Summary
Background and objectives
Lower heart rate variability implies increased risk of cardiovascular disease. This study aimed to evaluate the relationship between mineral metabolism and heart rate variability and longitudinal changes of heart rate variability after parathyroidectomy in stage 5 CKD patients.
Design, setting, participants, & measurements
This cross-sectional study included 118 stage 5 CKD patients, 87 controls, and a prospective study in two subgroups classified as successful (n=17) and unsuccessful (n=4) parathyroidectomy follow-up enrolled from March of 2011 to December of 2012. Blood examination and 24-hour Holter for heart rate variability were measured.
Results
Most heart rate variability indices were lower in stage 5 CKD patients. In multivariate stepwise regression models, serum intact parathyroid hormone was correlated with mean normal-to-normal R–R intervals, mean heart rate, and very low frequency, serum calcium was correlated with SD of 5-minute average of normal R–R intervals, and serum phosphorus was correlated with very low frequency and low frequency/high frequency. Compared with baseline, the successful parathyroidectomy subgroup had significant improvements in mean normal-to-normal R–R intervals, mean heart rate, SD of normal-to-normal R–R intervals, SD of 5-minute average of normal R–R intervals, very low frequency, high frequency, and low frequency/high frequency. There was no significant change of heart rate variability in patients after unsuccessful parathyroidectomy.
Conclusions
Disorders of mineral metabolism are associated with decreased heart rate variability in stage 5 CKD. Successful parathyroidectomy may contribute to reverse this cardiovascular disease risk in severe secondary hyperparathyroidism patients.
Introduction
Cardiovascular disease (CVD) is a leading cause of mortality and accounts for 39% of overall deaths in ESRD (1). The ability to identify patients at risk for death and prescribe therapy to reduce CVD risk is of considerable significance in the care of patients with ESRD.
Secondary hyperparathyroidism (SHPT) is universal in ESRD patients and characterized by derangement of calcium/phosphate homeostasis, elevated levels of parathyroid hormone (PTH), and hyperplastic parathyroid gland (PTG) (2–4). These patients present with various bone disorders and high risk of cardiovascular morbidity and mortality (5–8). Recent studies have shown the possibility that parathyroidectomy (PTX) can reduce the risk of CVD (9–12).
In the last decade, heart rate variability (HRV) has emerged as a powerful, noninvasive clinical tool for the assessment of sympathetic and parasympathetic control of the heart using standard electrocardiographic monitoring. Lower HRV has a significant predictive value for various CVDs, such as ventricular arrhythmias and sudden death (13–16).
Previous studies have shown a marked reduction of HRV indices in ESRD patients (17–20). To our knowledge, few studies have evaluated the correlation between SHPT and HRV in ESRD patients. Thus, the present study aimed to examine the associations of mineral metabolism with HRV indices in stage 5 CKD patients and investigate longitudinal changes of HRV in two subgroups with successful and unsuccessful PTX affected by severe SHPT.
Materials and Methods
Patients
We investigated 118 stable stage 5 CKD patients ages 20–75 years. Patients had an estimated GFR<15 ml/min per 1.73 m2 while awaiting commencement of dialysis or on maintenance dialysis (either hemodialysis or peritoneal dialysis). Hemodialysis treatment was performed for 12 hours weekly using bicarbonate as dialysate. Thirty-seven severe SHPT patients who underwent total PTX with forearm autotransplantation were enrolled. PTX was performed in patients with severe hyperparathyroidism (persistent serum levels of intact PTH [iPTH]>800 pg/ml [88.0 pmol/L]) associated with hypercalcemia and/or hyperphosphatemia according to the Kidney Disease Outcomes Quality Initiative guideline (21). Patients with PTX were refractory to medical therapy, including dietary phosphate restriction, low calcium dialysis, calcium-based phosphate binders, and oral calcitriol (3.0–7.0 µg), per hemodialysis for 3–5 months (21). Because vitamin D analogs and calcimimetics were unavailable in mainland China during the study period, none of the patients took these two drugs. Successful PTX was defined as normalization of serum calcium (Ca), phosphorus (P), and alkaline phosphatase (ALP) and maintenance of no more than two to three times serum iPTH compared with normal (ranging from 10 to 88 pg/ml). Because the PTX patients were derived from a wide geographical area, some of them dropped out after the operation because of transference to other dialysis units, inability to contact, or poor compliance. After ≥3 months after the surgery, 17 patients who underwent successful PTX were then subsequently followed up (median of 5.0 months). Four patients who underwent unsuccessful PTX were followed up for a median of 2.3 months. They were difficult to observe for a long period of time because of their strong desire for reoperation.
Participants were excluded if they had experienced episodes of acute myocardial infarction, stroke, a major surgical procedure within the past 2 months (15), malignant neoplasm, or any psychiatric diagnosis during the past 5 years (22,23) and suffered from severe congenital heart disease, atrial fibrillation or flutter, high-grade heart block, permanent pacemaker implantation, severe hepatic disease, chronic obstructive lung disease (24), or neurologic deficit (e.g., spinal cord injury or peripheral nerve trauma). We also excluded participants who were pregnant or had any infection, fever, other problems limiting their normal daily activities, or fasting blood glucose on the day of evaluation greater than 200 mg/dl, because they can decrease nerve conduction velocity and distort the results of autonomic testing (25).
Control Group
To compare HRV between stage 5 CKD patients and healthy individuals, we recruited 87 controls matched for age and sex. Exclusion criteria were the same as for the patients studied but also included any known renal disease.
Protocols
At enrollment, 5 ml venous whole blood was drawn after an overnight fast. Each participant underwent a 24-hour Holter monitoring within 1 week to allow assessment of baseline features. For hemodialysis patients, blood samples were collected before dialysis, and HRV was analyzed on a nondialysis day. For severe SHPT patients who underwent PTX, these studies were performed no more than 2 weeks before surgery. Patients were subsequently followed up after PTX, and examinations of laboratory value and HRV were repeated. Clinical characteristics, medical history, and use of medications were strictly recorded.
Between March of 2011 and December of 2012, all patients and controls gave informed consent, and the study protocols were approved by the Research Ethics Committee of The First Affiliated Hospital of Nanjing Medical University, People’s Republic of China.
Analysis of Laboratory Values
Blood routine tests were performed using an LH-750 Hematology Analyzer (Beckman Coulter, Inc., Fullerton, CA). Biochemical indices, such as serum albumin (Alb), lipid profile, Ca, P, and ALP, were measured using an Automatic Biochemical Analyzer (AU5400; Olympus Corporation, Tokyo, Japan). Serum iPTH levels were measured using a UniCel DxI800 Access Immunoassay System (Beckman Coulter, Inc., Fullerton, CA).
Analysis of HRV
Holter electrocardiogram was digitized to 12-bit data at 128 Hz with a scanner (MARS-PC; GE Company, Fairfield, CT) on which QRS complexes were collected automatically. The results were reviewed, and any errors in QRS labeling were edited manually. Computations of HRV measurements were performed using custom-made Marquetter analytical software.
Time domain variables included mean normal-to-normal R–R intervals (mean NN), SD of the normal-to-normal R–R intervals (SDNN), SD of 5-minute average of normal R–R intervals (SDANN), root mean square of differences between adjacent normal R–R intervals (rMSSD), and proportion of adjacent R–R intervals differing by >50 ms over 24 hours (pNN50%). For frequency domain analysis, the power spectrum was quantified by fast Fourier transformation for the following frequency bands: 0.0033 to <0.04 Hz (very low frequency [VLF]), 0.04 to <0.15 Hz (low frequency [LF]), and 0.15–0.4 Hz (high frequency [HF]). LF/HF was also calculated. Frequency domain measurements of HRV were transformed to natural logarithms, because their distributions were skewed (26).
Statistical Analyses
Categorical variables were presented as number and proportion, and continuous variables were presented as mean ± SD or median (interquartile range). Differences between groups were compared using independent samples t or Wilcoxon rank sum test for continuous variables and chi-squared or Fisher exact test for categorical variables. Baseline serum iPTH levels were transformed to natural logarithms, because they were not normally distributed. Multiple stepwise regression models were established, and they were nonadjusted and adjusted for iPTH to identify factors affecting 24-hour HRV parameters. A paired sample t test was used to assess the differences between values recorded before and after PTX. P<0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL).
Results
Patients Characteristics
We divided all stage 5 CKD patients (n=118) into a non-PTX group (n=81) and a PTX group (n=37). The latter included a nonfollow-up group (n=16), a successful PTX follow-up group (n=17), and an unsuccessful PTX follow-up group (n=4). Clinical characteristics and laboratory results in stage 5 CKD patients and healthy controls (n=87) were shown in Table 1. Stage 5 CKD patients had lower body mass index, hemoglobin (Hb), hematocrit (Hct), HDL, total cholesterol, and Alb. Prevalent disorders in mineral metabolism, especially serum P, ALP, and iPTH abnormality, were observed in stage 5 CKD patients (Table 1).
Table 1.
Clinical characteristics and laboratory results
| Variable | Control (n=87) | Stage 5 CKD Patients (n=118) | P | Non-PTX Group (n=81) | PTX Group (n=37) | |||
|---|---|---|---|---|---|---|---|---|
| Nonfollow-Up (n=16) | Successful PTX Follow-Up (n=17) | Unsuccessful PTX Follow-Up (n=4) | Total (n=37) | |||||
| Demographics | ||||||||
| Age (yr) | 47.1±12.4 | 50.3±13.2 | 0.08 | 52.6±13.4 | 41.9±9.3 | 48.8±12.1 | 44.5±8.9 | 45.2±11.1a |
| Men/women | 39/48 | 61/57 | 0.40 | 40/41 | 9/7 | 8/9 | 4/0 | 21/16 |
| Body mass index (kg/m2) | 23.7±2.9 | 21.9±3.2 | <0.001 | 22.0±3.2 | 20.3±3.5 | 22.4±2.6 | 22.2±3.6 | 21.6±3.2 |
| Systolic BP (mmHg) | 121.1±15.3 | 141.8±27.0 | <0.001 | 144.6±25.9 | 135.3±31.3 | 134.2±22.9 | 147.8±42.7 | 135.6±28.6 |
| Diastolic BP (mmHg) | 78.7±11.1 | 84.0±15.0 | 0.004 | 83.8±13.1 | 86.6±22.9 | 83.2±16.7 | 88.8±15.5 | 84.5±18.7 |
| Dialysis mode, n (%) | ||||||||
| Predialysis | 0 (0.0) | 5 (4.2) | <0.001 | 5 (6.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hemodialysis | 0 (0.0) | 95 (80.5) | <0.001 | 59 (72.8) | 16 (100.0) | 16 (94.1) | 4 (100.0) | 36 (97.3)a |
| Peritoneal dialysis | 0 (0.0) | 18 (15.3) | <0.001 | 17 (21.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) | 1 (2.7)b |
| Dialysis vintage (mo) | 0 (0.0–0.0) | 30.0 (6.0–86.3) | <0.001 | 10.0 (4.0–36.0) | 97.0 (60.0–128.3) | 87.0 (63.0–127.0)a | 91.0 (48.5–179.3) | 94.0 (63.0–126.5)a |
| Comorbidities, n (%) | ||||||||
| Diabetic mellitus | 0 (0.0) | 26 (22.0) | <0.001 | 24 (29.6) | 0 (0.0) | 1 (5.9) | 1 (25.0) | 2 (5.4)a |
| Hypertension | 11 (12.6) | 88 (74.6) | <0.001 | 64 (79.0) | 10 (62.5) | 13 (76.5) | 1 (25.0) | 24 (64.9) |
| Cause of ESRD, n (%) | ||||||||
| GN | 0 (0.0) | 72 (61.0) | <0.001 | 45 (55.6) | 11 (68.8) | 13 (76.5) | 3 (75.0) | 27 (73.0) |
| Diabetic nephropathy | 0 (0.0) | 12 (10.2) | <0.001 | 11 (13.6) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 1 (2.7) |
| Hypertensive nephropathy | 0 (0.0) | 2 (1.7) | <0.001 | 1 (1.2) | 0 (0.0) | 1 (5.9) | 0 (0.0) | 1 (2.7) |
| Polycystic kidney disease | 0 (0.0) | 6 (5.1) | <0.001 | 4 (4.9) | 1 (6.3) | 1 (5.9) | 0 (0.0) | 2 (5.4) |
| Other | 0 (0.0) | 26 (22.0) | <0.001 | 20 (24.7) | 4 (25.0) | 2 (11.8) | 0 (0.0) | 6 (16.2) |
| Medication history, n (%) | ||||||||
| Calcium channel blocker | 2 (2.3) | 76 (64.4) | <0.001 | 56 (69.1) | 9 (56.3) | 10 (58.8) | 1 (25.0) | 20 (54.1) |
| ACEI/ARB | 3 (3.4) | 39 (33.1) | <0.001 | 28 (34.6) | 5 (31.3) | 5 (29.4) | 1 (25.0) | 11 (29.7) |
| β-Receptor blocker | 0 (0.0) | 35 (29.7) | <0.001 | 24 (29.6) | 3 (18.8) | 8 (47.1) | 0 (0.0) | 11 (29.7) |
| Laboratory values | ||||||||
| Hemoglobin (g/L) | 143.2±16.8 | 95.6±22.9 | <0.001 | 92.6±22.0 | 100.3±30.4 | 102.5±17.1 | 102.3±15.9 | 102.3±23.7b |
| Hematocrit (%) | 42.7±4.4 | 29.2±7.0 | <0.001 | 28.0±6.6 | 31.8±9.8 | 31.5±5.0b | 32.0±5.5 | 31.8±7.4a |
| Glucose (mmol/L) | 5.4±0.8 | 5.3±2.6 | 0.57 | 5.6±2.7 | 4.0±0.5 | 4.9±3.4 | 4.8±1.4 | 4.5±2.3b |
| Creatinine (μmol/L) | 68.7±14.2 | 874.4±318.5 | <0.001 | 861.1±343.5 | 864.7±255.3 | 905.6±273.9 | 926.1±143.2 | 903.5±257.4 |
| Urea (mmol/L) | 5.7±1.2 | 23.5±8.6 | <0.001 | 24.4±9.1 | 20.2±7.1 | 23.0±7.8 | 18.9±2.4 | 21.7±7.2 |
| HDL cholesterol (mmol/L) | 1.4±0.3 | 1.1±0.3 | <0.001 | 1.1±0.3 | 1.2±0.4 | 1.1±0.3 | 1.1±0.4 | 1.1±0.3 |
| LDL cholesterol (mmol/L) | 2.8±0.6 | 2.6±0.9 | 0.20 | 2.8±1.0 | 2.4±0.7 | 2.3±0.5a | 2.3±0.6 | 2.4±0.6a |
| Total cholesterol (mmol/L) | 5.0±0.8 | 4.4±1.3 | <0.001 | 4.5±1.4 | 4.4±1.1 | 4.0±0.8 | 3.8±1.0 | 4.1±1.0 |
| Triglyceride (mmol/L) | 1.4±1.5 | 1.6±1.0 | 0.39 | 1.7±1.1 | 1.3±0.7 | 1.3±0.6 | 1.2±0.5 | 1.3±0.7b |
| Albumin (g/L) | 48.2±2.6 | 37.2±5.9 | <0.001 | 36.4±6.6 | 38.3±3.8 | 38.8±4.8 | 39.8±3.7 | 38.7±4.2b |
| Bone metabolism panel | ||||||||
| Calcium (mg/dl) | 9.4±0.4 | 9.3±1.3 | 0.38 | 8.8±1.2 | 10.2±1.0 | 10.5±0.6a | 10.4±1.0 | 10.3±0.8a |
| Phosphorus (mg/dl) | 3.7±0.5 | 6.1±1.7 | <0.001 | 5.8±1.6 | 6.5±1.9 | 6.9±1.8b | 6.2±1.6 | 6.7±1.9a |
| ALP (μ/L) | 73.2 (61.0–86.4) | 105.7 (78.9–220.2) | <0.001 | 89.0 (75.6–114.6) | 254.9 (145.4–957.6) | 527.3 (228.3–1006.8)a | 285.8 (152.6–570.0) | 351.5 (161.8–937.5)a |
| iPTH (pg/ml) | 37.0 (29.0–51.4) | 442.3 (191.3–1257.7) | <0.001 | 228.0 (156.5–530.6) | 1701.8 (1062.3–2384.0) | 1717.8 (1350.8–3209.7)a | 1639.9 (1491.3–2908.1) | 1717.5 (1294.9–2521.6)a |
| ln iPTH | 3.6±0.4 | 6.1±1.3 | <0.001 | 5.5±1.0 | 7.4±0.5 | 7.5±0.4a | 7.5±0.4 | 7.5±0.4a |
Data are mean ± SD, numbers and percentages, or median (25th to 75th percentile) as appropriate. Test of significance was by independent samples t or Wilcoxon rank sum test for continuous variables and chi-squared or Fisher exact test for categorical variables. PTX, parathyroidectomy; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; ALP, alkaline phosphatase; iPTH, intact parathyroid hormone.
P<0.01 versus non-PTX group.
P<0.05 versus non-PTX group.
Baseline Values and Determinants of HRV
As presented in Table 2, baseline HRV analysis in stage 5 CKD patients showed lower time and frequency domains than controls, except for HF. There were no statistically significant differences in baseline HRV parameters between the successful PTX follow-up group (n=17) and the nonfollow-up group (n=16). Variables including demographics (age, sex, body mass index, dialysis vintage, systolic pressure, and diastolic pressure), laboratory values (Hb, Hct, glucose, Alb, and triglyceride), and bone metabolism panel (Ca, P, ALP, and iPTH) were selected as independent variables in the multivariable models (Table 3). Taking all HRV indices as dependent variables, we observed a powerful association between mineral metabolism and HRV indices. Serum iPTH was independently correlated with mean NN, mean HR, and VLF. Serum Ca was independently correlated with SDANN, and serum P was independently correlated with VLF and LF/HF. The results remained significant after the adjustment for iPTH (data not shown).
Table 2.
Baseline heart rate variability parameters of study groups
| Variable | Control (n=87) | Stage 5 CKD Patients (n=118) | P | Non-PTX Group (n=81) | PTX Group (n=37) | |||
|---|---|---|---|---|---|---|---|---|
| Nonfollow-Up (n=16) | Successful PTX Follow-Up (n=17) | Unsuccessful PTX Follow-Up (n=4) | Total (n=37) | |||||
| Mean 24-h HR (beats/min) | 76.8±8.0 | 82.3±10.9 | <0.001 | 80.9±11.0 | 86.4±8.3 | 87.1±10.7 | 73.0±8.1 | 85.5±9.9 |
| Time domain measures | ||||||||
| Mean NN (ms) | 789.3±80.7 | 743.0±103.5 | <0.001 | 756.9±107.8 | 701.4±70.1 | 699.7±91.5 | 833.0±91.5 | 712.6±87.2 |
| SDNN (ms) | 140.9±34.9 | 82.7±48.4 | <0.001 | 85.8±54.9 | 80.9±33.1 | 67.8±24.6 | 94.0±27.0 | 76.1±29.3 |
| SDANN (ms) | 128.4±34.2 | 74.7±61.5 | <0.001 | 78.4±71.5 | 71.9±31.1 | 57.9±25.4 | 81.0±28.1 | 66.6±28.7 |
| rMSSD (ms) | 29.8±11.2 | 19.2±9.3 | <0.001 | 19.6±9.7 | 16.9±6.9 | 19.5±9.9 | 18.0±5.7 | 18.3±8.3a |
| pNN50% | 9.2±8.7 | 3.2±4.7 | <0.001 | 3.4±4.7 | 1.8±2.7 | 3.7±6.5 | 1.7±1.7 | 2.7±4.9 |
| Frequency domain measures | ||||||||
| VLF (ms2) | 1012.8 (646.0–1571.8) | 232.7 (112.1–473.6) | <0.001 | 210.1 (107.4–459.2) | 381.2 (139.4–472.4) | 164.4 (107.8–484.6) | 570.1 (154.8–1147.5) | 311.8 (119.6–485.4) |
| LF (ms2) | 177.5 (16.8–395.2) | 59.5 (21.6–150.5) | 0.02 | 50.8 (18.3–149.2) | 72.9 (24.8–124.4) | 79.1 (46.3–165.6) | 65.2 (9.2–533.5) | 74.2 (35.7–159.7) |
| HF (ms2) | 56.7 (1.6–189.0) | 26.0 (10.3–61.6) | 0.37 | 27.4 (9.6–63.4) | 21.0 (8.0–45.7) | 27.6 (18.5–81.4) | 22.1 (3.1–84.4) | 24.0 (12.1–56.1) |
| LF/HF | 4.0 (2.0–11.8) | 2.8 (1.5–5.3) | <0.001 | 2.3 (1.3–5.1) | 3.9 (2.7–5.9) | 3.1 (1.6–4.3) | 8.4 (2.0–15.3) | 3.5 (2.3–5.9) |
| ln VLF [ln(ms2)] | 6.9±0.6 | 5.5±1.0 | <0.001 | 5.4±1.1 | 5.7±1.1 | 5.4±1.0 | 6.1±1.1 | 5.6±1.0 |
| ln LF [ln(ms2)] | 4.5±1.7 | 4.0±1.4 | 0.03 | 3.9±1.4 | 4.2±1.5 | 4.4±1.0 | 4.0±2.1 | 4.3±1.3 |
| ln HF [ln(ms2)] | 2.9±2.6 | 3.0±1.6 | 0.67 | 3.0±1.7 | 2.8±1.8 | 3.4±1.1 | 2.4±2.4 | 3.1±1.6 |
| ln LF/HF | 1.6±1.0 | 1.0±1.0 | <0.001 | 0.9±1.0 | 1.4±0.8 | 1.0±0.8 | 1.6±1.6 | 1.2±0.9 |
Data are mean ± SD or median (25th to 75th percentile). Independent samples t test was used to compare stage 5 CKD patients (n=118) with controls (n=87) and nonfollow-up patients (n=16) with successful PTX follow-up patients (n=17). The Wilcoxon rank sum test was used to compare medians of untransformed frequency domain measures. Multiple linear regression analysis was used to compare the PTX group (n=37) with the non-PTX group (n=81) and the successful PTX follow-up group (n=17) with the non-PTX group (n=81) with adjustment for age, sex, dialysis vintage, diabetes, and hypertension. PTX, parathyroidectomy; HR, heart rate; mean NN, mean normal-to-normal R–R intervals; SDNN, SD of normal-to-normal R–R intervals; SDANN, SD of 5-minute average of normal R–R intervals; rMSSD, root mean square of differences between adjacent normal R–R intervals; pNN50%, proportion of adjacent R–R intervals differing by >50 ms over 24 hours; VLF, very low frequency; LF, low frequency; HF, high frequency.
P<0.05 versus non-PTX group.
Table 3.
Multiple stepwise regression models predicting 24-hour heart rate variability parameters (n=118)
| Dependent Variable | Independent Variable | Correlation Coefficient | Regression Coefficient (95% Confidence Interval) | P |
|---|---|---|---|---|
| Mean NN | iPTH | −0.25 | −0.03 (−0.05 to −0.01) | 0.007 |
| SDNN | DBP | −0.21 | −0.68 (−1.26 to −0.10) | 0.02 |
| SDANN | Hemoglobin | 0.22 | 0.58 (0.10 to 1.07) | 0.02 |
| SDANN | Calcium | −0.19 | −8.66 (−16.96 to −0.35) | 0.04 |
| rMSSD | Hemoglobin | 0.31 | 0.13 (0.06 to 0.20) | 0.001 |
| rMSSD | Triglyceride | −0.18 | −1.63 (−3.26 to −0.00) | 0.05 |
| pNN50% | SBP | −0.27 | −0.05 (−0.08 to −0.02) | 0.004 |
| pNN50% | Glucose | 0.19 | 0.33 (0.01 to 0.65) | 0.04 |
| Mean heart rate | iPTH | 0.26 | 0.00 (0.00 to 0.01) | 0.004 |
| ln VLF | Albumin | 0.22 | 0.04 (0.01 to 0.07) | 0.02 |
| ln VLF | Hemoglobin | 0.33 | 0.01 (0.01 to 0.02) | <0.001 |
| ln VLF | Phosphorus | 0.22 | 0.11 (0.02 to 0.21) | 0.02 |
| ln VLF | Triglyceride | −0.29 | −0.26 (−0.42 to −0.10) | 0.002 |
| ln VLF | Age | −0.26 | −0.02 (−0.03 to −0.01) | 0.005 |
| ln VLF | iPTH | −0.20 | 0.00 (0.00 to 0.00) | 0.04 |
| ln LF | Age | −0.35 | −0.04 (−0.05 to −0.02) | <0.001 |
| ln LF | Hemoglobin | 0.30 | 0.02 (0.01 to 0.03) | 0.001 |
| ln LF | Triglyceride | −0.22 | −0.27 (−0.50 to −0.04) | 0.02 |
| ln HF | BMI | −0.23 | −0.12 (−0.21 to −0.03) | 0.01 |
| ln LF/HF | Albumin | 0.32 | 0.05 (0.02 to 0.08) | <0.001 |
| ln LF/HF | Phosphorus | 0.22 | 0.12 (0.02 to 0.21) | 0.02 |
Mean NN, mean normal-to-normal R–R intervals; iPTH, intact parathyroid hormone; SDNN, SD of normal-to-normal R–R intervals; DBP, diastolic BP; SDANN, SD of 5-minute average of normal R–R intervals; rMSSD, root mean square of differences between adjacent normal R–R intervals; pNN50%, proportion of adjacent RR intervals differing by >50 ms over 24 hours; SBP, systolic BP; VLF, very low frequency; LF, low frequency; HF, high frequency; BMI, body mass index.
Baseline Information of Severe SHPT Patients with Successful PTX
PTX patients (n=37) tended to be younger, have longer dialysis vintage, and have a lower rate of diabetic mellitus than the non-PTX patients (n=81). Some PTX patients with severe anemia or hypoalbuminemia needed correction by blood transfusion or albumin infusion for preoperative preparation. Therefore, PTX patients (n=37) had higher baseline levels of serum Hb, Hct, and Alb compared with non-PTX patients (n=81). No significant differences were shown in laboratory results between the nonfollow-up group (n=16) and the successful PTX follow-up group (n=17).
Average age of successful PTX follow-up cohort (n=17) at baseline was 48.8±12.1 years. The mean duration of dialysis was 91.8 months. Thirteen patients were hypertensive, and one patient had both diabetes mellitus and coronary heart disease (Table 1).
Follow-Up Study of Laboratory Values in Successful PTX Patients
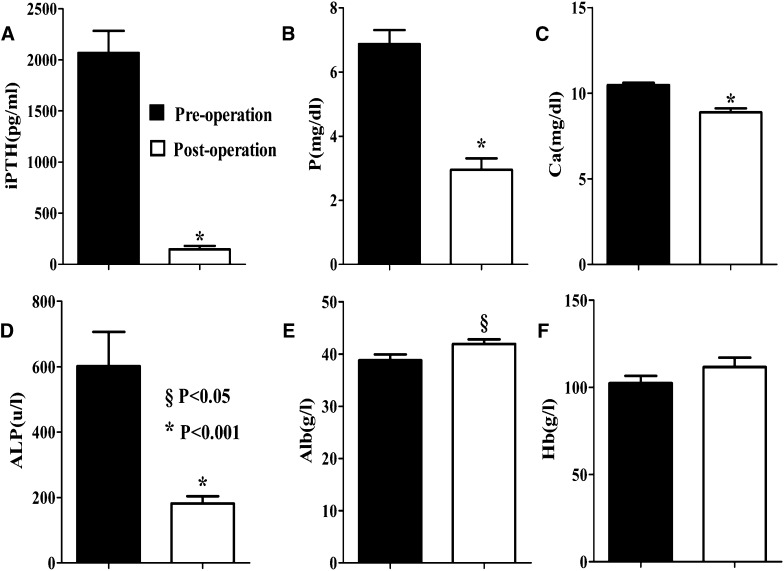
The pre- and postoperative serum indices of laboratory values are shown in Figure 1. Duration of follow-up was different in successful PTX patients, but the postoperative mineral metabolism indices were controlled at a satisfactory level, and no time trend was observed (data not shown). Serum iPTH levels were significantly lower (from 1717.8 [1350.8–3209.7] to 72.4 [42.7–242.5] pg/ml, P<0.001), and serum P levels were significantly decreased (from 6.9±1.8 to 2.9±1.5 mg/dl, P<0.001). There was also significant downregulation of serum Ca levels (10.5±0.6 versus 8.9±1.0 mg/dl, P<0.001) and serum ALP levels (527.3 [228.3–1006.8] versus 169.9 [111.0–244.8] μ/L, P<0.001). These results indicated a correction status of mineral metabolism in severe SHPT patients after total PTX with forearm transplantation. A significant increase in serum Alb levels (38.8±4.8 versus 41.9±3.7 g/L, P<0.05) was also observed in these patients.
Figure 1.
Comparison of laboratory values in severe SHPT patients before and after successful PTX. Serum levels of (A) iPTH, (B) P, (C) Ca, (D) ALP, (E) Alb, and (F) Hb pre- and postoperative. Data are mean ± SD (n=17). §P<0.05, *P<0.001. Alb, albumin; ALP, alkaline phosphatase; Ca, calcium; Hb, hemoglobin; iPTH, intact parathyroid hormone; P, phosphorus; PTX, parathyroidectomy; SHPT, secondary hyperparathyroidism.
Improvements of Decreased HRV in Successful PTX Patients
As presented in Table 4, we observed significant improvements in HRV parameters in severe SHPT patients after successful PTX. These parameters included mean 24-hour HR (76.8±8.1 versus 87.1±10.7 beats/min, P<0.001), mean NN (790.9±83.0 versus 699.7±91.5 ms, P<0.001), SDNN (84.2±28.6 versus 67.8±24.6 ms, P=0.003), SDANN (73.3±29.7 versus 57.9±25.4 ms, P=0.004), VLF (5.9±0.7 versus 5.4±0.9 [ln(ms2)], P=0.008), HF (1.7±2.8 versus 3.4±1.1 [ln(ms2)], P=0.02), and LF/HF (2.0±1.4 versus 1.0±0.8, P=0.008; all relative to baseline).
Table 4.
Heart rate variability in severe secondary hyperparathyroidism patients before and after parathyroidectomy
| Variable in Two Subgroups | Baseline | After PTX | Mean Difference | P |
|---|---|---|---|---|
| Successful PTX follow-up (n=17)a | ||||
| Mean 24-h heart rate (beats/min) | 87.1±10.7 | 76.8±8.1 | −10.4±8.2 | <0.001 |
| Time domain measures | ||||
| Mean NN (ms) | 699.7±91.5 | 790.9±83.0 | 91.2±74.5 | <0.001 |
| SDNN (ms) | 67.8±24.6 | 84.2±28.6 | 16.4±19.0 | 0.003 |
| SDANN (ms) | 57.9±25.4 | 73.3±29.7 | 15.4±18.6 | 0.004 |
| rMSSD (ms) | 19.5±9.9 | 23.5±9.2 | 3.9±10.9 | 0.16 |
| pNN50% | 3.7±6.5 | 5.8±7.9 | 2.1±9.7 | 0.40 |
| Frequency domain measures | ||||
| ln VLF [ln(ms2)] | 5.4±0.9 | 5.9±0.7 | 0.5±0.7 | 0.008 |
| ln LF [ln(ms2)] | 4.4±1.0 | 3.7±1.7 | −0.7±1.7 | 0.09 |
| ln HF [ln(ms2)] | 3.4±1.1 | 1.7±2.8 | −1.8±2.8 | 0.02 |
| ln LF/HF | 1.0±0.8 | 2.0±1.4 | 1.0±1.4 | 0.008 |
| Unsuccessful PTX follow-up (n=4)b | ||||
| Mean 24-h heart rate (beats/min) | 73.0±8.1 | 73.5±12.4 | 0.5±5.8 | 0.87 |
| Time domain measures | ||||
| Mean NN (ms) | 833.0±91.5 | 844.3±138.7 | 11.3±60.3 | 0.73 |
| SDNN (ms) | 94.0±27.0 | 93.3±11.8 | −0.8±19.3 | 0.94 |
| SDANN (ms) | 81.0±28.1 | 82.5±7.5 | 1.5±21.1 | 0.90 |
| rMSSD (ms) | 18.0±5.7 | 17.8±5.9 | −0.3±4.4 | 0.92 |
| pNN50% | 1.7±1.7 | 2.5±2.4 | 0.8±2.0 | 0.51 |
| Frequency domain measures | ||||
| ln VLF [ln(ms2)] | 6.1±1.1 | 5.7±1.0 | −0.3±0.4 | 0.17 |
| ln LF [ln(ms2)] | 4.0±2.1 | 3.4±1.5 | −0.6±3.3 | 0.73 |
| ln HF [ln(ms2)] | 2.4±2.4 | 2.3±2.6 | −0.2±4.5 | 0.95 |
| ln LF/HF | 1.6±1.6 | 1.1±1.9 | −0.5±1.2 | 0.51 |
Data are mean ± SD. PTX, parathyroidectomy; mean NN, mean normal-to-normal R–R intervals; SDNN, SD of normal-to-normal R–R intervals; SDANN, SD of 5-minute average of normal R–R intervals; rMSSD, root mean square of differences between adjacent normal R–R intervals; pNN50%, proportion of adjacent R–R intervals differing by >50 ms over 24 hours; VLF, very low frequency; LF, low frequency; HF, high frequency.
Patients were followed up for a median period of 5.0 months.
Patients were followed up for a median period of 2.3 months.
Changes of HRV in Unsuccessful PTX Patients
Four SHPT patients who underwent unsuccessful PTX showed no statistical significance in pre- and postoperative serum levels of iPTH (1639.9 [1491.3–2908.1] versus 1122.0 [911.2–2819.6] pg/ml, P=0.07), Ca (10.4±1.0 versus 9.8±1.8 mg/dl, P=0.50), and P (6.2±1.6 versus 5.4±1.8 mg/dl, P=0.18) in a median follow-up period of 2.3 months.
Average age of these patients at baseline was 44.5±8.9 years. The mean duration of dialysis was 106.3 months. One patient was hypertensive and had diabetes mellitus.
No statistically significant changes in HRV indices were observed in these patients before and after unsuccessful PTX (Table 4).
Discussion
In the last decade, decreased HRV has been established as a significant independent risk factor for higher mortality and cardiac death (15,16,27). It seemed to be caused by impairment of the cardiovascular autonomic control characterized by withdrawal in parasympathetic modulation and predominance in sympathetic tone (28). As in previous studies (17–20,29–33), our results showed that stage 5 CKD patients exhibited a marked reduction in HRV indices relative to age- and sex-matched controls, indicating that sympathetic hyperactivity may play an important role in increasing the risk of CVD among ESRD patients.
It has been proposed that CKD–mineral and bone disorder may be caused by disordered mineral metabolism (34,35), which is common among patients with stage 5 CKD and has been also detected in patients with stages 3 and 4 CKD (36). CVD, fractures, and mortality are its major outcomes. Increased PTH may diminish cardiac contractility, induce ventricular hypertrophy (37,38), and enhance coronary risk (39) and cardiovascular calcification (40,41). PTH also plays a role in disturbances in the autonomic nervous system—both sympathetic and parasympathetic. Wanic-Kossowska et al. (42) reported correlations between serum iPTH levels and SDNN, pNN50%, and rMSSD in 59 hemodialysis patients. Polak et al. (43) observed negative correlations between serum iPTH and both LF and HF. They also found total spectral power to be lower in patients with high serum levels of iPTH, which indicated deterioration in total autonomic activity. Here, we confirmed that disordered mineral metabolism, especially serum levels of iPTH, Ca, and P, was significantly associated with decreased HRV indices (Table 3). These results revealed that dysregulation in cardiovascular autonomic control raised by abnormal mineral metabolism could exert a cumulative effect on the risk of CVD.
Total PTX with forearm autograft transplantation remains the primary therapeutic means of addressing medication-refractory SHPT. A more recent investigation conducted by Sharma et al. (44) observed significantly reduced rates of all-cause and cardiovascular mortality in 150 dialysis patients who underwent near-total parathyroidectomy relative to 1044 non-PTX control patients. Accumulating evidence showed that successful PTX in severe SHPT patients led to substantial cardiovascular benefits, including improvement in BP (11,45), amelioration of uremic tumoral calcinosis (46,47), decrease in thickness of coronary artery intima media (10), and reduction in left ventricular mass index (48). However, whether successful PTX can affect cardiac autonomic outflow in severe SHPT remains unclear.
We then subjected 17 successful PTX patients to another 5.0 months of follow-up and observed a substantial correction of serum iPTH, Ca, P, and ALP and a significant increase in serum Alb (Figure 1). Improvements in HRV indices (mean HR, mean NN, SDNN, SDANN, VLF, HF, and LF/HF) (Table 4) indicated a decrease in sympathetic activity and an increase in parasympathetic activity. No significant changes of HRV were shown in patients after unsuccessful PTX (Table 4); however, the possibility of statistical error because of the small sample size and the short duration of follow-up demanded vigilance. The mechanisms by which successful PTX conferred benefits on HRV were not certain. The correction of mineral markers and serum Alb level may partially but crucially represent the impact of SHPT on HRV. Whether other factors contributed to the reversal of decreased HRV in these patients remained unknown. We also cannot exclude the possibility that PTX can improve HRV in cases of less severe SHPT. Sharma et al. (44) suggested that significantly corrected laboratory values, such as serum Alb, Hct, iPTH, Ca, and P, may have remarkable beneficial effects on cardiovascular outcomes. In our study, we speculate that the improvement of HRV may represent an important pathway linking PTX to better patient survival.
This study showed that successful PTX in severe SHPT patients could lead to amelioration of abnormal mineral metabolism and a relative physiologic sympathovagal balance. Patient selection during the follow-up study may have introduced some selection bias. However, there were no statistically significant differences between the successful PTX follow-up group and the nonfollow-up group. The lack of matched non-PTX controls was one limitation of the present study. Generally, severe SHPT patients can be scheduled for surgery shortly after they are confirmed refractory to medical therapy. Using untreated severe SHPT patients as controls during follow-up studies was not considered acceptable by medical ethicists. In the future, the longitudinal changes of HRV indices should be investigated in studies with larger sample sizes and longer observation periods.
Our data have shown a significant reduction in baseline HRV parameters in stage 5 CKD patients relative to age- and sex-matched controls. This result may reflect dysfunction of the cardiac autonomic nervous system as sympathetic hyperactivity. Disorders of mineral metabolism were correlated with decreased HRV in stage 5 CKD. Successful PTX in severe SHPT patients may contribute to reverse this high CVD risk by blunting sympathetic hyperactivity and enhancing parasympathetic activity as indicated by HRV parameters. Our research provides a novel insight into the mechanism linking PTX to CVD risk and the potential therapeutic avenue in severe SHPT patients.
Disclosures
None.
Acknowledgments
The authors thank Chengjing Yan and Qiaodi Zhang for assistance with the measurement of laboratory indices, Yun Xia, Zhihui Song, and Jing Tu for assistance with the analysis of heart rate variability, Feng Chen and Min Cai for assistance with statistical analyses, and Cuiping Liu for the management of blood samples.
This study was funded by National Natural Science Foundation of China (81270408), ZX07 200908, RC201162, 2010(IB10), and LJ201125, PAPD.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 59[1 Suppl 1]: e1–e420, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Goodman WG, Quarles LD: Development and progression of secondary hyperparathyroidism in chronic kidney disease: Lessons from molecular genetics. Kidney Int 74: 276–288, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Cunningham J, Locatelli F, Rodriguez M: Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 6: 913–921, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G, Kidney Disease: Improving Global Outcomes (KDIGO) : Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Moe SM, Drüeke TB: Management of secondary hyperparathyroidism: The importance and the challenge of controlling parathyroid hormone levels without elevating calcium, phosphorus, and calcium-phosphorus product. Am J Nephrol 23: 369–379, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Raggi P, Kleerekoper M: Contribution of bone and mineral abnormalities to cardiovascular disease in patients with chronic kidney disease. Clin J Am Soc Nephrol 3: 836–843, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Kestenbaum B, Andress DL, Schwartz SM, Gillen DL, Seliger SL, Jadav PR, Sherrard DJ, Stehman-Breen C: Survival following parathyroidectomy among United States dialysis patients. Kidney Int 66: 2010–2016, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Naranda J, Ekart R, Pečovnik-Balon B: Total parathyroidectomy with forearm autotransplantation as the treatment of choice for secondary hyperparathyroidism. J Int Med Res 39: 978–987, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Coen G, Calabria S, Bellinghieri G, Pecchini F, Conte F, Chiappini MG, Ferrannini M, Lagona C, Mallamace A, Manni M, DiLuca M, Sardella D, Taggi F: Parathyroidectomy in chronic renal failure: Short- and long-term results on parathyroid function, blood pressure and anemia. Nephron 88: 149–155, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Costa-Hong V, Jorgetti V, Gowdak LH, Moyses RM, Krieger EM, De Lima JJ: Parathyroidectomy reduces cardiovascular events and mortality in renal hyperparathyroidism. Surgery 142: 699–703, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Chandra P, Sands RL, Gillespie BW, Levin NW, Kotanko P, Kiser M, Finkelstein F, Hinderliter A, Pop-Busui R, Rajagopalan S, Saran R: Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant 27: 700–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battipaglia I, Scalone G, Macchione A, Pinnacchio G, Laurito M, Milo M, Pelargonio G, Bencardino G, Bellocci F, Pieroni M, Lanza GA, Crea F: Association of heart rate variability with arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ J 76: 618–623, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Fukuta H, Hayano J, Ishihara S, Sakata S, Mukai S, Ohte N, Ojika K, Yagi K, Matsumoto H, Sohmiya S, Kimura G: Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant 18: 318–325, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Oikawa K, Ishihara R, Maeda T, Yamaguchi K, Koike A, Kawaguchi H, Tabata Y, Murotani N, Itoh H: Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int J Cardiol 131: 370–377, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Wei CY, Chung TC, Wu SC, Chung CF, Wu WP: The subjective sleep quality and heart rate variability in hemodialysis patients. Ren Fail 33: 109–117, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Chan CT, Levin NW, Chertow GM, Larive B, Schulman G, Kotanko P, Frequent Hemodialysis Network Daily Trial Group : Determinants of cardiac autonomic dysfunction in ESRD. Clin J Am Soc Nephrol 5: 1821–1827, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CT, Hanly P, Gabor J, Picton P, Pierratos A, Floras JS: Impact of nocturnal hemodialysis on the variability of heart rate and duration of hypoxemia during sleep. Kidney Int 65: 661–665, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Steinberg AA, Mars RL, Goldman DS, Percy RF: Effect of end-stage renal disease on decreased heart rate variability. Am J Cardiol 82: 1156–1158, 1998 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 22.Kojima M, Hayano J, Fukuta H, Sakata S, Mukai S, Ohte N, Seno H, Toriyama T, Kawahara H, Furukawa TA, Tokudome S: Loss of fractal heart rate dynamics in depressive hemodialysis patients. Psychosom Med 70: 177–185, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Roumelioti ME, Ranpuria R, Hall M, Hotchkiss JR, Chan CT, Unruh ML, Argyropoulos C: Abnormal nocturnal heart rate variability response among chronic kidney disease and dialysis patients during wakefulness and sleep. Nephrol Dial Transplant 25: 3733–3741, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayano J, Takahashi H, Toriyama T, Mukai S, Okada A, Sakata S, Yamada A, Ohte N, Kawahara H: Prognostic value of heart rate variability during long-term follow-up in chronic haemodialysis patients with end-stage renal disease. Nephrol Dial Transplant 14: 1480–1488, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Schroeder EB, Chambless LE, Liao D, Prineas RJ, Evans GW, Rosamond WD, Heiss G, Atherosclerosis Risk in Communities (ARIC) study : Diabetes, glucose, insulin, and heart rate variability: The Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 28: 668–674, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology : Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17: 354–381, 1996 [PubMed] [Google Scholar]
- 27.Chan CT: Heart rate variability in patients with end-stage renal disease: An emerging predictive tool for sudden cardiac death? Nephrol Dial Transplant 23: 3061–3062, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Ranpuria R, Hall M, Chan CT, Unruh M: Heart rate variability (HRV) in kidney failure: Measurement and consequences of reduced HRV. Nephrol Dial Transplant 23: 444–449, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Yang YW, Wu CH, Tsai MK, Kuo TB, Yang CC, Lee PH: Heart rate variability during hemodialysis and following renal transplantation. Transplant Proc 42: 1637–1640, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Furuland H, Linde T, Englund A, Wikström B: Heart rate variability is decreased in chronic kidney disease but may improve with hemoglobin normalization. J Nephrol 21: 45–52, 2008 [PubMed] [Google Scholar]
- 31.Giordano M, Manzella D, Paolisso G, Caliendo A, Varricchio M, Giordano C: Differences in heart rate variability parameters during the post-dialytic period in type II diabetic and non-diabetic ESRD patients. Nephrol Dial Transplant 16: 566–573, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Kurata C, Uehara A, Sugi T, Ishikawa A, Fujita K, Yonemura K, Hishida A, Ishikawa K, Tawarahara K, Shouda S, Mikami T: Cardiac autonomic neuropathy in patients with chronic renal failure on hemodialysis. Nephron 84: 312–319, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Deligiannis A, Kouidi E, Tourkantonis A: Effects of physical training on heart rate variability in patients on hemodialysis. Am J Cardiol 84: 197–202, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Martin KJ, González EA: Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: What is normal, when to start, and how to treat? Clin J Am Soc Nephrol 6: 440–446, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Lishmanov A, Dorairajan S, Pak Y, Chaudhary K, Chockalingam A: Elevated serum parathyroid hormone is a cardiovascular risk factor in moderate chronic kidney disease. Int Urol Nephrol 44: 541–547, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Nasri H, Baradaran A: Close association between parathyroid hormone and left ventricular function and structure in end-stage renal failure patients under maintenance hemodialysis. Bratisl Lek Listy (Tlacene Vyd) 105: 368–373, 2004 [PubMed] [Google Scholar]
- 38.Akmal M, Barndt RR, Ansari AN, Mohler JG, Massry SG: Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney Int 47: 158–163, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Grandi NC, Breitling LP, Hahmann H, Wüsten B, März W, Rothenbacher D, Brenner H: Serum parathyroid hormone and risk of adverse outcomes in patients with stable coronary heart disease. Heart 97: 1215–1221, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Perkovic V, Hunt D, Griffin SV, du Plessis M, Becker GJ: Accelerated progression of calcific aortic stenosis in dialysis patients. Nephron Clin Pract 94: c40–c45, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Peiris AN, Youssef D, Grant WB: Secondary hyperparathyroidism: Benign bystander or culpable contributor to adverse health outcomes? South Med J 105: 36–42, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Wanic-Kossowska M, Guzik P, Lehman P, Czekalski S: Heart rate variability in patients with chronic renal failure treated by hemodialysis. Pol Arch Med Wewn 114: 855–861, 2005 [PubMed] [Google Scholar]
- 43.Polak G, Strózecki P, Grześk G, Manitius J, Grabczewska Z, Przybył R: Effect of parathormone on heart rate variability in hemodialysis patients. Auton Neurosci 115: 94–98, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Sharma J, Raggi P, Kutner N, Bailey J, Zhang R, Huang Y, Herzog CA, Weber C: Improved long-term survival of dialysis patients after near-total parathyroidectomy. J Am Coll Surg 214: 400–407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evenepoel P, Claes K, Kuypers D, Maes B, Vanrenterghem Y: Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: A single centre study. Nephrol Dial Transplant 20: 1714–1720, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Zouboulis CC, Blume-Peytavi U, Lennert T, Stavropoulos PG, Schwarz A, Runkel N, Trautmann C, Orfanos CE: Fulminant metastatic calcinosis with cutaneous necrosis in a child with end-stage renal disease and tertiary hyperparathyroidism. Br J Dermatol 135: 617–622, 1996 [PubMed] [Google Scholar]
- 47.Goldsmith DJ, Covic A, Sambrook PA, Ackrill P: Vascular calcification in long-term haemodialysis patients in a single unit: A retrospective analysis. Nephron 77: 37–43, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Chow KM, Szeto CC, Kum LC, Kwan BC, Fung TM, Wong TY, Leung CB, Li PK: Improved health-related quality of life and left ventricular hypertrophy among dialysis patients treated with parathyroidectomy. J Nephrol 16: 878–885, 2003 [PubMed] [Google Scholar]