Summary
Background and objectives
Patients with ESRD experience a fivefold higher incidence of hip fracture than the age- and sex-matched general population. Despite multiple changes in the treatment of CKD mineral bone disorder, little is known about long-term trends in hip fracture incidence, treatment patterns, and outcomes in patients on dialysis.
Design, setting, participants, & measurements
Fourteen annual cohorts (1996–2009) of older patients (≥67 years) initiating dialysis in the United States were studied. Eligible patients had Medicare fee-for-service coverage for ≥2 years before dialysis initiation and were followed for ≤3 years for a first hip fracture. Type of treatment (internal fixation or partial or total hip replacement) was ascertained along with 30-day mortality. Cox and modified Poisson regressions were used to describe trends in study outcomes.
Results
This study followed 409,040 patients over 607,059 person-years, during which time 17,887 hip fracture events were recorded (29.3 events/1000 person-years). Compared with patients incident for ESRD in 1996, adjusted hip fracture rates increased until the 2004 cohort (+41%) and declined thereafter. Surgical treatment included internal fixation in 56%, partial hip replacement in 29%, and total hip replacement in 2%, which remained essentially unchanged over time; 30-day mortality after hip fracture declined from 20% (1996) to 16% (2009).
Conclusions
Hip fracture incidence rates remain higher today than in patients reaching ESRD in 1996, despite multiple purported improvements in the management of CKD mineral bone disorder. Although recent declines in incidence and steady declines in associated short-term mortality are encouraging, hip fractures remain among the most common and consequential noncardiovascular complications of ESRD.
Introduction
Hip fracture is a major health concern in the general older population, resulting in substantial mortality, morbidity, and significant economic burden (1,2). Patients with CKD, especially those patients with ESRD requiring maintenance dialysis or kidney transplantation, are particularly burdened by an increased risk of hip fracture. The increased risk may be explained, at least in part, by the alterations in bone metabolism caused by impaired kidney function, including changes in phosphorus handling, vitamin D metabolism, and changes in parathyroid hormone production and secretion. With more than one half of a million Americans with ESRD and more than 110,000 new patients initiating dialysis every year (3), one half of whom are now older than 64 years, little is known about secular trends in hip fracture risk in the dialysis population, the treatment of affected patients, and their prognosis after hip fracture.
Hence, we examined secular trends in the incidence, treatment, and short-term outcomes of hip fracture among older individuals initiating dialysis in the United States (1996–2009). We hypothesized that hip fracture incidence and corresponding case fatality in older patients on dialysis had decreased over time.
Materials and Methods
Study Population
We used data from the US Renal Data System (USRDS) on all individuals ages 67 years or older who commenced treatment for ESRD in the United States between 1996 and 2009. We retained those individuals who had uninterrupted Medicare fee-for-service coverage for at least 2 years before their reported ESRD incidence (first service) date. In primary analyses, patients were also excluded if they had recorded history of hip fracture before their ESRD incidence date.
Outcomes
Hip fracture was ascertained from the presence of a corresponding International Classification of Diseases; 9th Revision code in a hospital billing claim to the Centers for Medicare and Medicare Services (formerly Health Care Financing Administration). The specific codes used are listed in Supplemental Table 1; such an approach to identify hip fracture has previously been validated and was found to have a sensitivity of 97% and a positive predictive value of 98% (4).
We also ascertained the treatment for hip fracture received within 3 days by affected patients, which were grouped as follows: partial hip replacement, total hip replacement, internal fixation, and other (which includes reduction, repair not otherwise specified, death, or no procedure). The specific procedure codes used are listed in Supplemental Table 1. Finally, we ascertained case fatality rates as indicated by 30-day mortality. We also tabulated the reported cause of these deaths.
Predictor
The key independent variable was the year of ESRD incidence, which ranged from 1996 to 2009. Year was considered categorical to allow flexibility for a potential nonlinear association between year of initiation and the hazard of hip fracture, with the cohort of older patients reaching ESRD in 1996 serving as the reference.
Other Characteristics
We ascertained each patient’s age at ESRD incidence, sex, and race (white, black, Asian, American Indian, or other). Information on Hispanic ethnicity, Quetélet (body mass) index, serum albumin, and estimated GFR (eGFR) at baseline was derived from each patient’s Medical Evidence Report. From all claims preceding the index date by 2 years, we ascertained a large number of comorbidities and other conditions, requiring presence of at least two outpatient or one inpatient diagnosis. The detailed algorithms are shown in Supplemental Table 2; although they were used for analysis, we also collapsed some of these categories for presentation of baseline characteristics in this work.
Statistical Analyses
Patient characteristics were summarized using counts (percentages) or medians (interquartile ranges) for all patients as well as by era of ESRD incidence (1996–1999, 2000–2004, or 2005–2009). Missing values were observed in 30.6% of the cohort. The most frequently missing variable was serum albumin, with a missing rate of 26.6%. Other variables with missing values included sex (<0.1%), race (0.1%), ethnicity (2.4%), body mass index (5.9%), and eGFR (4.1%). We used multiple imputation methods by chain equation using the ICE procedure in Stata with five imputed datasets (5).
Because of the presence of potential competing events (i.e., death, transplantation, and loss of Medicare coverage), we estimated the subdistribution hazard ratios (HRs) and 95% confidence intervals (95% CIs) from Cox proportional regression methods. Patients who did not experience a hip fracture event within 3 years were censored at the end of the 3-year follow-up or the end of the study period (December 31, 2009).
Temporal trends of 30-day case fatality after hip fracture were examined using relative risks estimated from Poisson regression methods. We performed bootstrap resampling (n=5000) to estimate the within- and between-imputation variance to calculate the overall standard error of the mean for each analytic model.
For both analyses, we fit a series of increasingly adjusted models, beginning with unadjusted models with index year as the only independent variable, then using demographics-adjusted models, and finally, ending with models that adjusted for all measured characteristics. We used two-sided inference tests throughout without adjustments for multiple testing.
Finally, among patients who experienced hip fracture, we conducted a descriptive analysis of the surgical procedures performed within 3 days from their date of hip fracture diagnoses. We grouped the procedures into four categories as detailed above and tabulated the proportions by index year.
Additional sensitivity analyses included analyses of individuals who had data available on all variables used (complete case) as well as time-to-event analyses estimating cause-specific HRs (not accounting for competing risk). In addition, we conducted sensitivity analyses that included patients with a history of hip fracture before ESRD. We excluded 6101 (34.1%) of all hip fracture events that coincided with coded fractures of the scull or upper extremity long bones (trying to separate traumatic from fragility fractures, although orthopedic experience teaches that even hip fractures from low-energy falls can often lead to accompanying skull or upper extremity fractures) and used Poisson instead of Cox regression. None of these sensitivity analyses altered the main findings in any material way (results not shown).
This study was approved by an Institutional Review Board of Stanford University School of Medicine. All analyses were conducted using SAS, Version 9.3 (The SAS Institute, Cary, NC), Stata, Version 12.1 (StataCorp, College Station, TX), and R, Version 2.14 (The R Project for Statistical Computing, Vienna, Austria).
Results
We identified 418,470 older patients who had at least 2 years of uninterrupted Medicare fee-for-service insurance before the initiation of treatment for ESRD between 1996 and 2009 (Supplemental Figure 1). Of these patients, 9430 (2.3%) were found to have had a history of hip fracture and excluded. The remaining 409,040 patients were followed over 607,059 person-years, during which time 17,887 hip fracture events were recorded, for an unadjusted incidence rate of 29.3 events per 1000 person-years. This rate increased slightly with time from ESRD incidence (27.4, 30.4, and 33.3 events per 1000 person-years in the first, second, and third years of follow-up, respectively).
Baseline characteristics of the study patients are shown in Table 1 (detailed descriptions in Supplemental Table 3). In brief, patients initiating treatment for ESRD in more recent years were older, more likely to be men, white, and Asian, and had generally higher rates of previously diagnosed comorbidities; body mass index and eGFR at initiation were higher in more recent years, whereas serum albumin was essentially unchanged.
Table 1.
Characteristics of study patients by era
| Characteristic | N (%) or Median (Interquartile Range) | |||
|---|---|---|---|---|
| All Patients | 1996–1999 | 2000–2004 | 2005–2009 | |
| Total (row %) | 409,040 (100.0) | 105,565 (25.8) | 152,790 (37.4) | 150,685 (36.8) |
| Age (yr) | 76.0 (71.0, 81.0) | 75.0 (71.0, 80.0) | 76.0 (72.0, 81.0) | 77.0 (72.0, 82.0) |
| Sex (N missing=4) | ||||
| Men | 212,398 (51.9) | 53,117 (50.3) | 78,212 (51.2) | 81,069 (53.8) |
| Women | 196,638 (48.1) | 52,448 (49.7) | 74,575 (48.8) | 69,615 (46.2) |
| Race (N missing=219) | ||||
| White | 312,829 (76.5) | 79,629 (75.4) | 116,265 (76.1) | 116,935 (77.6) |
| Black | 81,168 (19.8) | 22,289 (21.1) | 30,842 (20.2) | 28,037 (18.6) |
| Asian | 9861 (2.4) | 2082 (2.0) | 3426 (2.2) | 4353 (2.9) |
| Other | 4963 (1.2) | 1509 (1.4) | 2173 (1.4) | 1281 (0.9) |
| Ethnicity (N missing=9979) | ||||
| Non-Hispanic | 368,876 (90.2) | 94,087 (89.1) | 137,683 (90.1) | 137,106 (91.0) |
| Hispanic | 30,185 (7.4) | 7454 (7.1) | 11,790 (7.7) | 10,941 (7.3) |
| Body mass index (kg/m2; N missing=24,015) | ||||
| <18.5 | 23,661 (5.8) | 8807 (8.3) | 8575 (5.6) | 6279 (4.2) |
| 18.5–24.9 | 160,627 (39.3) | 43,567 (41.3) | 63,410 (41.5) | 53,650 (35.6) |
| 25.0–29.9 | 112,819 (27.6) | 23,977 (22.7) | 43,890 (28.7) | 44,952 (29.8) |
| ≥30.0 | 87,918 (21.5) | 13,953 (13.2) | 32,496 (21.3) | 41,469 (27.5) |
| Albumin (g/dl; N missing=108,787) | 3.2 (2.7, 3.6) | 3.2 (2.8, 3.6) | 3.2 (2.7, 3.6) | 3.2 (2.7, 3.6) |
| eGFR (ml/min per 1.73 m2; N missing=16,776) | 9.6 (7.1, 13.0) | 8.1 (6.1, 10.8) | 9.5 (7.1, 12.9) | 10.8 (8.1, 14.3) |
| Comorbid conditions | ||||
| Cerebrovascular disease | 132,395 (32.4) | 32,885 (31.2) | 48,980 (32.1) | 50,530 (33.5) |
| Cardiovascular disease | 88,594 (21.7) | 22,112 (20.9) | 34,203 (22.4) | 32,279 (21.4) |
| Atrial fibrillation | 118,556 (29.0) | 26,696 (25.3) | 44,422 (29.1) | 47,438 (31.5) |
| Other arrhythmias | 99,712 (24.4) | 23,850 (22.6) | 37,062 (24.3) | 38,800 (25.7) |
| Substance abuse | 10,191 (2.5) | 2383 (2.3) | 3809 (2.5) | 3999 (2.7) |
| Dementia | 36,611 (9.0) | 7616 (7.2) | 13,735 (9.0) | 15,260 (10.1) |
| Psychiatric disease | 57,279 (14.0) | 12,488 (11.8) | 21,420 (14.0) | 23,371 (15.5) |
| Diabetes | 237,735 (58.1) | 56,420 (53.4) | 88,516 (57.9) | 92,799 (61.6) |
| Hypertension | 381,270 (93.2) | 95,667 (90.6) | 141,516 (92.6) | 144,087 (95.6) |
| Heart failure | 272,601 (66.6) | 70,941 (67.2) | 102,247 (66.9) | 99,413 (66.0) |
| Hyperparathyroidism | 13,253 (3.2) | 913 (0.9) | 3338 (2.2) | 9002 (6.0) |
| Cancer | 83,017 (20.3) | 20,132 (19.1) | 30,416 (19.9) | 32,469 (21.5) |
| Liver disease | 24,998 (6.1) | 4904 (4.6) | 8819 (5.8) | 11,275 (7.5) |
| Lung disease | 159,547 (39.0) | 37,713 (35.7) | 59,076 (38.7) | 62,758 (41.6) |
| Peripheral vascular disease | 148,836 (36.4) | 36,686 (34.8) | 55,695 (36.5) | 56,455 (37.5) |
| Rheumatologic disease | 22,142 (5.4) | 5646 (5.3) | 8064 (5.3) | 8432 (5.6) |
| Tobacco use | 20,013 (4.9) | 3689 (3.5) | 7645 (5.0) | 8679 (5.8) |
eGFR, estimated GFR.
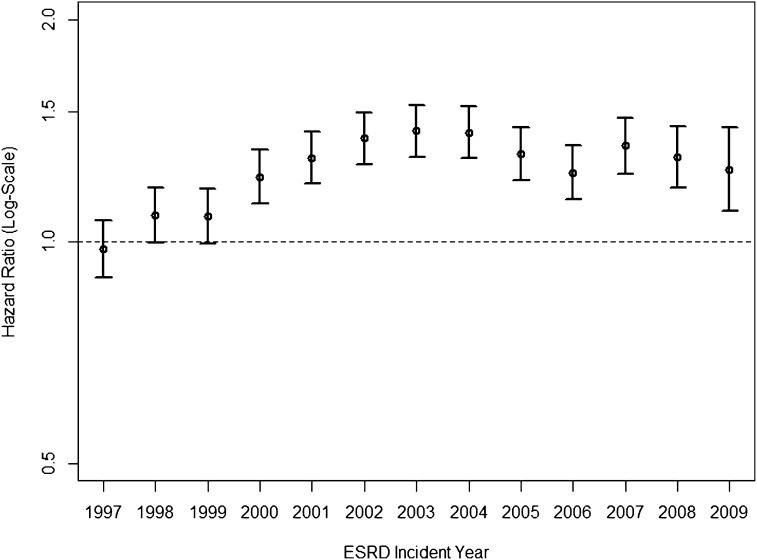
Compared with the time to hip fracture in older patients initiating treatment for ESRD in 1996, the HRs increased considerably over the subsequent years and were 43% (95% CI=32% to 55%) higher in patients initiating dialysis in 2004 in unadjusted analyses. Thereafter, the HRs declined but remained 27% (95% CI=12% to 44%) higher in patients in 2009 compared with 1996. Similar, albeit slightly attenuated, patterns were observed after adjustment for demographic factors (age, sex, race, and ethnicity) or when additionally adjusting for all baseline comorbidities (Figure 1). Estimates of subdistribution and cause-specific HRs were almost identical (Supplemental Table 4).
Figure 1.
Relative hazards of incident hip fracture in annual cohorts of older patients new to dialysis. Note that multivariable hazard ratios (HRs) adjusted for age, sex, race, Hispanic ethnicity, body mass index, serum albumin concentration, estimated GFR at initiation of dialysis, and all comorbidities are listed in Supplemental Table 3. Patients initiating dialysis in 1996 served as the reference group for all annual comparisons. The average incidence rate of hip fracture over up to 3 years of follow-up in this cohort was 29.3 events per 1000 person-years.
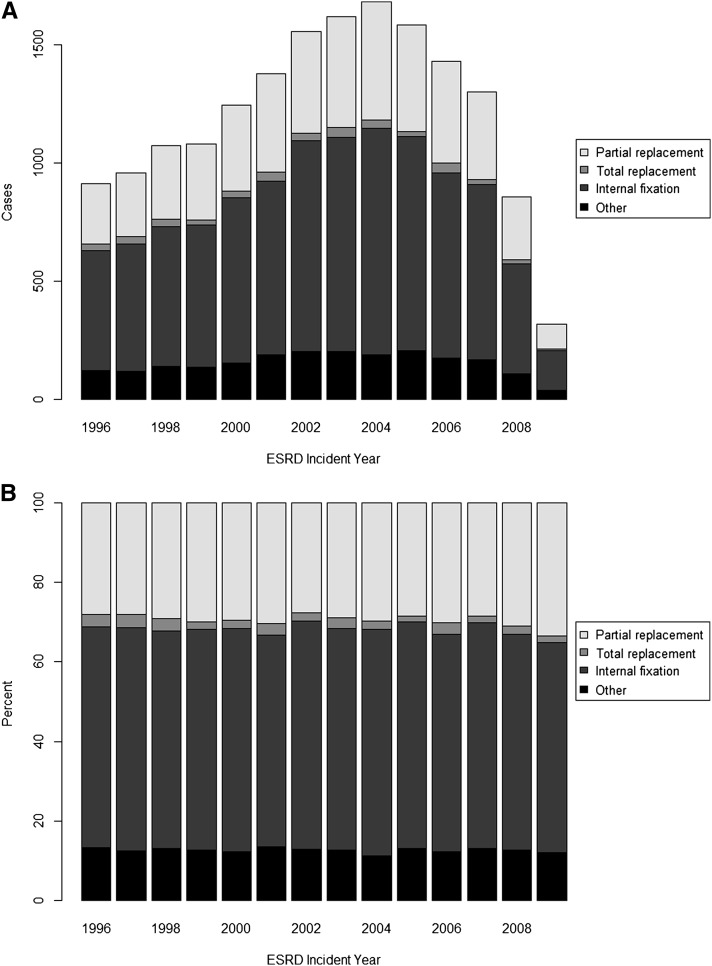
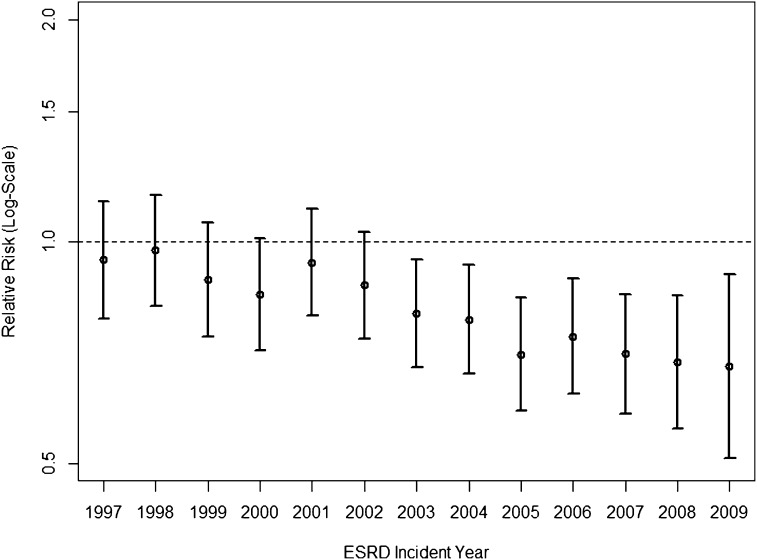
Treatment for most individuals with hip fracture across all years was internal fixation (55.9%), whereas 29.2% of individuals underwent partial hip replacement, and 2.4% of individuals received a total hip replacement. Only a small percentage of patients were treated conservatively or died within 3 days of the hip fracture without any evidence of hip surgery. We did not observe a noticeable trend in treatment patterns; the proportions of each treatment group were fairly consistent across the study period (Figure 2). Among 17,887 individuals with hip fracture, 17.4% (95% CI=16.9% to 18.0%) died within 30 days. However, case fatality decreased considerably over the 14 years of study: compared with patients experiencing a hip fracture event who had initiated ESRD treatment in 1996, those patients who did in 2009 had 32% (95% CI=10% to 49%) lower risks of 30-day mortality after multivariable adjustment (Figure 3). Cause of death was coded as cardiac or vascular in the majority of cases (50.9%) and infectious in 10.8%. Of note, the proportion of cardiovascular causes declined in more recent years (from 57% in 1996 to 40% in 2009) (Supplemental Figure 2). Results from multiple imputation-based analyses were comparable with those results from complete case analyses (Supplemental Table 4).
Figure 2.
Trends in the treatment for hip fracture in older dialysis patients. (A) Counts. (B) Percentages.
Figure 3.
Trends in the 30-day mortality after hip fracture in older patients on dialysis. Note that multivariable relative risks adjusted for age, sex, race, Hispanic ethnicity, body mass index, serum albumin concentration, estimated GFR at initiation of dialysis, and all comorbidities are listed in Supplemental Table 3. All relative risks compare the 30-day mortality after hip fracture of patients incident for ESRD in the respective year versus those incident for ESRD in 1996.
Discussion
We found that hip fracture incidence showed an initial increase from the 1996 to the 2003–2004 incident ESRD cohorts. Thereafter, the hip fracture incidence decreased again but remained at a 27% higher adjusted rate in patients initiating dialysis in 2009 compared with patients initiating dialysis in 1996. Most interestingly and not previously described in the dialysis population, there was the high 30-day case fatality, which was as high as 19.7% in 1996, but it declined to 15.9% in 2009 for a case mix-adjusted relative reduction of 32%.
Previous studies on the epidemiology of hip fracture in US dialysis patients were based on patients observed before 2004. Alem et al. (6) studied the risk of hip fracture among Caucasian hemodialysis patients who initiated dialysis between 1989 and 1996 and compared their experience with the general population. The work by Alem et al. (6) found that the incidence of hip fracture was 7.5/1000 person-years for men and 13.6/1000 person-years for women on dialysis, and there was an overall relative risk of 4.4 compared with age- and sex-matched individuals from the general population (6). These findings are difficult to compare with our findings, because the work by Alem et al. (6) considered patients of all ages, whereas we studied only patients ages 67 years and older. We did so to obtain detailed, valid, claims-based data on comorbidity that are not routinely available in younger patients without up-front Medicare eligibility. The Dialysis Outcomes and Practice Patterns Study (DOPPS) collected data between 2002 and 2004 in 12 countries and found the hip fracture incidence in the United States to be 9.9/1000 person-years (7). The DOPPS was also unrestricted by age and may have underascertained the outcome of interest because of its data collection design (per facility questionnaire every 4 months rather than continuous monitoring) that may have missed hip fracture events that led to early subsequent deaths. However, patients waitlisted for a transplant (2.9/1000 person-years) as well as prior transplant recipients (3.3/1000 person-years) had a lower incidence of hip fracture (8). Our study shows an incidence rate higher than these studies, with an average rate of 29.3/1000 person-years, in part because of the restriction to patients over the age of 67 years.
There are several reasons that support a priori considerations of changing trends in hip fracture. First, increasingly sicker and frailer patients have been accepted over the past decades into dialysis programs, which can be gleaned from trends in baseline characteristics in our study. Indeed, there is a substantial burden of conventional hip fracture risk factors in the dialysis population, including advanced age, female sex, white race, peripheral arterial disease, hypoalbuminemia, low body mass index, and abnormal parathyroid hormone (PTH) levels (PTH was unavailable in USRDS data) (7,9,10). Second, although targets for serum phosphorus, calcium, and PTH concentrations have been established in clinical practice guidelines, treatment strategies have changed considerably in the United States over the time frame covered by the study. Over these years, the use of intravenous vitamin D sterols (1,25-dihydroxy vitamin D and synthetic vitamin D derivatives) has markedly increased. Noncalcium-containing phosphate binders have been widely used (since 2000) alone or in conjunction with calcium-based phosphate binders. Calcimimetics became commercially available in 2004. In the DOPPS, Blayney and Tentori (11) reported that the median serum phosphorus and calcium values have decreased to ranges recommended by European best practice guidelines. Also, the electronic collection laboratory data national trend reports for 2002–2011 by ESRD network showed similar trends, with increases of the proportions of patients with mean serum phosphorus and calcium concentrations in the recommended target ranges (12). Lower parathyroidectomy rates (13), especially in the era of calcimimetics, also suggest better PTH control.
Our results in the older ESRD population differ from the results in older adults in the general population. Brauer et al. (14) studied incidence of hip fracture in US Medicare beneficiaries ages >65 years from 1985 to 2005 and reported that the age-adjusted hip fracture incidence steadily declined by 24.5% from 1995 to 2005. Wright et al. (15) used more recent data (also on Medicare beneficiaries >65 years old from 2000 to 2009) and found an overall continuation of the decreasing trend of hip fracture incidence by 8% in men and 14% in women. The increasing use of bisphosphonates was proposed as a possible factor for this encouraging trend in the general population. Because bisphosphonates are contraindicated in patients with advanced CKD, one could speculate that the discrepant findings in hip fracture incidence among patients undergoing dialysis and the general population may partly be explained by differences in bisphosphonate or other medication use.
In general, the mainstay of treatment for hip fracture is surgical repair of the fracture or arthroplasty. The modality of treatment chosen in a patient on dialysis, however, may vary from the general population because of the higher prevalence of comorbidities. The majority of patients (56% overall) underwent internal fixation, whereas 31.6% underwent total or partial hip replacement. We could not identify any clear time trends in the relative use of these treatments. Based on the fact that partial or complete hip replacement is used for intracapsular femoral neck fractures and that internal fixation is used mainly for peritrochanteric fractures, one can deduce that there was no change in regard to the overall type of hip fracture sustained over time in this population. This finding would stand to reason, because there was no identifiable factor in these patients’ overall care that would favor a trend to one type of fracture over another about the proximal femur.
The current study showed that about one in eight (12.5%) patients may have been treated conservatively. This fraction is comparable with the findings in a Canadian study (1992–1998), which showed that 11% of individuals in the general population who experienced a hip fracture were treated nonoperatively (16). However, in a Scottish national audit, the proportion of patients managed conservatively was as low as 2.6% (17).
Our study yielded additional important insights. Case fatality after hip fracture was (and remains) extremely high (at almost 20% in 1996), although 30-day mortality has declined considerably over the 14 years of study. This finding contrasts with the mortality trend in the overall population of older patients initiating dialysis, which is approximately 35% in the first year (or 30-day mortality of <3%) and has remained unchanged between 1996 and 2006 (18). Although the 30-day case fatality has shown a favorable trend, it still remains high (at 15.9% in 2009) compared with the general population. The 30-day mortality from 1995 to 2005 was stable at approximately 10% in the US Medicare population (14), which is markedly lower than in the ESRD population. Although the cause of death after hip fracture in the general population after surgical treatment is mainly caused by cardiac complications, thromboembolism, pneumonia, and sepsis, one can only speculate about the causes of this improvement in outcomes after hip fracture in older patients on dialysis. Putative improvements in anesthesia and surgical techniques and adoption of early mobilization and aggressive early rehabilitation regimens may have played a role in this development.
Our study has certain limitations that require disclosure and discussion. Because we based our event (or outcome) definition on administrative claims data, there may be underascertainment of hip fractures, because only hospitalized patients were included. However, most hip fractures require hospitalization, and hip fracture has been found to be reliably coded in other administrative databases (19). Approaches similar to our approach have been used in numerous studies, and they have been validated and found to possess high accuracy and positive predictive value (4,19,20). There may also have been overascertainment of hip fractures; however, approximately 90% of fracture events were accompanied by billings for corresponding surgical treatments, indicating that hip fractures were, indeed, present. The proportion of patients with a hip fracture who had no evidence of surgical intervention was small, and comparable with other reports, and constant over the 14 years of study. Also, because we looked for claims within 3 days of incidence of hip fracture, we may have missed patients who received surgical treatment at a later date. Finally, despite the availability of unusually rich baseline information from pre-ESRD Medicare claims, residual confounding may be present by concurrent temporal changes of other risk factors for hip fractures, which were unavailable or imprecisely measured.
In summary, despite several purported advances in the management of CKD mineral bone disorder, hip fracture remains a common and highly consequential complication of ESRD. Incidence rates remain high above corresponding rates in the age-matched general population. Fortunately, hip fracture incidence has declined since 2005, and case fatality has been slowly but steadily declining since 1996. Although adherence to clinical practice guidelines—based largely on opinion and limited evidence—can be argued to have improved practice, serum phosphorus, calcium, and PTH are intermediate markers. Fractures along with their associated morbidity and disability are what matter more to patients. Carefully conducted large-scale clinical trials considering adjudicated hip and/or other serious fractures as key primary or secondary end points will be required to define optimal management strategies for CKD mineral bone disorder.
Disclosures
G.M.C. has received research support from Amgen and is an advisor to Ardelyx and Keryx. W.C.W. has served as an advisor to Amgen, Keryx, and Vifor-Fresenius Medical Care Renal Pharma. The other authors have no conflicts to report.
Supplementary Material
Acknowledgments
This study was funded by an unrestricted research fellowship grant from Sanofi (to S.S.N.). G.M.C. was supported for this work by National Institutes of Health Grant K24 DK085446.
Data were provided through a data use agreement between the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) and W.C.W. An NIDDK officer reviewed this manuscript for privacy and approved of its publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10901012/-/DCSupplemental.
References
- 1.Braithwaite RS, Col NF, Wong JB: Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc 51: 364–370, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A: Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22: 465–475, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis 59: e1–420, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Ray WA, Griffin MR, Fought RL, Adams ML: Identification of fractures from computerized Medicare files. J Clin Epidemiol 45: 703–714, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Royston P, White IR: Multiple imputation by chained equations (MICE): Implementation in Stata. J Stat Softw 45: 1–20, 2011 [Google Scholar]
- 6.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C: Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58: 396–399, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, Kestenbaum BR, Stehman-Breen C: Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288: 3014–3018, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Stehman-Breen CO, Sherrard DJ, Alem AM, Gillen DL, Heckbert SR, Wong CS, Ball A, Weiss NS: Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 58: 2200–2205, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Coco M, Rush H: Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 36: 1115–1121, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Blayney MJ, Tentori F: Trends and consequences of mineral bone disorder in haemodialysis patients: Lessons from The Dialysis Outcomes and Practice Patterns Study (DOPPS). J Ren Care 35[Suppl 1]: 7–13, 2009 [DOI] [PubMed] [Google Scholar]
- 12.National Elab 2011 and Trends Report, Renal Network of the Upper Midwest, St. Paul, MN. Available at: http://www.esrdnet11.org/assets/pdf/elab_national_and_trends_2011.pdf. Accessed April 12, 2013
- 13.Li S, Chen YW, Peng Y, Foley RN, St Peter WL: Trends in parathyroidectomy rates in US hemodialysis patients from 1992 to 2007. Am J Kidney Dis 57: 602–611, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB: Incidence and mortality of hip fractures in the United States. JAMA 302: 1573–1579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright NC, Saag KG, Curtis JR, Smith WK, Kilgore ML, Morrisey MA, Yun H, Zhang J, Delzell ES: Recent trends in hip fracture rates by race/ethnicity among older US adults. J Bone Miner Res 27: 2325–2332, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Jain R, Basinski A, Kreder HJ: Nonoperative treatment of hip fractures. Int Orthop 27: 11–17, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scottish Hip Fracture Audit National Trend Analysis. 1998–2004. ISD Scotland Publications, NHS National Services Scotland, Edinburgh, Scotland, 2005. Avaliable at: http://www.shfa.scot.nhs.uk/AnnualReport/SHFA_Trend_Analysis.pdf. Accessed April 12, 2013.
- 18.Winkelmayer WC, Liu J, Chertow GM, Tamura MK: Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern Med 171: 1371–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virnig B, Durham SB, Folsom AR, Cerhan J: Linking the Iowa Women’s Health Study cohort to Medicare data: Linkage results and application to hip fracture. Am J Epidemiol 172: 327–333, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baron JA, Lu-Yao G, Barrett J, McLerran D, Fisher ES: Internal validation of Medicare claims data. Epidemiology 5: 541–544, 1994 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.