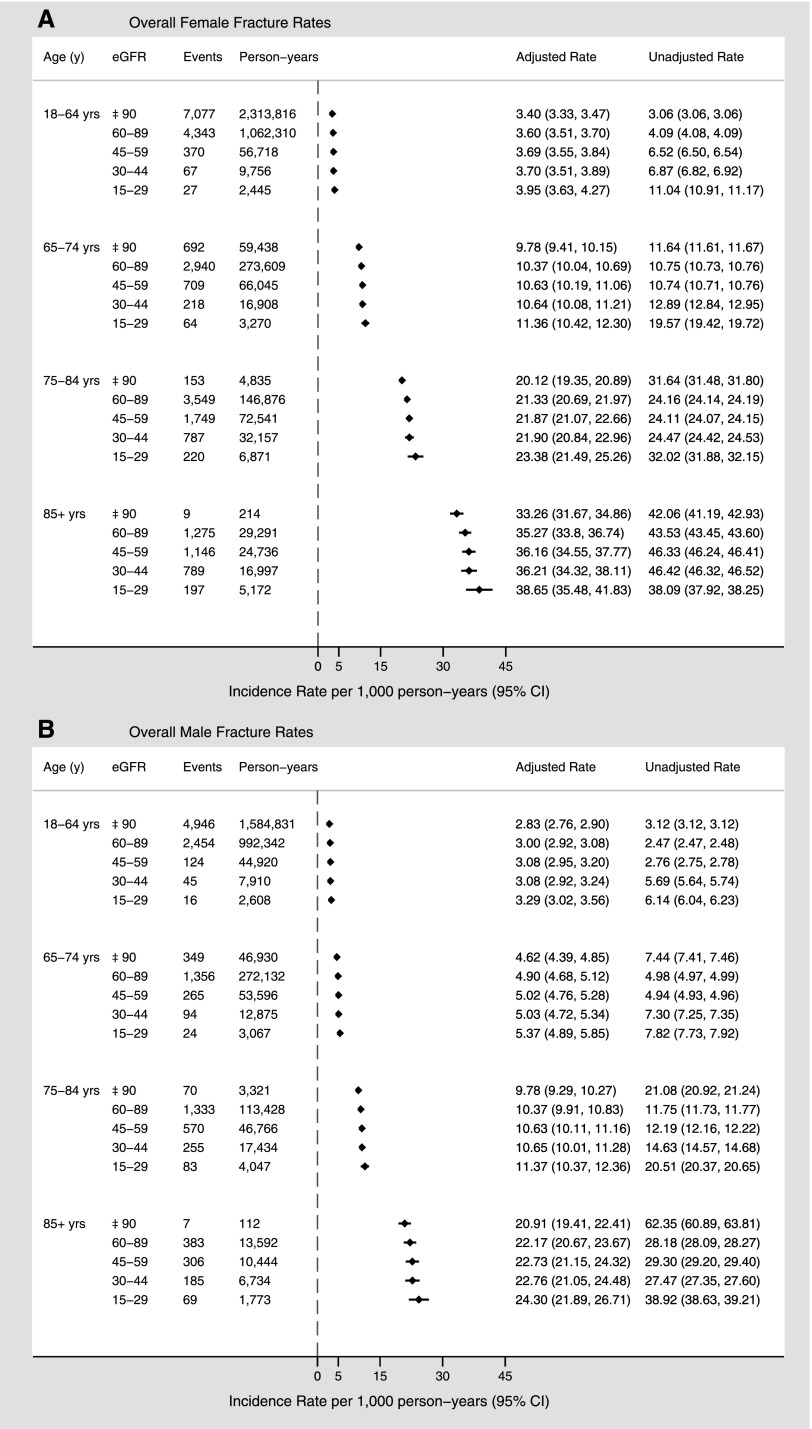
Crude and adjusted fracture rates for all three sites combined are depicted in Figure 4. As with fracture rates assessed at each site individually, the overall adjusted rates increased with advancing age in both sexes but were higher in women than in men. Within each age category, fracture rates were similar across eGFR levels.
Figure 4.
Sex-specific overall fracture rates (per 1000 person-years) by age and eGFR. Overall fracture rates for women (A) and men (B). Adjusted for sociodemographic characteristics, diabetes, hypertension, Charlson comorbidities, and episode of kidney stones.
Sensitivity Analyses
We obtained similar results in a sensitivity analysis, which excluded 262 hip, 449 wrist, and 939 vertebrae fractures from trauma due to transport accidents.
Discussion
In this community-based cohort of >1.8 million adults, we found that reduced eGFR was not associated with increased adjusted rates of incident hip, wrist, and vertebral fractures compared with normal renal function. The fracture rates varied by age and sex for all three fracture sites, which highlights the importance of considering both age and sex in studies of fracture risk.
Why was reduced eGFR not associated with increased fracture risk, in contrast to the excess fracture risk observed in patients with ESRD? The increased fracture risk among ESRD patients may relate to the more advanced underlying metabolic bone disease (CKD–mineral and bone disorder) observed in this population than in those with earlier stages of CKD (12,28). Of note, most of our patients had eGFR ≥30 ml/min per 1.73 m2, and although we did not have access to measures of mineral metabolism, other studies would suggest that even nondialysis patients with eGFR <30 ml/min per 1.73 m2 may not develop disordered mineral metabolism (29,30). In addition, a significant proportion of ESRD patients meet criteria for frailty (31), which could predispose these patients to a higher risk of falls and fall-related morbidity including fractures than in the general population of a similar age (32). Although our unadjusted fracture rates suggest increased fracture risk (particularly at the hip) with declining eGFR, these results were attenuated after adjustment for additional demographic variables and comorbidities. These findings suggest common underlying risk factors in addition to age and sex in those with reduced eGFR (particularly in the larger population of CKD patients with eGFR ≥30 ml/min per 1.73 m2) and the general population, rather than unique pathophysiology as seen in patients with ESRD. However, because we did not have bone biopsy data or other measures of mineral metabolism from patients, this remains speculative (33). A recent prospective study that included patients with earlier stages of CKD (83% had an eGFR between 45 and 59.9 ml/min per 1.73 m2) further supports these conclusions (34). This study demonstrated that CKD patients with osteoporosis as defined by bone density criteria had a 2-fold higher risk of nonspine fragility fracture than those with normal bone density, which was similar to the risk in osteoporotic non-CKD patients.
Our results differ from existing studies (15,16,35–37), which suggested an increased fracture risk among patients with CKD. A nested case-cohort study of 9704 women aged ≥65 years reported an increasing risk of hip but not vertebral fractures with declining GFR (estimated using the Cockcroft–Gault equation), which was highest with eGFR <45 ml/min per 1.73 m2 after adjustment for age, weight, and bone mineral density (P for trend = 0.02) (16). However, there was no increase in risk of either hip or vertebral fractures when eGFR was estimated using the Modified Diet in Renal Disease (MDRD) study equation, and it is unclear whether patients with ESRD were excluded from this study. A study of 33,091 male veterans aged 50–90 years reported a nearly 4-fold higher risk of hip fracture among men with stage 4 CKD compared with men with normal renal function after multivariable adjustment (hazard ratio, 3.65 [95% CI, 1.87 to 7.13]), with no increased risk of hip fracture among those with stage 3 CKD (36). This study, however, was limited because its main findings were based on 11 fracture events in people with stage 4 CKD.
These and other previous studies reporting fracture rates in patients with CKD vary with respect to reporting of fracture site and mechanism (i.e., traumatic versus nontraumatic), estimation and classification eGFR, and consideration of age and sex as potential effect modifiers, which may explain some of the observed differences in fracture risk (13,15,16,35,37). In comparison to earlier studies, we examined fracture incidence at three distinct sites within a large, diverse cohort inclusive of both sexes and over a wide range of adult ages and eGFRs. We stratified fracture rates at the three sites by age and sex given the potential for effect modification of these variables. Furthermore, we estimated GFR based on index serum creatinine according to the CKD-EPI equation. Although this equation performs similarly to the MDRD formula at lower eGFR, it is more accurate in determining eGFR at higher levels (22). Interestingly, our results suggest that patients with eGFR >90 ml/min per 1.73 m2 may have slightly higher rates of fracture than those with eGFR in lower ranges. This is particularly evident among the unadjusted hip fracture rates in the older age groups, with tests for U-shaped trends being statistically significant only among adjusted hip fracture rates in select age categories. Similar findings were previously described in association with other conditions including pneumonia and pneumonia-related complications (21) and may relate to overestimation of eGFR in patients with low muscle mass and chronic illness, both of which are known risk factors for falls and fractures. Despite the lack of a clearly demonstrated association between eGFR and fracture rates, our findings demonstrate both consistency and strength of association between fracture incidence and increasing age.
Our study has limitations that need to be considered, primarily related to its observational design. First, our cohort was limited to adults who had at least one serum creatinine measured as part of their medical care in Alberta (with baseline kidney function defined by their first measurement) and as such may include a greater proportion of adults with underlying comorbidity. Second, despite adjustment for a number of clinical and demographic covariates, we cannot eliminate the possibility of residual confounding by unmeasured variables that could affect fracture risk. These include, for example, menopausal status, patient weight, laboratory measures of mineral metabolism, and concurrent medication use. We were able to account for menopausal status in part through stratification by age and sex, and further adjustment for mineral metabolism parameters and medications is unlikely to negate the findings of this study. Third, because we used administrative data to identify fractures, there is potential for misclassification of fracture outcomes, particularly with respect to mechanism (i.e., traumatic versus nontraumatic). Because many vertebral fractures remain clinically undetected, reliable database coding of such fractures can be especially problematic in observational studies (38). However, we used validated case definitions to identify our outcomes (Supplemental Table 1) (27), and our primary analysis included all fractures, irrespective of mechanism. Furthermore, our sensitivity analysis, which excluded traumatic fractures from transport accidents, produced similar results and supports the validity of our findings. Finally, the median follow-up of 4.4 years may have been inadequate for the observed outcome of incident fractures. However, existing studies on incident fractures in CKD patients, including those demonstrating increased risk, have similar follow-up periods.
Future studies are needed to clarify the association between GFR and fracture risk, with consideration given to using measured GFR to categorize renal function given the limitations of serum creatinine-based GFR estimation equations. Further understanding of the role of underlying mineral bone disease in modifying this risk will also be important in individualizing targeted therapies for patients with CKD and fractures. One such study, Fracture Risk Assessment in Chronic Kidney Disease, Prospective Testing Under Real World Environments, is currently underway (39). The results of this study, which aims to prospectively characterize prognostic factors for bone loss in patients with stages 3–5 CKD, including assessment of bone density and turnover via imaging modalities and bone biopsy, will greatly enhance our understanding of CKD and fracture risk.
In summary, we found that lower levels of eGFR were not associated with increased rates of fractures of the hip, wrist, and vertebrae relative to normal renal function. Rather, our findings suggest that sex and factors associated with aging have more important effects on fracture rates than lower levels of renal function.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by an interdisciplinary team grant from Alberta Innovates–Health Solutions (AI-HS). M.T. and B.J.M. were supported by career salary awards from AI-HS. B.R.H. was supported by the Roy and Vi Baay Chair in Kidney Research. This study is based in part by data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta.
B.R.H. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09130912/-/DCSupplemental.
See related editorial, “Fracture Risk in CKD,” on pages 1282–1283.
References
- 1.Johnell O, Kanis J: Epidemiology of osteoporotic fractures. Osteoporos Int 16[Suppl 2]: S3–S7, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Coco M, Rush H: Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 36: 1115–1121, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, Kestenbaum BR, Stehman-Breen C: Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288: 3014–3018, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C: Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58: 396–399, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz K-G, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Kaneko TM, Foley RN, Gilbertson DT, Collins AJ: Clinical epidemiology of long-bone fractures in patients receiving hemodialysis. Clin Orthop Relat Res 457: 188–193, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Stehman-Breen CO, Sherrard DJ, Alem AM, Gillen DL, Heckbert SR, Wong CS, Ball A, Weiss NS: Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 58: 2200–2205, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Atsumi K, Kushida K, Yamazaki K, Shimizu S, Ohmura A, Inoue T: Risk factors for vertebral fractures in renal osteodystrophy. Am J Kidney Dis 33: 287–293, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM: PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 47: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, Chauncey R, Nikkel L, Yin MT, Liu XS, Boutroy S, Staron RB, Leonard MB, McMahon DJ, Dworakowski E, Shane E: Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol 22: 1560–1572, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craver L, Marco MP, Martínez I, Rue M, Borràs M, Martín ML, Sarró F, Valdivielso JM, Fernández E: Mineral metabolism parameters throughout chronic kidney disease stages 1-5—achievement of K/DOQI target ranges. Nephrol Dial Transplant 22: 1171–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Coen G, Mazzaferro S, Ballanti P, Sardella D, Chicca S, Manni M, Bonucci E, Taggi F: Renal bone disease in 76 patients with varying degrees of predialysis chronic renal failure: A cross-sectional study. Nephrol Dial Transplant 11: 813–819, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Kinsella S, Chavrimootoo S, Molloy MG, Eustace JA: Moderate chronic kidney disease in women is associated with fracture occurrence independently of osteoporosis. Nephron Clin Pract 116: c256–c262, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Nickolas TL, McMahon DJ, Shane E: Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17: 3223–3232, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dukas L, Schacht E, Stähelin HB: In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int 16: 1683–1690, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Ensrud KE, Lui L-Y, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR, Osteoporotic Fractures Research Group : Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167: 133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Schumock GT, Sprague SM: Clinical and economic burden of fractures in patients with renal osteodystrophy. Clin Nephrol 67: 201–208, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Doan QV, Gleeson M, Kim J, Borker R, Griffiths R, Dubois RW: Economic burden of cardiovascular events and fractures among patients with end-stage renal disease. Curr Med Res Opin 23: 1561–1569, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the Alberta Kidney Disease Network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M, Alberta Kidney Disease Network : Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 21.James MT, Quan H, Tonelli M, Manns BJ, Faris P, Laupland KB, Hemmelgarn BR, Alberta Kidney Disease Network : CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 54: 24–32, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hux JE, Ivis F, Flintoft V, Bica A: Diabetes in Ontario: Determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 25: 512–516, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Quan H, Khan N, Hemmelgarn BR, Tu K, Chen G, Campbell N, Hill MD, Ghali WA, McAlister FA, Hypertension Outcome and Surveillance Team of the Canadian Hypertension Education Programs : Validation of a case definition to define hypertension using administrative data. Hypertension 54: 1423–1428, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC: Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 4: 804–811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lix LM, Azimaee M, Acan Osman B, Caetano P, Morin S, Metge C, Goltzman D, Kreiger N, Prior J, Leslie WD: Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health 12: 301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coen G, Ballanti P, Bonucci E, Calabria S, Costantini S, Ferrannini M, Giustini M, Giordano R, Nicolai G, Manni M, Sardella D, Taggi F: Renal osteodystrophy in predialysis and hemodialysis patients: Comparison of histologic patterns and diagnostic predictivity of intact PTH. Nephron 91: 103–111, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Johansen KL, Chertow GM, Jin C, Kutner NG: Significance of frailty among dialysis patients. J Am Soc Nephrol 18: 2960–2967, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Cook WL, Tomlinson G, Donaldson M, Markowitz SN, Naglie G, Sobolev B, Jassal SV: Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol 1: 1197–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Miller PD: The role of bone biopsy in patients with chronic renal failure. Clin J Am Soc Nephrol 3[Suppl 3]: S140–S150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, Harris TB, Newman AB, Cauley JA, Fried LF, Health, Aging, and Body Composition Study : Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol 7: 1130–1136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaCroix AZ, Lee JS, Wu L, Cauley JA, Shlipak MG, Ott SM, Robbins J, Curb JD, Leboff M, Bauer DC, Jackson RD, Kooperberg CL, Cummings SR, Women’s Health Initiative Observational : Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc 56: 1434–1441, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dooley AC, Weiss NS, Kestenbaum B: Increased risk of hip fracture among men with CKD. Am J Kidney Dis 51: 38–44, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, Sarnak M, Siscovick D, Harris T, Cauley J, Newman AB, Robbins J: Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 18: 282–286, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ, 3rd: Incidence of clinically diagnosed vertebral fractures: A population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res 7: 221–227, 1992 [DOI] [PubMed] [Google Scholar]
- 39.West SL, Lok CE, Jamal SA: Fracture Risk Assessment in Chronic Kidney Disease, Prospective Testing Under Real World Environments (FRACTURE): A prospective study. BMC Nephrol 11: 17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.