Summary
Background and objectives
Cell-based therapy with natural (CD4+CD25hiCD127lo) regulatory T cells to induce transplant tolerance is now technically feasible. However, regulatory T cells from hemodialysis patients awaiting transplantation may be functionally/numerically defective. Human regulatory T cells are also heterogeneous, and some are able to convert to proinflammatory Th17 cells. This study addresses the suitability of regulatory T cells from hemodialysis patients for cell-based therapy in preparation for the first clinical trials in renal transplant recipients (the ONE Study).
Design, setting, participants, & measurements
Healthy controls and age- and sex-matched hemodialysis patients without recent illness/autoimmune disease on established, complication-free hemodialysis for a minimum of 6 months were recruited. Circulating regulatory T cells were studied by flow cytometry to compare the regulatory T cell subpopulations. Regulatory T cells from members of each group were compared for suppressive function and plasticity (IL-17–producing capacity) before and after in vitro expansion with and without Rapamycin, using standard assays.
Results
Both groups had similar total regulatory T cells and subpopulations I and III. In each subpopulation, regulatory T cells expressed similar levels of the function-associated markers CD27, CD39, HLA-DR, and FOXP3. Hemodialysis regulatory T cells were less suppressive, expanded poorly compared with healthy control regulatory T cells, and produced IL-17 in the absence of Rapamycin. However, Rapamycin efficiently expanded hemodialysis regulatory T cells to a functional and stable cell product.
Conclusions
Rapamycin-based expansion protocols should enable clinical trials of cell-based immunotherapy for the induction of tolerance to renal allografts using hemodialysis regulatory T cells.
Introduction
Although solid-organ transplantation is the treatment of choice for end stage kidney disease (ESKD), the use of broad-spectrum immunosuppressive drugs results in accelerated mortality (1), is toxic to transplants, and does not prevent chronic rejection (2). Thus, the establishment of clinical tolerance to engrafted tissues to minimize or eliminate immunosuppression is a key research goal.
Natural CD4+CD25hiCD127loFOXP3+ regulatory T cells (Tregs) that physiologically prevent autoimmune diseases by inhibiting target cells, including responder (CD4+CD25−) T cells (Tresps) and antigen-presenting cells (3), can prevent allograft rejection in animal models (4,5). Experimental induction of immunologic transplant tolerance in animal models is associated with increased Treg numbers in both the transplant and regional lymphoid tissue (6,7). This finding mirrors recent human data correlating Treg infiltration of renal transplants and outcome in borderline rejection (8).
Functional Tregs from healthy individuals can now be selected and expanded polyclonally in vitro under good manufacturing practice (GMP) conditions (4,9,10). Thus, clinical cell therapy with Tregs is a realistic possibility. Indeed, case series and phase I studies have shown beneficial outcomes in type 1 diabetes mellitus (11) and the prevention or treatment of post-bone marrow transplantation graft versus host disease in humans (9,12,13).
Human Tregs are heterogeneous, being divided into three functionally distinct populations based on differential expression of the naïve cell marker CD45RA, CD25, and the transcription factor FOXP3 (14): populations I (CD4+CD25hiCD127loCD45RA+), II (CD4+CD25brightCD127loCD45RA−), and III (CD4+CD25hiCD127loCD45RA−). Population I is the most abundant in umbilical cord blood and matures to populations III and II on activation. The latter are effector Tregs that are highly suppressive and short-lived, whereas the former are circulating memory-type Tregs (14). Other surface markers have also been used to delineate functional Treg subsets, such as costimulatory molecules (CD27) (15), ectoenzymes (CD39) (16), HLA-DR (17), and the memory T cell marker CD45RO (14,18). Treg phenotypes can be plastic (19), and some Treg subpopulations (especially those subpopulations in population III) (14) have the capacity to express proinflammatory cytokines and transcription factors more typically seen in Th1 and Th17 lineages (14,20,21). Consequently, successful programs of cell therapy will be critically dependent on the selection of the most appropriate Treg populations for infusion into humans.
ESKD patients awaiting transplantation are immunologically unique in showing features of both immunodeficiency (22–24) and chronic inflammation/immune dysregulation (25–27). ESKD patients have reduced peripheral blood Tregs (28) and naïve T cells (29), implying a deficiency of naïve Tregs, the population most resistant to Th17 conversion in vitro (14). Here, we sought to characterize the phenotype and function of Tregs from ESKD patients on hemodialysis (HD) compared with healthy controls (HCs) and determine if Tregs from these patients could be expanded ex vivo in GMP-compatible conditions.
Materials and Methods
Participant Selection
Fourteen patients with ESKD established on HD for at least 6 months without HD-associated complications and fourteen age- and sex-matched HCs were recruited after informed consent (Institutional Review Board approval 09/H0707/86). Exclusion criteria included recent illness (within the previous 2 months), significant anemia, autoimmune disease, current/previous immunosuppressive medicines, and previous transplantation. HCs were clinically well for at least 2 months. Participant characteristics are summarized in Table 1.
Table 1.
Participant demographics
| Characteristic | HCs | HD Patients | P |
|---|---|---|---|
| Number | 14 | 14 | N/A |
| Sex (men:women ) | 7:7 | 7:7 | N/A |
| Age (mean ± SD; yr) | 49±12 | 49±9.9 | 0.64 |
| Time on dialysis (median [25th and 75th percentiles]; mo) | N/A | 50 (28.7, 77.0) | N/A |
| Cause of ESKD | |||
| Diabetes mellitus type 2 | N/A | 5 | N/A |
| Renovascular | N/A | 2 | N/A |
| Hypertension | N/A | 2 | N/A |
| Urological | N/A | 1 | N/A |
| IgA nephropathya | N/A | 1 | N/A |
| FSGS | N/A | 1 | N/A |
| Unknownb | N/A | 2 | N/A |
N/A, not applicable; ESKD, end stage kidney disease
This patient initially had ESKD of unknown etiology, which was later assumed to be caused by IgA nephropathy. He has been retained in the analysis.
Both assumed to be secondary to hypertensive nephropathy.
CD4+ T Cell Enrichment
PBMCs isolated from fresh blood as previously described (30) were enriched for CD4+ T cells using the MACS CD4+ T Cell Isolation Kit II (Miltenyi Biotech, Germany) according to the manufacturer’s instructions. Enrichment was typically to >90% CD4+ lymphocytes.
Flow Cytometry
Flow cytometry using CD4+ T cells stained with CD4-Qdot 605, CD39-FITC, CD127-PerCP-Cy5.5, HLA-DR-EF450 (eBioscience, United Kingdom), CD25-PE (MA251), CD27-APC-H7, CD45RO-PECy7 (all Becton-Dickinson, San Jose, CA), and CD45RA-AF700 (Biolegend, United Kingdom) was carried out on an FACSCalibur or LSR II flow cytometer (Becton-Dickinson). Appropriate isotype controls and Fluorescence Minus One controls were used to assign gates. Gating strategies used to identify Treg subpopulations are shown in Supplemental Figure 1 (14).
Intracellular staining for FOXP3 used the kit and anti–FOXP3-FITC from eBioscience according to the manufacturer’s instructions. For double–double staining with IL-17-PE (eBioscience), PMA (50 ng/ml; Sigma), ionomycin (1 mM; Sigma), and Brefeldin A (3 μg/ml; eBioscience) were added to cell cultures 4.5 hours before intracellular staining.
Ex Vivo Treg Expansion
PBMCs were depleted for CD8+ followed by enrichment for CD25+ using magnetic beads (Miltenyi). Enriched cells were typically >95% CD25hiFOXP3+ (data not shown). Cells were plated at 106/ml in X-VIVO 15 Medium (Lonza, MD) containing 5% human AB serum (Biosera, United Kingdom) ±100 nM Rapamycin (31,32) (Rapamune, Wyeth, United Kingdom) and activated with anti-CD3/CD28–coated microbeads (2:1 bead:cell ratio; Dynabeads; Invitrogen, United Kingdom). HuRecombinant IL-2 (500 IU/ml; Proleukin; Novartis, United Kingdom) was added at day 4 and replenished every second day. Every 10–12 days, beads were removed by magnetic adherence, and cells were restimulated as before with fresh Rapamycin and IL-2.
Suppression Assay
Suppression of Tresp proliferation by Tregs was assessed using the standard carboxyfluorescein succinimidyl ester dilution method (33) comparing allogeneic Tresp from normal volunteers cultured alone with Tresp cocultured 1:1 with Tregs at Treg:Tresp ratios of 1:1, 1:5, and 1:10 (constant Tresp numbers). For comparison of suppression of Tresps by Tregs, the Tregs:Tresp ratio needed to cause 50% suppression (IC50; the higher the IC50, the less suppressive the Tregs) and maximum suppression (Smax) were calculated for each population as per the methods described in refs. 34 and 35.
IL-17 Production
To assess IL-17 production, Tregs were cultured in the presence of IL-2 (10 IU/ml), IL-1β (10 ng/ml), IL-6 (4 ng/ml), and TGF-β (5 ng/ml; R&D Systems) as described in ref. 32 before harvest of supernatants at 5 days.
ELISA
ELISA for human IL-17 was carried out using the Duo-Set ELISA Kit from R&D (Abingdon, United Kingdom) according to manufacturer’s instructions and expressed as IL-17 concentration per million live cells in the assay.
Data Analysis
Data analysis used GraphPad Prism (LaJolla, CA). Data are presented as mean ± SD or median (interquartile range) for parametric and nonparametric data, respectively, and compared using paired t or Wilcoxon signed rank tests as appropriate. A P value<0.05 was considered statistically significant.
Results
Participant Demographics
Fourteen HD patients and fourteen age- and sex-matched HCs participated in this study based on the inclusion and exclusion criteria listed in Materials and Methods. Demographic data are summarized in Table 1.
HD Patients Have Treg Populations Corresponding to HCs
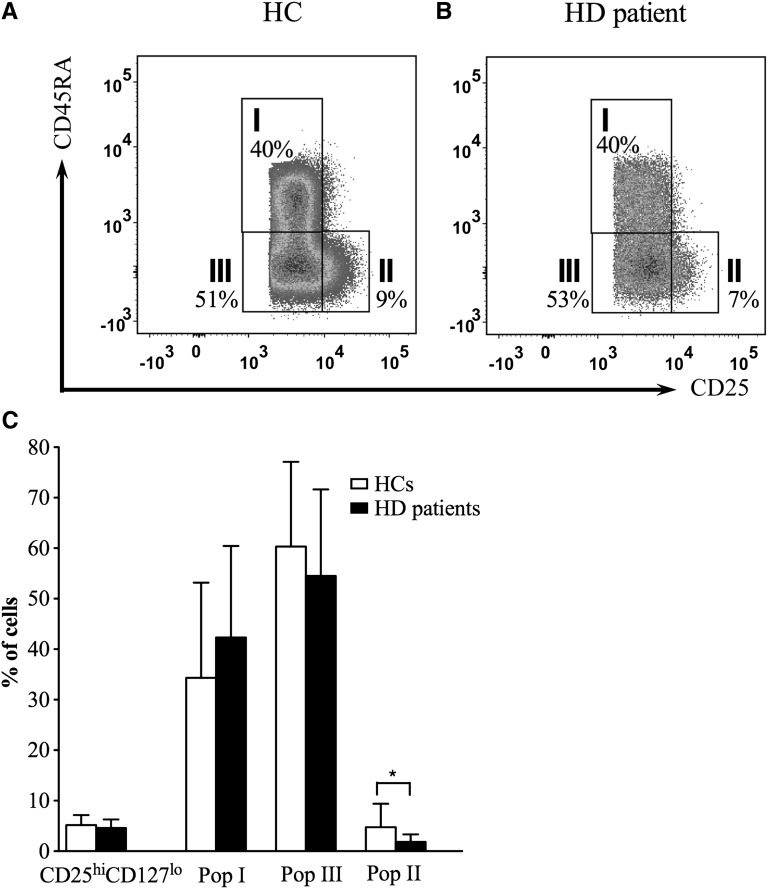
To compare circulating Tregs between HD patients and HCs, enriched CD4+ T cells were phenotyped by flow cytometry. The percentage of Tregs in the total CD4+ population did not differ between the two cohorts (5.2%±2.0% compared with 4.6%±1.7% for HCs and HD patients, respectively, P=0.41) (Figure 1 and Table 2). Because previous reports had suggested that patients on HD should have fewer naïve Tregs relative to HCs (29), we next compared the three populations described in the work by Miyara et al. (14) in HCs and patients on HD. Population III was the largest subpopulation of Tregs in both groups (Figure 1 and Table 2). Although the proportions of Treg populations I and III were similar between both groups, a paucity of cells in population II was seen in HD patients (Figure 1).
Figure 1.
Comparison of regulatory T cell (Treg) numbers between healthy controls (HCs) and hemodialysis (HD) patients. (A and B) Representative examples from 14 participants of Treg populations I–III in (A) HCs and (B) patients on HD. Please note that the different appearance of the dot plots is because of data acquisition on different FACS machines on separate days. (C) Pooled data from 14 participants in each cohort showing mean percentage (± SD) of total Tregs (CD4+CD25hiCD127lo) in the CD4+ population and the percentage of Treg subpopulations I–III within that population. *P<0.05.
Table 2.
Treg subpopulations in HCs and HD patients
| Population | Mean Percent (± SD) of Cells in HCs (n=14) | Mean Percent (± SD) of Cells in HD Patients (n=14) | P |
|---|---|---|---|
| CD4+CD25hiCD127lo | 5.2±2.0 | 4.6±1.7 | 0.41 |
| Population I | 34.3±18.8 | 42.3±18.1 | 0.27 |
| Population II | 4.7±4.7a,b | 1.8±1.5b | <0.05 |
| Population III | 60.3±16.8a | 54.5±17.1 | 0.37 |
n=14 for both groups.
P<0.05 compared with population I.
P<0.05 compared with population III.
Naïve CD45RA+ Tregs decrease with age, with a reciprocal increase in the proportion of CD45RO+ Tregs (36), which may influence the feasibility of isolating certain Treg populations for therapeutic use. To determine whether significant age-dependent changes in Treg subsets occurred in HD patients, the effect of age on Treg populations I and III was examined by linear regression. Neither group showed a significant reduction in population I Tregs (HC: R2=0.02, P=0.71; HD: R2=0.004, P=0.86) nor an appreciable increase in population III Tregs (HC: R2=0.06, P=0.49; HD: R2=0.004, P=0.86) with age.
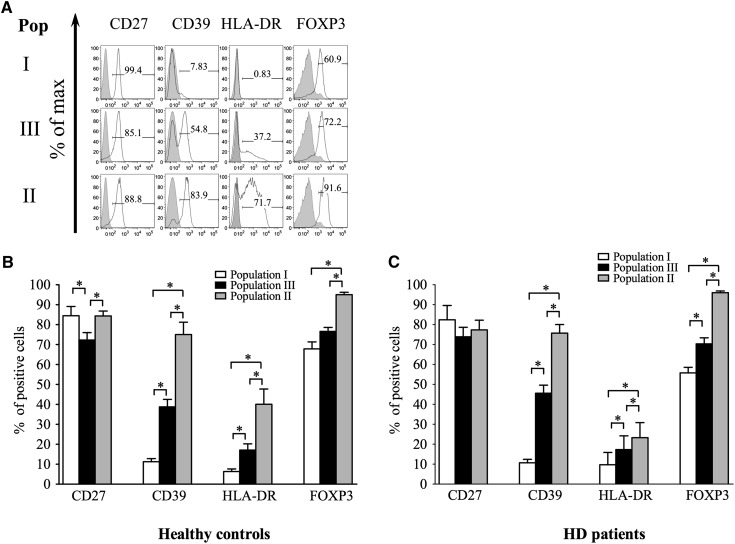
To see whether differences existed in Treg activation and maturation between HCs and HD patients, the expression of Treg activation/maturation markers was compared between Treg populations in both groups (Figure 2) (14–18). As expected, in both cohorts, stepwise increases in the percentage of cells expressing CD39, HLA-DR, and FOXP3 between populations I, III, and II were seen, correlating with maturation status (Figure 2, B and C). Expression of these markers on Treg subpopulations did not, however, differ between patients on HD and HCs, indicating that Treg maturation and activation within individual subsets is similar between HD patients and HCs.
Figure 2.
Expression of FOXP3 and surface markers on gated populations I–III. A shows one representative example from 14 HCs. Numbers on gates show frequency (percentage) of parent population. (B and C) Pooled data from 14 (B) HCs and (C) HD patients showing mean percentage expression ± SD of each marker in the three Treg populations. *P<0.05.
Tregs from Dialysis Patients Can Be Expanded Using Existing GMP-Compliant Protocols While Retaining Suppressive Function
Having established that HD patients have the same numbers of Tregs as HCs with normal expression of maturation and activation markers within subsets, albeit with slight reduction in population II, we next sought to determine whether they could be expanded in vitro under the GMP-compatible conditions (13,32) as required for a program of cell therapy.
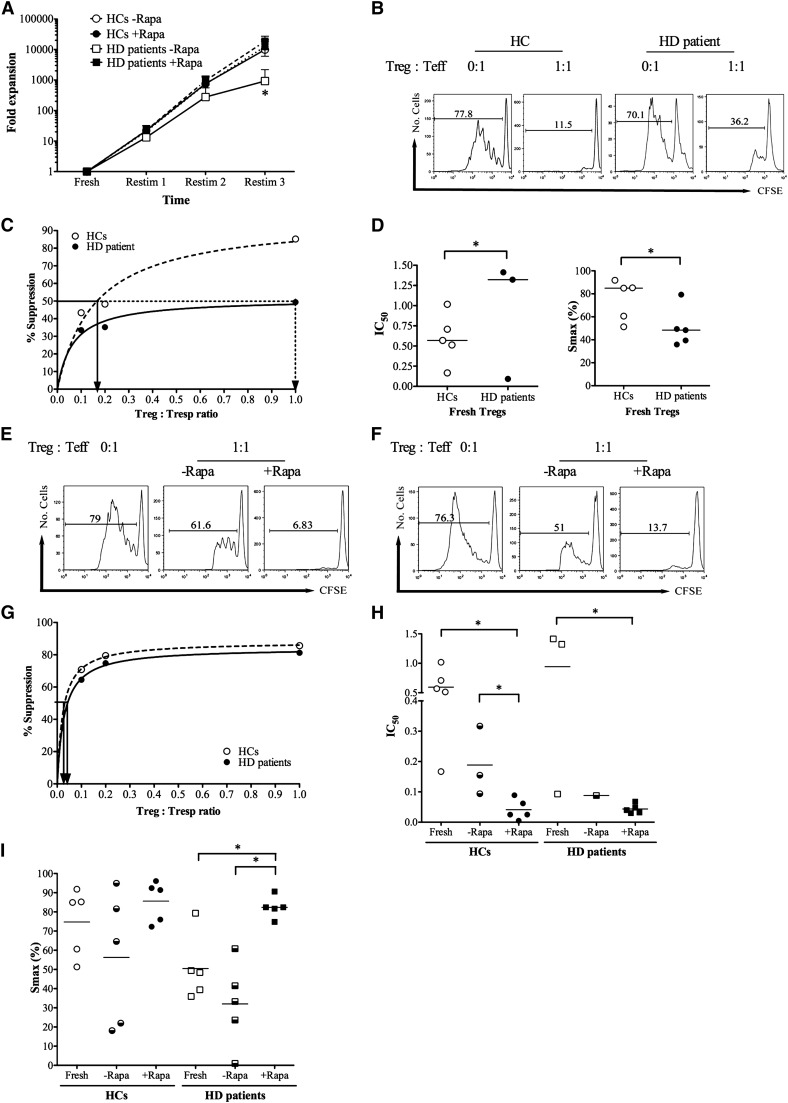
Freshly isolated Tregs from five HD patients and five HCs were expanded in vitro as described in Materials and Methods in the presence or absence of Rapamycin. Baseline CD25 and FOXP3 expression was similar in Tregs isolated from both cohorts (Supplemental Figure 2, A and B). Tregs from both groups expanded rapidly in the presence or absence of Rapamycin (Figure 3A). Although Tregs from HD patients proliferated slower than the Tregs from HCs in the absence of Rapamycin, no significant difference was observed between HC and HD Tregs in the presence of Rapamycin (Figure 3A). Expression of FOXP3 and CD25 was comparable at the end of expansion (Supplemental Figure 2, C and D).
Figure 3.
In vitro expansion of Tregs from HD patients under good manufacturing practice (GMP) -compatible conditions and suppressive function before and after expansion. (A) Fold expansion of Tregs per restimulation (restim) in the presence and absence of Rapamycin. *P<0.05 relative to no Rapamycin. (B–D) Baseline suppression of carboxyfluorescein succinimidyl ester (CFSE) -labeled autologous responder T cells (Tresps). (B) A representative example of suppression of CFSE dilution in the absence (0:1) and presence (1:1) of Tregs at 1:1 ratio. (C) Representative regression plot of percent suppression against Treg:Tresp ratio for the calculation of the Treg:Tresp ratio sufficient for 50% suppression (IC50); straight lines show IC50 for HCs, and dotted lines show IC50 for HD patients. (D) Cumulative IC50 and maximum suppression (Smax) for Tregs from five HCs and five patients on HD. Please note that only three IC50 values could be calculated for HD Tregs, because the other two were poorly suppressive. (E–I) Suppressive capacity of Tregs after expansion in vitro in the absence (−Rapa) and presence (+Rapa) of Rapamycin. (E and F) Representative examples of suppression of CFSE dilution in the absence (0:1) and presence (1:1) of Tregs at 1:1 ratio for (E) HC and (F) HD Tregs. (G) Representative regression plot of percent suppression against Treg:Tresp ratio; straight lines show IC50 for HCs, and dotted line show IC50 for paired HD patient. The example is taken from a Rapamycin-treated pair of Tregs. (H and I) IC50 and Smax for Tregs expanded with and without Rapamycin compared with freshly isolated Tregs. Please note that missing IC50 values are because of poor suppressive ability. *P<0.05.
Carboxyfluorescein succinimidyl ester dilution assays were performed to assess the ability of freshly isolated and ex vivo expanded Tregs from HCs and HD patients to suppress proliferation of allogeneic Tresps (Figure 3B). To compare suppressive capacity of HC Tregs against those Tregs of HD patients at baseline, the IC50 (the higher the IC50, the less suppressive the Tregs) and Smax were calculated from nonlinear regression plots (Figure 3C) as previously described (35).
Freshly isolated HD Tregs had impaired ability to suppress Tresp proliferation relative to the HCs, which was evidenced by a higher median IC50 and a lower Smax than their counterparts (Figure 3, C and D), consistent with previous reports (28). In vitro expansion in the presence of Rapamycin resulted in highly suppressive Tregs from HD patients, comparable in suppressive ability with freshly isolated Tregs from HCs (Figure 3, E–I). In vitro expansion in the absence of Rapamycin resulted in poorly suppressive Tregs from both HC and HD groups. Indeed, only three HC Tregs and one HD Treg expanded without Rapamycin had maximal suppressive capability of 50% or more at 1:1 ratio. Consequently, although freshly isolated Tregs from HD patients are functionally impaired, GMP-compatible ex vivo expansion in the presence of Rapamycin efficiently expands these Tregs and produces potently suppressive cells.
Rapamycin Effectively Prevents IL-17 Production by Expanded HD Tregs
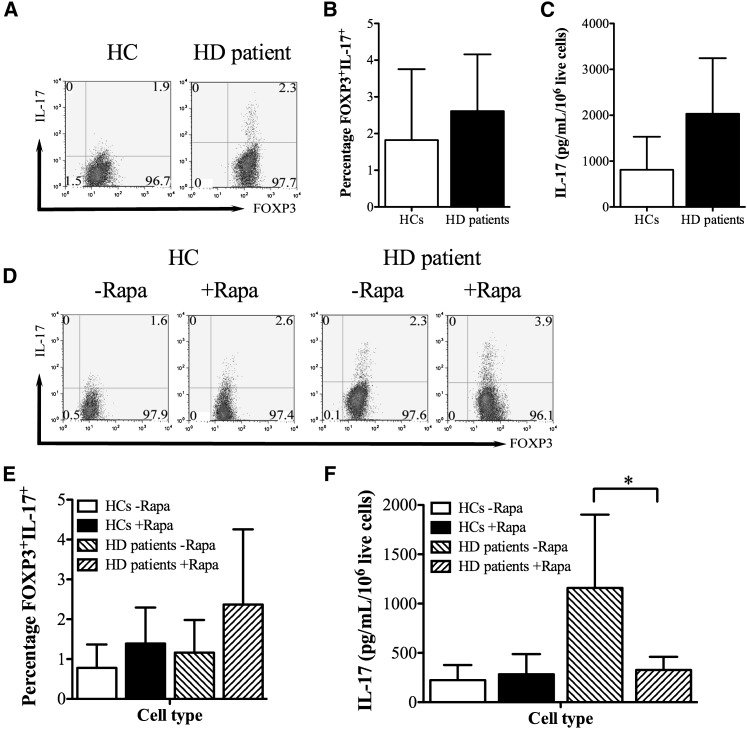
Because Treg plasticity to a Th17 phenotype after infusion in humans may confer an undesirable proinflammatory phenotype, we next evaluated the ability of these cells to produce IL-17 in a proinflammatory milieu. Freshly isolated Tregs from HD patients stained marginally more positively for IL-17 and after activation in IL-17–inducing cytokines (IL-2, IL-1β, IL-6, and TGF-β [32]), produced more IL-17 than those Tregs from HCs, but these differences did not reach statistical significance (Figure 4, A–C). After expansion ex vivo, low-level staining for IL-17 was observed in Tregs from both HCs and HD patients, with HD Tregs, as before, being slightly more positive for IL-17 (Figure 4, D and E). Rapamycin did not significantly alter IL-17 staining, although there was a tendency for slightly higher IL-17 expression in Tregs expanded in the presence of the drug (Figure 4, D and E) (P>0.05 for all comparisons). However, when IL-17 production was analyzed in 5-day supernatants by ELISA, Tregs from patients on HD expanded without Rapamycin produced substantial quantities of IL-17 (Figure 4F), with Rapamycin clearly inhibiting this production (Figure 4F).
Figure 4.
IL-17 production by ex vivo-expanded HD Tregs. (A and B) IL-17 and FOXP3 staining in freshly isolated Tregs from HCs and HD patients showing (A) representative and (B) cumulative data from five independent experiments. (C) IL-17 production from freshly isolated HC and HD Tregs is activated in the presence of inflammatory cytokines; cumulative data from five independent experiments are shown. (D and E) IL-17 staining in HC and HD Tregs expanded ex vivo in the presence or absence of Rapamycin, showing (D) representative and (E) cumulative data from five independent experiments. (F) IL-17 production from HC and HD Tregs expanded ex vivo in the presence or absence of Rapamycin, showing cumulative data from five independent experiments. *P<0.05.
Taken together, these findings suggest that expansion of Tregs from HD patients with Rapamycin decreases their capacity to produce IL-17 under inflammatory conditions.
Discussion
Tregs are ideal candidates for cell-based therapy to induce solid organ transplant tolerance. Such strategies have been successful in animal models (4,5). Experimental induction of transplant tolerance is characterized by increased Treg frequency within both the transplanted organ and the regional lymphoid tissue (6,7). The likelihood of positive outcomes using Treg-based therapies in humans is suggested by data from human renal transplantation, where Treg infiltration of transplants correlates with better outcomes from borderline rejection (8), and demonstrations that GMP-grade functional Tregs can be expanded from PBMCs of healthy donors (4,9,10).
For Treg cellular therapy to be viable, three technical challenges need to be overcome: Tregs need to be present in peripheral blood in sufficient numbers to be isolated, they need to be expandable in vitro, and they need to be functionally stable. Although these requirements have been shown for Tregs from healthy donors (4,9,10), they have not been shown for patients with ESKD on HD awaiting a renal transplant. Indeed, these individuals show features of chronic inflammation/immune dysregulation (25–27). Although some of the chronic inflammation is attributable to excessive immune system activation (37), abnormalities in Tregs have also been described, particularly a defect in Treg numbers and function (28,38); some of this defect is ascribed to high apoptosis in the presence of uremic factors, particularly oxidized LDL (38). However, these studies did not include strict age and sex matching of patients to controls (one of them did not include HCs at all) (28), included patients with immunologic and inflammatory diseases (28), and did not address important phenotypic characteristics required of Tregs for cell-based therapy.
In the present study, a comprehensive analysis of Treg phenotypes in patients with ESKD compared with age- and sex-matched controls was performed. We accept that the study is limited by the expansion of only five Treg lines from each group, although this number is in keeping with the majority of previous literature in the field. We showed that patients on HD have equivalent Treg subsets I and III to their matched controls and express almost identical Treg surface markers on these subsets (CD27, CD39, HLA-DR, and FOXP3). Population II Tregs are effector Tregs, with potent suppressive function, that die quickly on activation (14), and therefore, the deficiency of population II observed in HD patients may be the result of increased apoptosis (38) or impaired generation of effector Tregs in the context of chronic inflammation. In either case, population II does not expand ex vivo and therefore, is not amenable to use for cell therapy. We did not find paucity in naïve (population I) Tregs as suggested previously (29). Furthermore, an additional analysis of naïve and memory subsets of Tresps did not support the difference reported in the previous publication (29) (data not shown). Importantly, because population I Tregs are the subset most resistant to Th17 conversion in vitro (14), Tregs expanded from this population may minimize the potential for immune-mediated graft loss during cell therapy (18).
Although FOXP3 expression was similar in freshly isolated Tregs from patients and controls, impaired Treg-mediated suppression of autologous Tresps was seen in HD patients. Because this finding was apparent in the absence of uremic serum, we believe that the majority of the functional defect is cell-intrinsic (i.e., the Tregs are defective) rather than cell-extrinsic (uremic toxins, oxidized LDL, and hypercytokinemia). However, HD Tregs were easy to expand in vitro, particularly in the presence of Rapamycin, to a number sufficient for programs of cell therapy, and Rapamycin rescued the suppressive function of HD Tregs, making them suitable for cell-based therapy. Using the Rapamycin-based protocol in this paper, we predict being able to achieve sufficient Treg expansion to administer between 3×106 and 5×106 cells/kg on a single occasion (as predicated by the ONE Study). The number that may actually be required for tolerance induction will depend on their in vivo survival, their migratory ability, and the degree of infectious tolerance imparted by the infusion of the Tregs (39).
Finally, we observed that Rapamycin effectively diminished the capacity of Tregs from HD patients to produce or secrete (40,41) IL-17 in the presence of an inflammatory stimulus. Rapamycin promotes the expansion of phenotypically and functionally stable Tregs in GMP-compatible systems while impairing the proliferation of CD4+CD25− T effectors (13,32,42). Thus, in the absence of Rapamycin treatment, the elevated IL-17 production by HD Tregs may be attributed to increased contaminating Th17 cells or a greater propensity for these cells to differentiate into Th17 cells (consistent with the chronic inflammation seen in HD and/or with vitamin D insufficiency/deficiency in ESKD, because this vitamin prevents Th17 differentiation) (43). Nevertheless, this finding is the clearest indication yet that Treg-based cell therapy for renal allograft transplantation should use a Rapamycin-based protocol. Rapamycin is an ideal choice for Treg-based therapy in solid organ transplantation, because it preferentially expands the most stable Treg subpopulation (population I) and inhibits IL-17 production from other subpopulations (32,44). Rapamycin interferes with early T cell signaling events (Akt/mammalian target of rapamycin), which are critical in T cell fate decisions (45). In addition to preferentially expanding population I Tregs, Rapamycin also induces expression of CD62L and CD27 on Tregs (44), which are permissive for homing to sites of antigen encounter after transplantation and also markers for the most suppressive of Tregs in vivo and in vitro (46–49).
Together, these data are a significant advance on prior publications, including the work by of Berglund et al. (50), which did not analyze Treg subpopulations in HD, baseline suppressive function, GMP-based clinical grade expansion, or Treg plasticity. We have shown, for the first time, that Tregs from patients on HD have similar subpopulations to age- and sex-matched healthy donors and can be expanded ex vivo under GMP-compatible conditions in the presence of Rapamycin to a functional and stable cell product that can be used in clinical trials of Treg-based immunotherapy for the induction of tolerance to renal allografts. The results support the rationale behind the use of Treg-based immunotherapy among patients receiving kidney transplants as planned in the ONE Study.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Institute for Health Research (NIHR; to B.A., J.B.C., and G.L.), the Medical Research Council (to B.A., S.J., and G.L.), the Academy of Medical Sciences (to B.A.), the Wellcome Trust (to B.A.), the ONE Study (to C.S.), the British Heart Foundation, the Kidney Patients’ Association, and the Guy’s and St. Thomas’ Charity (to R.I.L. and G.L.). The research was also funded by the NIHR Biomedical Research Centre based at Guy's and St. Thomas' NHS Foundation Trust and King's College London. The authors acknowledge the support of the Medical Research Council Centre for Transplantation.
The views expressed are the views of the authors and not necessarily the views of the NHS, the NIHR, or the Department of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12931212/-/DCSupplemental.
References
- 1.Halloran PF: Immunosuppressive drugs for kidney transplantation. N Engl J Med 351: 2715–2729, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Safinia N, Afzali B, Atalar K, Lombardi G, Lechler RI: T-cell alloimmunity and chronic allograft dysfunction. Kidney Int Suppl 119: S2–S12, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Miyara M, Costantino CM, Hafler DA: FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10: 490–500, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Sagoo P, Lombardi G, Lechler RI: Regulatory T cells as therapeutic cells. Curr Opin Organ Transplant 13: 645–653, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Golshayan D, Wyss JC, Abulker CW, Schaefer SC, Lechler RI, Lehr HA, Pascual M: Transplantation tolerance induced by regulatory T cells: In vivo mechanisms and sites of action. Int Immunopharmacol 9: 683–688, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW: Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med 201: 1037–1044, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS: Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 7: 652–662, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Taflin C, Nochy D, Hill G, Frouget T, Rioux N, Vérine J, Bruneval P, Glotz D: Regulatory T cells in kidney allograft infiltrates correlate with initial inflammation and graft function. Transplantation 89: 194–199, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, Myśliwska J, Hellmann A: First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol 133: 22–26, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL: Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One 4: e5994, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A, Mysliwska J, Trzonkowski P: Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 35: 1817–1820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velardi A, Aversa F, Martelli MF: Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117: 3921–3928, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE, Blazar BR: Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med 3: 83ra41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S: Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30: 899–911, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F: Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 201: 1793–1803, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robson SC, Sévigny J, Zimmermann H: The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal 2: 409–430, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baecher-Allan C, Wolf E, Hafler DA: MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol 176: 4622–4631, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M: Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood 108: 4260–4267, 2006 [DOI] [PubMed] [Google Scholar]
- 19.O’Shea JJ, Paul WE: Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327: 1098–1102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afzali B, Mitchell P, Lechler RI, John S, Lombardi G: Translational mini-review series on Th17 cells: Induction of interleukin-17 production by regulatory T cells. Clin Exp Immunol 159: 120–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hori S: Regulatory T cell plasticity: Beyond the controversies. Trends Immunol 32: 295–300, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Rautenberg P, Proppe D, Schütte A, Ullmann U: Influenza subtype-specific immunoglobulin A and G responses after booster versus one double-dose vaccination in hemodialysis patients. Eur J Clin Microbiol Infect Dis 8: 897–900, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Girndt M, Pietsch M, Kohler H: Tetanus immunization and its association to hepatitis B vaccination in patients with chronic renal failure. Am J Kidney Dis 26: 454–460, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Kreft B, Klouche M, Kreft R, Kirchner H, Sack K: Low efficiency of active immunization against diphtheria in chronic hemodialysis patients. Kidney Int 52: 212–216, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Sester U, Sester M, Hauk M, Kaul H, Kohler H, Girndt M: T-cell activation follows Th1 rather than Th2 pattern in haemodialysis patients. Nephrol Dial Transplant 15: 1217–1223, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Girndt M, Sester U, Kaul H, Köhler H: Production of proinflammatory and regulatory monokines in hemodialysis patients shown at a single-cell level. J Am Soc Nephrol 9: 1689–1696, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Girndt M, Sester U, Sester M, Kaul H, Kohler H: Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transplant 14: 2807–2810, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Hendrikx TK, van Gurp EAFJ, Mol WM, Schoordijk W, Sewgobind VDKD, Ijzermans JNM, Weimar W, Baan CC: End-stage renal failure and regulatory activities of CD4+CD25bright+FoxP3+ T-cells. Nephrol Dial Transplant 24: 1969–1978, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Yoon J-W, Gollapudi S, Pahl MV, Vaziri ND: Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int 70: 371–376, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Yates J, Rovis F, Mitchell P, Afzali B, Tsang JY-S, Garin M, Lechler RI, Lombardi G, Garden OA: The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol 19: 785–799, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG: Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 177: 8338–8347, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Tresoldi E, Dell’Albani I, Stabilini A, Jofra T, Valle A, Gagliani N, Bondanza A, Roncarolo MG, Battaglia M: Stability of human rapamycin-expanded CD4+CD25+ T regulatory cells. Haematologica 96: 1357–1365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venken K, Thewissen M, Hellings N, Somers V, Hensen K, Rummens JL, Stinissen P: A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Methods 322: 1–11, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Monk CR, Spachidou M, Rovis F, Leung E, Botto M, Lechler RI, Garden OA: MRL/Mp CD4+,CD25- T cells show reduced sensitivity to suppression by CD4+,CD25+ regulatory T cells in vitro: A novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum 52: 1180–1184, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Afzali B, Mitchell PJ, Scotta C, Canavan J, Edozie FC, Fazekasova H, Lord GM, John S, Barber LD, Hernandez-Fuentes MP, Lechler RI, Lombardi G: Relative resistance of human CD4(+) memory T cells to suppression by CD4(+) CD25(+) regulatory T cells. Am J Transplant 11: 1734–1742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santner-Nanan B, Seddiki N, Zhu E, Quent V, Kelleher A, Fazekas de St Groth B, Nanan R: Accelerated age-dependent transition of human regulatory T cells to effector memory phenotype. Int Immunol 20: 375–383, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I: Disturbances of acquired immunity in hemodialysis patients. Semin Dial 20: 440–451, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Meier P, Golshayan D, Blanc E, Pascual M, Burnier M: Oxidized LDL modulates apoptosis of regulatory T cells in patients with ESRD. J Am Soc Nephrol 20: 1368–1384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H: “Infectious” transplantation tolerance. Science 259: 974–977, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, Hong S, Berry LS, Reichelt S, Ferreira M, Tavaré S, Inoki K, Shimizu S, Narita M: Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332: 966–970, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pani G: From growing to secreting: New roles for mTOR in aging cells. Cell Cycle 10: 2450–2453, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Battaglia M, Stabilini A, Roncarolo MG: Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105: 4743–4748, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L, Christakos S, Youssef S: 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol 31: 3653–3669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scotta C, Esposito M, Fazekasova H, Fanelli G, Edozie FC, Ali N, Xiao F, Peakman M, Afzali B, Sagoo P, Lechler RI, Lombardi G: Differential effects of rapamycin and retinoic acid on expansion, stability and suppressive qualities of human CD4+CD25+FOXP3+ Treg subpopulations. Haematologica 2012, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peter C, Waldmann H, Cobbold SP: mTOR signaling and metabolic regulation of T cell differentiation. Curr Opin Immunol 22: 655–661, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Issa F, Hester J, Goto R, Nadig SN, Goodacre TE, Wood K: Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation 90: 1321–1327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koenen HJPM, Fasse E, Joosten I: CD27/CFSE-based ex vivo selection of highly suppressive alloantigen-specific human regulatory T cells. J Immunol 174: 7573–7583, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Koenen HJPM, Smeets RL, Vink PM, van Rijssen E, Boots AMH, Joosten I: Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112: 2340–2352, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, Schiopu A, Taggart DP, Wood KJ: In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med 16: 809–813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berglund D, Korsgren O, Lorant T, Schneider K, Tufveson G, Carlsson B: Isolation, expansion and functional assessment of CD4+CD25+FoxP3+ regulatory T cells and Tr1 cells from uremic patients awaiting kidney transplantation. Transpl Immunol 26: 27–33, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.