Key Points
The MD Anderson Symptom Inventory for CML can be used to collect patient-reported symptoms for research and clinical practice.
Thirty percent of patients in chronic-phase CML and on TKIs experience moderate symptoms that interfere with daily functioning.
Abstract
We developed a module of the MD Anderson Symptom Inventory (MDASI) for patients with chronic myeloid leukemia (CML). To develop the MDASI-CML, we identified CML-specific symptoms from qualitative interviews with 35 patients. A list of candidate symptoms was reduced by a panel of patients, caregivers, and clinicians to the 13 core MDASI symptom items and 6 CML-specific items; these items were subsequently administered to 30 patients. Cognitive debriefing confirmed that the items were clear, relevant, and easy to use. One additional CML-specific symptom item was added, for a total of 7. The refined MDASI-CML was administered to 152 patients once every 2 weeks for 1 year. The content, concurrent, known-group, and construct validity of the MDASI-CML were evaluated. The internal consistency and test-retest reliabilities of the module were adequate. Longitudinal analysis showed relatively stable symptom severity scores over time. The most severe symptoms were fatigue, drowsiness, disturbed sleep, muscle soreness and cramping, and trouble remembering things. Approximately one-third of the patients who completed the MDASI-CML reported persistent moderate-to-severe symptoms. The MDASI-CML is a valid and reliable symptom assessment instrument that can be used in clinical studies of symptom status in patients with CML. This trial was registered at www.clinicaltrials.gov as #NCT01046305.
Introduction
The prevalence of survivors with chronic myeloid leukemia (CML) has increased by 4000 to 5000 per year since the approval of oral tyrosine kinase inhibitor (TKI) therapy in 2001 and is expected to plateau at 181 000 by 2050.1 Despite excellent survival results obtained with TKIs, patients frequently experience chronic adverse events. In a recent study of 448 patients with CML treated with the first-generation TKI imatinib, 25% or more of the patients reported severe symptoms, including edema, musculoskeletal pain, muscle cramps, and fatigue.2 Because chronic adverse events may affect adherence to therapy,3 measures that reflect the symptom burden experienced by patients with CML are necessary to improve care, facilitate individual treatment decisions,4 and evaluate the efficacy of emerging cancer therapies.5
Symptom assessment is dependent on patient report, necessitating psychometrically validated self-report measures that are sensitive to change, easy to use, and quick to administer. Early symptom studies in CML were based on clinician notes or Common Terminology Criteria for Adverse Events (CTCAE) ratings by researchers6; however, use of the CTCAE for assessing symptoms underrepresents the occurrence and frequency of symptoms that patients experience.7 The Princess Margaret Hospital Symptom Experience Report,8 the Functional Assessment of Cancer Therapy (FACT) for biological response modifiers (BRM),9,10 the FACT for Leukemia (Leu),11,12 the MD Anderson Symptom Inventory (MDASI),13 and an ad-hoc symptom measure2 have been used to capture patient-reported CML symptoms. However, the FACT-BRM and FACT-Leu are health-related quality-of-life (QOL) instruments that, although including symptom items, are not symptom instruments per se. Except for the MDASI, the other instruments are not psychometrically validated.
The MDASI14 assesses the severity and interference with daily activities of common symptoms of cancer and its treatment. The MDASI has several advantages over other measures15,16: it is comprehensive yet brief enough to avoid being a burden to answer, it assesses both the severity of cancer-related symptoms and the level of symptom interference with functioning, its 0 to 10 numeric scale is readily understood by patients, easy to translate into other languages, and adaptable for telephone and electronic administration, and it is recommended as a patient-reported outcome measure for use in clinical effectiveness research. Additional symptom items can be appended to the 13 symptom and 6 interference items of the core MDASI to form disease- or treatment-specific modules.17-23 The number of additional module-specific symptom items is minimized to keep the module concise and to facilitate repeated measurement.
The primary goal of our study was to psychometrically validate a MDASI module specific to CML (MDASI-CML). Early-phase development of 2 other patient-reported scales to measure symptoms in CML have been reported in abstract form: the Comprehensive Symptom Profile for CML24 and a European Organization for Research and Treatment of Cancer (EORTC) CML questionnaire.25 Because few studies have followed health-related QOL longitudinally in patients with CML,9,11,26 and no study has longitudinally compared symptoms related to various TKIs, our secondary goal was to explore the occurrence of symptoms reported by patients with CML over a 1-year period.
Materials and methods
Three cohorts were used to develop, validate, and administer longitudinally the MDASI-CML. Cohort 1 generated potential symptom items from patient report of symptom experiences. Cohort 2 reduced the number of potential symptom items to those that were most relevant to patients. Cohort 3 established the reliability and validity of the final MDASI-CML and reported symptoms regularly for 1 year. Patient involvement was critical in all stages of MDASI-CML development to ensure its adequacy and content validity.27
This study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center. All patients provided written consent in accordance with the Declaration of Helsinki. Analysis software was not used for the qualitative analysis. Quantitative data were analyzed using SPSS version 17.0.2. Correlations, means, SDs, ranges, 95% confidence limits, and frequencies were computed for all symptoms and subscales of all MDASI versions used. Statistical significance was assumed at a 2-tailed α level of .05.
Cohort 1
Cohort 1 comprised English-speaking patients 18 years of age or older with Philadelphia chromosome–positive CML who were seen in the Leukemia Center at MD Anderson during November and December 2009. Sampling for this cohort was purposive, to select patients with a broad range of demographic and disease characteristics and treatment experiences that could impact symptoms.
Data collection methods and measures.
The patients participated in single, individual, in-depth, taped 30-minute interviews about their symptom experiences since diagnosis. Trained qualitative research interviewers conducted all interviews and recorded observational notes after each interview. The interview guide included open-ended questions about the patient’s current experience of CML and about past experiences since the time of diagnosis. Additional probe questions were asked to elicit detailed information about symptoms mentioned by the patient and to confirm that all important experiences had been discussed.
After the interview, patients provided sociodemographic information and completed the core MDASI. The MDASI asks patients to rate the worst severity of symptoms during the past 24 hours on an 11-point scale ranging from 0 (not present) to 10 (as bad as you can imagine).14 Patients also rate the degree to which symptoms interfere with various aspects of life (general activity, mood, work [including work around the house], relations with other people, walking, and enjoyment of life) during the past 24 hours. The interference items are rated on an 11-point scale ranging from 0 (did not interfere) to 10 (interfered completely).
Patients also answered a single question rating overall QOL in the last week on a scale of 0 (as bad as it can be) to 10 (as good as it can be).28,29
Disease-related and treatment-related information and Eastern Cooperative Oncology Group performance status (ECOG PS) were collected from the patient’s medical record.
The taped recordings were transcribed verbatim, and accuracy was verified by the interviewer.
Data analysis.
Descriptive statistics were used to summarize the patients’ demographic characteristics, disease- and treatment-related variables, ECOG PS, comorbidities, and QOL.
Descriptive exploratory content analysis was used to examine the transcripts of the qualitative interviews and to identify symptoms.30 The number of patients reporting each symptom was recorded. Two clinicians and 2 researchers reviewed the list of symptoms and made suggestions for combining or modifying symptoms. The researchers and clinicians jointly agreed on the final list of symptoms. Descriptive statistics were used to summarize patient responses to the core MDASI.
Cohort 2
Cohort 2 was a panel of content experts comprising physicians and nurses from the United States with at least 5 years’ experience caring for patients with CML, patients 18 years or older who had been treated for CML for at least 1 year, and family caregivers of these patients. Physician and nurse experts were suggested by 2 clinicians at MD Anderson. Patients and family caregivers were contacted in the Leukemia Center.
Data collection methods and measures.
Packets containing an explanation of the study, a consent-to-participate statement, an Expert Symptom Relevance (ESR) form, and a return envelope were mailed or given to the content experts in January 2010. Experts returned the signed consent statement and completed ESR form by mail. If forms were not returned within 3 weeks, the expert was excluded from the study.
The ESR form included the 13 symptoms from the core MDASI and the additional symptoms identified by cohort 1. The relevance of each symptom to patients with CML was rated from 1 (not relevant) to 4 (very relevant). The experts could suggest other relevant symptoms as well.
Data analysis.
Mean ratings of the individual symptom items were calculated. All core MDASI items and any additional symptom that had a mean rating of ≥3 and was endorsed by 10 or more participants in cohort 1 was retained in a provisional MDASI-CML for testing in cohort 3.
Cohort 3
Cohort 3 consisted of English-speaking patients 18 years or older with CML who were not receiving active treatment of a second malignancy and were being followed in MD Anderson’s Leukemia Center. All patients meeting the inclusion criteria from March to November 2010 were approached for study participation.
Data collection methods and measures.
Patients completed a paper version of the provisional MDASI-CML and a QOL question. A clinical research coordinator collected demographic and clinical information from each patient’s medical record. All patients were scheduled to complete the MDASI-CML by a computer-based interactive voice response system (IVRS) every 2 weeks for 1 year. Each IVRS call directed patients to record the severity of their symptoms using their telephone keypad.
The demographic questionnaire and QOL question were the same as those used for cohort 1. Patients’ disease history and current clinical and treatment information were obtained as described in cohort 1.
The first 30 patients participated in a cognitive debriefing interview about the MDASI-CML after completing it. Cognitive debriefing ensures that the instrument is complete and is usable by members of the intended population. The debriefing included 7 questions about ease of completion, comprehensibility, acceptability, redundancy, scoring, clarity, and content of the MDASI-CML, scored from 0 (best) to 10 (worst). Subquestions allowed patients to identify items that they found problematic. Further changes to symptom items could be made on the basis of the cognitive debriefing results.
Data analysis.
Descriptive statistics were used to summarize the patient characteristics and demographics of cohort 3.
Cronbach α values were calculated to estimate the internal consistency reliability of each of 3 MDASI-CML subscales: the core subscale (13 core MDASI symptom items), the CML-specific subscale (7 CML-specific symptom items), and the interference subscale (6 interference items). A Cronbach α value of .70 or higher indicates good internal consistency reliability.31
Test-retest reliability refers to the ability of an instrument to produce the same result over time when no changes in the concept being measured are expected. We calculated intraclass correlations for the 3 MDASI-CML subscales from cohort 3 assessments made 2 weeks apart to evaluate test-retest reliability.
Item reduction identifies the set of items that best represent the concept of interest. Hierarchical cluster analysis was used to determine redundancy among the CML-specific items and their redundancy with core MDASI symptoms. Descriptive statistics were used to determine floor effects (a large number of scores clustering at a set lower limit and lacking variation) for all CML-specific symptom items. These analyses identified symptom items that provided little additional information or were not relevant to patients with CML and could be removed from the final MDASI-CML. Clinicians were consulted before an item was removed to verify that it was not clinically relevant.
Content validity refers to the ability of an instrument to measure the concept of interest. The content validity of the MDASI-CML was established by patient input into item generation and selection and confirmed by cognitive debriefing in the final validation stage.
Concurrent validity refers to the correlation of an instrument with an instrument that measures a related but not identical concept. Concurrent validity was examined with Spearman correlations of mean MDASI-CML symptom and interference scores and mean QOL scores for the same patients.
Known-group validity is defined as the ability of an instrument to identify outcomes of patients in specific groups using the instrument’s subscales or items when such group differences are expected. We tested the MDASI-CML’s sensitivity to different TKIs to establish known-group validity. Effect sizes (ESs) were calculated to estimate the magnitude of differences in the symptom severity scores reported by patients receiving 1 of the FDA-approved TKIs: imatinib, dasatinib, or nilotinib.32,33
Construct validity was tested using factor analysis with direct oblimin rotation to determine the constructs being represented by MDASI-CML symptom items. We evaluated model fit by examining the residuals and using Harman criteria.34 To evaluate model fit, we examined the differences between the reproduced correlations based on the factor solution identified and the observed correlations in the sample. A solution was considered adequate if the SD of the residuals was less than or equal to the reciprocal of the square root of our sample size. We hypothesized a 2-factor structure (a general severity factor and a CML-specific factor).
Longitudinal data were explored by group-based trajectory analysis that identified groups of patients by reports of symptom severity over 1 year. Mean symptom severity and interference for the groups were compared using descriptive analysis and t tests.
Results
Demographic and clinical characteristics of study cohorts
Demographic and clinical characteristics of cohort 1 (n = 35) and cohort 3 (n = 152) are summarized in Table 1. Almost all were in chronic phase and being treated with TKIs (94% and 93%, respectively). Cohort 2 comprised 4 physicians, 4 nurses, 3 patients, and 4 family caregivers; 1 physician, 1 nurse, and 1 patient failed to return required forms and were excluded from the study.
Table 1.
Demographic and clinical characteristics of CML patients in study cohorts 1 and 3 at study entry
| Patient characteristics, mean (SD) | Cohort 1, n = 35 | Cohort 3, n = 152 | ||
|---|---|---|---|---|
| Age, y | 52.7 (13.8) | 51.1 (13.6) | ||
| Education level, y | 14.7 (1.90) | 14.7 (2.3) | ||
| Time since diagnosis, mo | 70.8 (64.8) | 72.5 (56.4) | ||
| Patient characteristics, no. (%) | ||||
| Sex | ||||
| Men | 16 (46) | 71 (47) | ||
| Women | 19 (54) | 81 (53) | ||
| Race and ethnicity | ||||
| Hispanic, black non-Hispanic, Asian, and Native American | 6 (17) | 37 (24) | ||
| White non-Hispanic | 29 (83) | 115 (76) | ||
| Marital status | ||||
| Married | 27 (77) | 115 (76) | ||
| Not married | 8 (23) | 37 (24) | ||
| Employment status | ||||
| Employed outside the home | 20 (57) | 91 (60) | ||
| Not employed | 15 (43) | 61 (40) | ||
| ECOG PS score | ||||
| Good (1 or lower) | 34 (97) | 149 (98) | ||
| Poor (2 or higher) | 1 (3) | 3 (2) | ||
| CML phase | ||||
| Chronic phase | 32 (91) | 148 (97) | ||
| Accelerated phase | 1 (3) | 1 (1) | ||
| Blast crisis | 2 (6) | 3 (2) | ||
| Disease response | ||||
| Hematologic | 34 (97) | 131 (86) | ||
| Complete cytogenetic | 26 (74) | 131 (86) | ||
| Complete or major molecular | 60 (21) | 53 (80) | ||
| Previous therapy | ||||
| Yes | 28 (80) | 116 (76) | ||
| No | 7 (20) | 36 (24) | ||
| Treatment | Previous | Current | Previous | Current |
| Imatinib | 23 (66) | 7 (20) | 57 (38) | 71 (47) |
| Dasatinib | 6 (17) | 16 (46) | 13 (9) | 34 (22) |
| Nilotinib | 4 (11) | 4 (11) | 9 (6) | 22 (14) |
| Bosutinib | 5 (14) | 1 (3) | 4 (3) | 6 (4) |
| Ponatinib | 0 | 3 (8) | 0 | 5 (3) |
| Bafetinib | 1 (3) | 1 (3) | 0 | 1 (1) |
| Rebastinib | 0 | 1 (3) | 0 | 3 (2) |
| Other drug therapy | 22 (63) | 1 (3) | 88 (58) | 3 (2) |
| HSCT in last 3 mo | 5 (14) | 0 (0) | 7 (5) | 1 (1) |
| No treatment | 0 | 1 (3) | 0 | 7 (5) |
HSCT, hematopoietic stem cell transplant.
Cohort 1: generation of potential symptom items from patient report
Forty-four symptoms occurring over the disease course were identified from content analysis of the 35 qualitative interviews; of these, 22 were reported by at least 20% of patients. Although not mentioned spontaneously during the qualitative interviews, 2 of the core MDASI symptom items, drowsiness and dry mouth, were reported as currently present by 60% and 57% of the patients, respectively, when they completed the MDASI after the interview. Candidate symptom items were generated using terms consistent with words used by patients in the interviews. Two clinicians and 2 researchers reviewed the symptom item list, and a final candidate list of 39 symptoms for rating by the expert panel was developed by consensus of the researchers and clinicians.
Cohort 2: reduction of symptom items by expert panel rating
Nineteen symptom items, including 6 CML-specific items, met the criteria for inclusion in the MDASI-CML (Table 2).
Table 2.
Cohort 3 MDASI-CML symptom scores and QOL ratings
| Item | Mean (SD) | |
|---|---|---|
| Test, n = 152 | Retest, n = 140 | |
| Fatigue (tiredness) | 2.67 (2.53) | 2.53 (2.34) |
| Muscle soreness and cramping* | 2.23 (2.58)‡ | 2.07 (2.25)‡ |
| Feeling drowsy (sleepy) | 2.18 (2.29) | 2.14 (2.21) |
| Disturbed sleep | 2.13 (2.44) | 2.06 (2.50) |
| Problem with remembering things | 1.82 (1.96) | 1.78 (1.91) |
| Pain | 1.75 (2.49) | 1.57 (2.36) |
| Swelling of the hands, legs, feet, abdomen, and around the eyes* | 1.74 (2.13)‡ | 1.64 (2.02)‡ |
| Feeling of malaise (not feeling well)* | 1.72 (2.14)‡ | 1.50 (1.93)‡ |
| Feeling distressed (upset) | 1.66 (2.26) | 1.33 (1.90) |
| Having a dry mouth | 1.66 (2.25) | 1.50 (2.27) |
| Numbness or tingling | 1.47 (2.20) | 1.28 (1.93) |
| Feeling sad | 1.43 (2.14) | 1.36 (2.00) |
| Shortness of breath | 1.28 (1.74) | 1.09 (1.60) |
| Rash or skin changes* | 1.25 (1.77)‡ | 1.19 (1.47)‡ |
| Headache† | 1.21 (1.78)‡ | 1.20 (1.84)‡ |
| Diarrhea* | 1.18 (2.10)‡ | 0.96 (1.86)‡ |
| Bruising easily and bleeding* | 1.14 (1.79)‡ | 0.85 (1.30)‡ |
| Nausea | 0.81 (1.63) | 0.55 (1.18) |
| Problem with lack of appetite | 0.74 (1.54) | 0.58 (1.30) |
| Vomiting | 0.36 (0.93) | 0.20 (0.65) |
| Mean combined core symptoms | 1.54 (1.45) | 1.38 (1.32) |
| Mean combined 5 most severe core symptoms | 2.11 (1.92) | 2.03 (1.82) |
| Mean combined CML symptoms | 1.49 (1.39) | 1.34 (1.22) |
| Mean combined 5 most severe CML symptoms | 1.63 (1.51) | 1.52 (1.38) |
| Work (including work around the house) | 2.01 (2.37) | 1.78 (2.17) |
| General activity | 1.81 (2.23) | 1.48 (1.96) |
| Walking | 1.66 (2.31) | 1.36 (2.06) |
| Mood | 1.62 (2.10) | 1.39 (2.04) |
| Enjoyment of life | 1.55 (2.20) | 1.23 (1.93) |
| Relations with other people | 1.25 (1.92) | 1.02 (1.67) |
| Mean combined interference items | 1.65 (1.94) | 1.38 (1.71) |
| Mean combined physical interference items | 1.83 (2.15) | 1.54 (1.89) |
| Mean combined affective interference items | 1.47 (1.94) | 1.21 (1.76) |
| Single-item QOL | 8.14 (2.05) | Not done |
CML-specific items were retained for psychometric testing after expect panel review.
This CML-specific item was added for psychometric testing after patient recommendation during cohort 3 testing.
P < .05 when tested against mean = 0 for floor effect.
Cohort 3: psychometric validation of the MDASI-CML
Cognitive debriefing.
The participants rated the provisional MDASI-CML questions easy to understand and complete, and did not find them overly repetitive (all mean ratings <1). All patients reported the form easy to read and not too long. All patients reported the 0 to 10 scoring system easy to understand and use and were comfortable grading symptoms that way.
Several participants reported experiencing headache, which had not been included in the initial CML module. Headache was added to the list of CML-specific symptoms, bringing the total number of symptom items for testing to 20. Eighty patients had been accrued and had completed the MDASI-CML at least once when headache was added. An assessment in which each patient had the opportunity to respond to the 20 symptom items was selected for the validation. This resulted in a validation sample of 152 patients and 140 patients who completed the MDASI-CML 2 weeks later for the retest.
Description of MDASI-CML scores and item reduction.
Means and SDs for the individual symptom and interference items and all subscales for both the test and retest scores are in Table 2. The 5 symptoms with the highest mean severity were fatigue, drowsiness, disturbed sleep, muscle soreness or cramping, and difficulty remembering. Four of these symptoms had the highest moderate-to-severe (rating ≥5) incidence (fatigue, 21%; disturbed sleep, 18%; drowsiness, 17%; and muscle soreness and cramping, 16%), with swelling of the hands, legs, feet, abdomen, or around the eyes (14%) having the fifth-highest moderate-to-severe incidence.
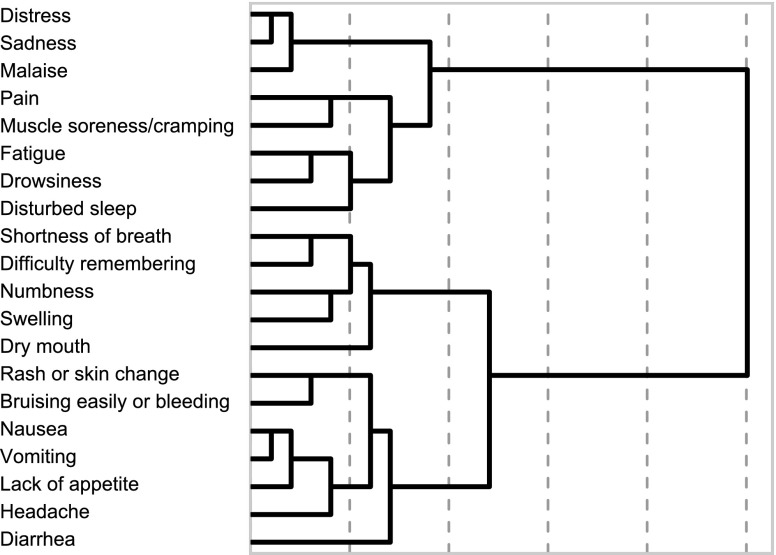
Hierarchical cluster analysis showed that, although items clustered, none of the CML-specific items were redundant with core MDASI symptom items (Figure 1). A test of the mean severity scores for all CML-specific symptom items demonstrated that the means were significantly higher than 0, indicating that there were no floor effects (all symptom severity means were significantly different from 0). Therefore, all 7 CML-specific items were retained in the final version of the MDASI-CML.
Figure 1.
Dendrogram of hierarchical cluster analysis of symptom items from the MDASI-CML.
Reliability.
The MDASI-CML symptom and interference scales and subscales showed good internal consistency reliability (Table 3). The intraclass correlations of the MDASI-CML total symptom and interference scales and subscales administered 2 weeks apart (n = 140) indicated good test-retest reliability. All values were at least 0.89 (Table 3).
Table 3.
Internal consistency and test-retest reliability of the MDASI-CML and correlation of MDASI-CML ratings with single-item QOL scores
| Scale or subscale | Cronbach coefficient α internal consistency | Test-retest* | Mean | SD | QOL, Mean (SD) | Correlation | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test sample, n = 152 | Retest sample, n = 140 | Intraclass correlation | P | Pearson | Spearman | P | ||||
| Core symptoms | 0.91 | 0.90 | 0.92 | <.001 | 1.54 | 1.45 | 8.20 (1.87) | –.315 | –.411 | <.001 |
| CML module symptoms | 0.80 | 0.79 | 0.89 | <.001 | 1.49 | 1.39 | 8.20 (1.87) | –.209 | –.269 | <.01 |
| Total symptoms | 0.93 | 0.93 | 0.92 | <.001 | 1.52 | 1.38 | 8.20 (1.87) | –.289 | –.382 | <.001 |
| Interference | 0.95 | 0.94 | 0.93 | <.001 | 1.65 | 1.94 | 8.20 (1.87) | –.364 | –.436 | <.001 |
The time interval between test and retest was 2 weeks.
Validity.
Results of cognitive debriefing provided evidence of content validity. Concurrent validity was established by correlating mean total symptom scores from the MDASI-CML with mean QOL scores for the same patients. Spearman correlations were between –0.269 and –0.436 and were statistically significant (Table 3). Symptom severity was moderately correlated to overall QOL.
Although differences in symptom scores between TKI treatment groups were nonsignificant, some mean individual symptom scores and the mean score for the CML-specific symptom subscale showed moderate to large effect-size differences between imatinib and each of the other TKIs (Table 4), which may be clinically significant35 and suggest known-group validity. The difference in mean severity of distress (1.86 vs 1.03, ES = 0.45), vomiting (0.50 vs 0.09, ES = 0.45), and headache (1.19 vs 0.56, ES = 0.48) between nilotinib and dasatinib, respectively, also showed moderate effects.
Table 4.
Moderate and large effect sizes of differences in symptom severity means of patients with CML treated with different TKIs
| Drug 1, mean (SD) | Drug 2, mean (SD) | Difference | Effect size | |
|---|---|---|---|---|
| Imatinib (drug 1) vs nilotinib (drug 2) | ||||
| Nausea | 1.03 (1.64) | 0.41 (1.10) | 0.62 | 0.40 |
| Dry mouth | 1.94 (2.31) | 1.14 (2.10) | 0.81 | 0.36 |
| Numbness and tingling | 1.49 (2.01) | 0.91 (1.54) | 0.58 | 0.31 |
| Diarrhea | 1.68 (2.37) | 0.55 (1.37) | 1.13 | 0.52 |
| Swelling | 2.17 (2.08) | 1.18 (1.71) | 0.99 | 0.49 |
| Muscle soreness | 2.55 (2.54) | 1.59 (2.30) | 0.96 | 0.39 |
| Mean CML-module items | 1.75 (1.45) | 1.20 (1.46) | 0.55 | 0.38 |
| Mean 5 most severe symptoms | 2.39 (2.05) | 1.73 (1.84) | 0.66 | 0.33 |
| Imatinib (drug 1) vs dasatinib (drug 2) | ||||
| Nausea | 1.03 (1.64) | 0.44 (1.52) | 0.59 | 0.37 |
| Distress | 1.73 (2.31) | 1.03 (1.59) | 0.70 | 0.33 |
| Lack of appetite | 0.73 (1.52) | 0.18 (0.52) | 0.56 | 0.43 |
| Dry mouth | 1.94 (2.31) | 1.26 (1.91) | 0.68 | 0.31 |
| Feeling drowsy | 2.44 (2.42) | 1.53 (1.66) | 0.91 | 0.41 |
| Muscle soreness | 2.55 (2.54) | 1.68 (2.31) | 0.87 | 0.35 |
| Mean all symptom items | 1.66 (1.41) | 1.42 (1.96) | 0.48 | 0.38 |
| Mean CML-module Items | 1.75 (1.45) | 1.08 (1.01) | 0.66 | 0.50 |
| Dasatinib (drug 1) vs nilotinib (drug 2) | ||||
| Distress | 1.03 (1.59) | 1.86 (2.23) | 0.83 | 0.45 |
| Vomiting | 0.09 (0.29) | 0.50 (1.41) | 0.41 | 0.45 |
| Headache | 0.56 (0.93) | 1.19 (1.78) | 0.63 | 0.48 |
Principal axis factoring for construct validity showed a 3-factor solution for the symptom items of the MDASI-CML—a generalized symptom factor, an organ-specific symptom factor, and a gastrointestinal symptom factor—instead of our hypothesized 2-factor solution (Table 5). We used Harman criteria to test the adequacy of the 3-factor solution.34 The SD of the residuals (0.045) was smaller than the reciprocal of the square root of our sample size (0.081), indicating an acceptable fit with the 3-factor solution.
Table 5.
Factor analysis of MDASI-CML symptom items
| Pattern matrix | |||
|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | |
| Malaise | .999 | –.133 | .018 |
| Distress | .851 | –.081 | .134 |
| Fatigue | .847 | .127 | –.162 |
| Sadness | .845 | –.177 | .152 |
| Drowsiness | .705 | .217 | –.141 |
| Disturbed sleep | .625 | .247 | –.105 |
| Lack of appetite | .550 | .005 | .271 |
| Pain | .544 | .285 | –.058 |
| Muscle soreness | .487 | .408 | –.098 |
| Headache | .423 | .150 | .081 |
| Bruising easily or bleeding | .334 | .104 | .206 |
| Numbness | –.085 | .710 | .058 |
| Swelling | .174 | .638 | –.024 |
| Rash or skin change | –.047 | .552 | .108 |
| Shortness of breath | .165 | .522 | .008 |
| Difficulty remembering | .349 | .408 | .017 |
| Dry mouth | .307 | .384 | .060 |
| Diarrhea | .117 | .289 | .263 |
| Vomiting | –.011 | .286 | .692 |
| Nausea | .509 | –.042 | .517 |
Longitudinal analysis.
Using the IVRS, 121 patients (80%) completed at least 50% of the every-other-week assessments for 1 year, and 78 patients (51%) completed 80% of the assessments.
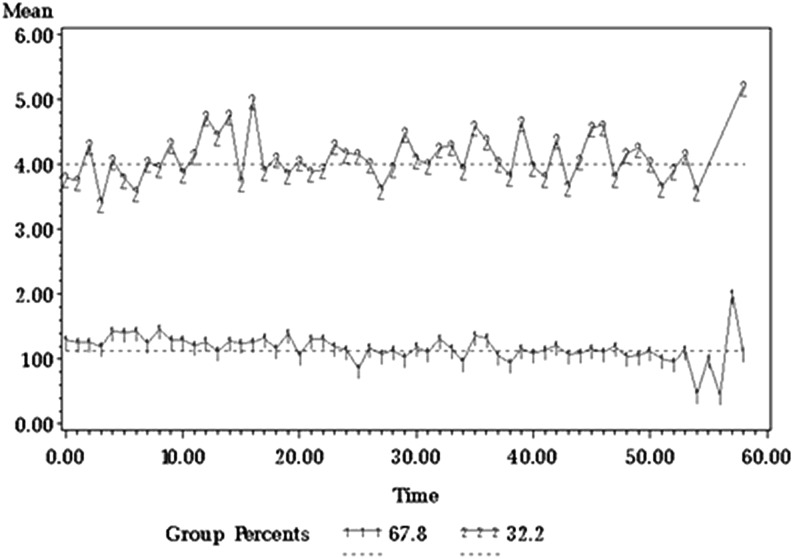
Group-based trajectory analysis of the mean severity of the 5 most severe symptoms over 1 year identified 2 patient groups that remained stable over time (Figure 2). The group reporting high symptom severity (n = 49, 32%) at the start of the study continued to report similar symptom severity throughout the study (mean 5 most severe symptoms = 4.21, SD = 1.58; mean interference = 3.22, SD = 1.97). The group initially reporting low symptom severity (n = 103, 68%) consistently reported low severity throughout the year (mean 5 most severe symptoms = 1.23, SD = 1.05; mean interference = 0.74, SD = 1.04). The 5 most severe symptoms for the low-severity group were fatigue, drowsiness, muscle soreness and cramping, disturbed sleep, and difficulty remembering. For the high-severity group, pain replaced difficulty remembering as the fifth-most-severe symptom in all quarters except the last quarter. Logistic regression analysis showed that patients who were married or working were more likely to be in the low-severity group (P = .047 and .01, respectively). No other demographic, disease, or treatment factors were associated with group membership. Means of all interference items and interference subscales were significantly higher for the high-severity group than the low-severity group (P < .001). Overall QOL was significantly lower for the high-severity group (6.74, SD = 2.30) than for the low-severity group (8.63, SD = 1.73, P < .001).
Figure 2.
Trajectory of the 5 most severe symptoms in the high- and low-symptom groups over 1 year.
We used t tests to explore differences in mean symptom severity by disease- and treatment-related variables. Patients receiving imatinib reported significantly more severe diarrhea (2.06, SD = 2.32 vs 0.45, SD = 1.41; P < .001), muscle soreness and cramping (2.44, SD = 2.62 vs 1.42, SD = 2.12; P = .017), swelling (2.08, SD = 2.43 vs 1.27, SD = 1.99; P = .044), and overall CML-specific symptom severity (1.75, SD = 1.54 vs 1.04, SD = 0.98; P = .002) than did patients receiving the second-generation TKIs dasatinib or nilotinib.
Discussion
In this study, we developed and performed initial psychometric testing of a CML-specific module of the MDASI. Our results provide psychometric evidence for the use of the MDASI-CML. All the symptom severity and interference subscales showed high test-retest reliability in tests given 2 weeks apart in this sample of patients with stable symptom severity. Cognitive debriefing confirmed that participants found that the MDASI-CML included the most important symptoms related to CML and was easy to use and understand. All subscales had acceptable internal consistency reliability. The CML-specific symptom items were able to detect severity differences among patients receiving different generations of TKIs that have clinically reported variations in toxicities and tolerances, as the diarrhea, muscle soreness and cramping, and swelling from the second-generation TKIs were less severe. Our results demonstrated that multiple and frequent symptom assessment may be used to describe differences in treatment-related symptom burden. Our intent was to develop an instrument that could be used to monitor symptom burden associated with therapy for CML across the board. We are currently conducting additional research to compare symptoms across various TKIs in larger samples of patients.
Most patients with CML receive TKI therapy and remain in chronic phase for years. Many experience few symptoms. However, we identified a significant portion (approximately one-third) of patients who experience moderate-to-severe levels of symptoms consistently over a year, including fatigue, drowsiness, disturbed sleep, muscle soreness and cramping, and difficulty remembering as the most severe symptoms. Pain also persisted over time. It is likely that these symptoms are treatment-related because >50% of our sample had complete or major molecular remission. However, the physiological mechanism behind these symptoms is unknown.
Our findings show that the severe symptoms identified in 25% to 30% of patients with CML receiving imatinib in the cross-sectional study by Efficace and colleagues2 remain consistently problematic over time and occur with all TKIs. The stability and chronicity of these symptoms is especially troubling, as even a mild symptom that persists for years can become problematic. Moderate-to-severe symptoms that are present for years can profoundly affect patients’ functional status and QOL. This may lead patients to be noncompliant with therapy or abandon treatment entirely.3
Symptomatic patients should be identified and have routine symptom monitoring and management. We have shown that automated telephone monitoring is acceptable to this patient population, who have infrequent clinic visits. Our findings deserve more investigation to determine whether there are factors that may predict which patients will experience more severe symptoms, the effect of symptoms on compliance with therapy, and best methods to manage symptoms. As expected, patients in the high-severity group reported significantly more symptom interference with function and poorer QOL than did patients in the low-severity group.
Our study had limitations. First, cohort 3 was a homogenous group of patients with well-controlled disease and stable symptom severity, which limited our ability to psychometrically evaluate the instrument’s sensitivity to change over time. Most patients had been diagnosed and received current therapy for an extended period of time. We expect that patients in blast crisis would report more severe symptoms, as they experience worse health-related QOL.12 Additional studies evaluating symptoms in untreated patients and symptoms that occur early in the course of therapy and may resolve with time would be highly desirable and are needed to test the broad applicability of the MDASI-CML. Second, all of our study participants were drawn from a single comprehensive cancer center. Therefore, our results may not be representative of patients with CML in general.
Our study also had strengths. We had access to a large population of patients with CML and completed accrual in <1 year. Because therapy for CML is rapidly evolving and new therapies are emerging, longer studies run the risk of changes in therapy interfering with the interpretability of results. We collected symptom reports consistently over 1 year. This is the first study to report symptoms of CML patients receiving different TKIs and to begin evaluating the symptom burden experienced by these patients. Finally, unlike previous measures used to report symptoms in patients with CML, the MDASI-CML is based on the concept of symptom burden rather than health-related QOL. Measures of symptom burden may provide patients, clinicians, and regulators with more information for making treatment decisions and evaluating new therapies.
The MDASI-CML is a valid, reliable, and sensitive instrument for assessing the severity of symptoms of CML and their interference in patients’ daily functioning. Most patients receive therapy with TKIs for many years or for life, and chronic mild symptoms are common but generally poorly quantitated in the literature. A significant proportion (approximately one-third) of patients with CML experience moderate-to-severe symptoms that require particular attention to manage symptoms and maintain functioning.
Acknowledgments
The authors acknowledge the editorial support of Bryan F. Tutt, MA, ELS, and Jeanie F. Woodruff, ELS, both of whom are employed by The University of Texas MD Anderson Cancer Center and are compensated from institutional funds.
This work was supported by Novartis Pharmaceuticals (L.A.W., J.E.C.) and in part by grants from the National Institutes of Health (CA124787; C.S.C.) and Anderson Cancer Center Support Grant CA016672.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Novartis Pharmaceuticals or the National Institutes of Health.
Footnotes
Presented at the 2010 annual meeting of the American Society of Clinical Oncology Chicago, IL, June 4-8, 2010, and the 16th Congress of the European Hematology Society London, United Kingdom, June 9-12, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.A.W., T.R.M., C.S.C., and J.E.C. designed the study; L.A.W., H.M.K., and J.E.C. performed research; A.G.G.G. and P.A. collected the data; L.A.W., T.R.M., M.L.S., C.S.C., A.N., and J.E.C. analyzed and interpreted the data; J.L.W. and F.H. performed statistical analysis; and L.A.W., T.R.M., C.S.C., and J.E.C. wrote the manuscript.
Conflict-of-interest disclosure: L.A.W. and J.E.C. have research funding from Novartis, Inc. J.E.C. and H.M.K. have research funding from Bristol-Myers Squibb, Pfizer, Ariad, and Chemgenex. J.E.C. has research funding from Deciphera and is a paid consultant for Ariad, Pfizer, and Teva. The remaining authors declare no competing financial interests.
Correspondence: Loretta A. Williams, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 1450, Houston, TX 77030; e-mail: loriwilliams@mdanderson.org.
References
- 1.Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118(12):3123–3127. doi: 10.1002/cncr.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efficace F, Baccarani M, Breccia M, et al. GIMEMA. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood. 2011;118(17):4554–4560. doi: 10.1182/blood-2011-04-347575. [DOI] [PubMed] [Google Scholar]
- 3.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinilla-Ibarz J, Cortes J, Mauro MJ. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: Definitions and clinical implications. Cancer. 2011;117(4):688–697. doi: 10.1002/cncr.25648. [DOI] [PubMed] [Google Scholar]
- 5.Postel-Vinay S, Gomez-Roca C, Molife LR, et al. Phase I trials of molecularly targeted agents: should we pay more attention to late toxicities? J Clin Oncol. 2011;29(13):1728–1735. doi: 10.1200/JCO.2010.31.9236. [DOI] [PubMed] [Google Scholar]
- 6.Savage DG, Szydlo RM, Goldman JM. Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period. Br J Haematol. 1997;96(1):111–116. doi: 10.1046/j.1365-2141.1997.d01-1982.x. [DOI] [PubMed] [Google Scholar]
- 7.Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–1632. doi: 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiss TL, Abdolell M, Jamal N, Minden MD, Lipton JH, Messner HA. Long-term medical outcomes and quality-of-life assessment of patients with chronic myeloid leukemia followed at least 10 years after allogeneic bone marrow transplantation. J Clin Oncol. 2002;20(9):2334–2343. doi: 10.1200/JCO.2002.06.077. [DOI] [PubMed] [Google Scholar]
- 9.Aziz Z, Iqbal J, Aaqib M, Akram M, Saeed A. Assessment of quality of life with imatinib mesylate as first-line treatment in chronic phase-chronic myeloid leukemia. Leuk Lymphoma. 2011;52(6):1017–1023. doi: 10.3109/10428194.2011.560310. [DOI] [PubMed] [Google Scholar]
- 10.Hahn EA, Glendenning GA, Sorensen MV, et al. IRIS Investigators. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus low-dose cytarabine: results from the IRIS Study. J Clin Oncol. 2003;21(11):2138–2146. doi: 10.1200/JCO.2003.12.154. [DOI] [PubMed] [Google Scholar]
- 11.Trask PC, Cella D, Besson N, Kelly V, Masszi T, Kim DW. Health-related quality of life of bosutinib (SKI-606) in imatinib-resistant or imatinib-intolerant chronic phase chronic myeloid leukemia. Leuk Res. 2012;36(4):438–442. doi: 10.1016/j.leukres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Trask PC, Cella D, Powell C, Reisman A, Whiteley J, Kelly V. Health-related quality of life in chronic myeloid leukemia. Leuk Res. 2013;37(1):9–13. doi: 10.1016/j.leukres.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Efficace F, Cartoni C, Niscola P, et al. Predicting survival in advanced hematologic malignancies: do patient-reported symptoms matter? Eur J Haematol. 2012;89(5):410–416. doi: 10.1111/ejh.12004. [DOI] [PubMed] [Google Scholar]
- 14.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 16.Kirkova J, Davis MP, Walsh D, et al. Cancer symptom assessment instruments: a systematic review. J Clin Oncol. 2006;24(9):1459–1473. doi: 10.1200/JCO.2005.02.8332. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT) [published correction appears in J Neurooncol. 2006;80(1):37]. J Neurooncol. 2006;80(1):27–35. doi: 10.1007/s11060-006-9135-z. [DOI] [PubMed] [Google Scholar]
- 18.Gning I, Trask PC, Mendoza TR, et al. Development and initial validation of the thyroid cancer module of the M. D. Anderson Symptom Inventory. Oncology. 2009;76(1):59–68. doi: 10.1159/000178809. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal DI, Mendoza TR, Chambers MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck. 2007;29(10):923–931. doi: 10.1002/hed.20602. [DOI] [PubMed] [Google Scholar]
- 20.Wang XS, Williams LA, Eng C, et al. Validation and application of a module of the M. D. Anderson Symptom Inventory for measuring multiple symptoms in patients with gastrointestinal cancer (the MDASI-GI). Cancer. 2010;116(8):2053–2063. doi: 10.1002/cncr.24920. [DOI] [PubMed] [Google Scholar]
- 21.Mendoza TR, Wang XS, Lu C, et al. Measuring the symptom burden of lung cancer: the validity and utility of the lung cancer module of the M. D. Anderson Symptom Inventory. Oncologist. 2011;16(2):217–227. doi: 10.1634/theoncologist.2010-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fadol A, Mendoza T, Gning I, et al. Psychometric testing of the MDASI-HF: a symptom assessment instrument for patients with cancer and concurrent heart failure. J Card Fail. 2008;14(6):497–507. doi: 10.1016/j.cardfail.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Williams LA, Couriel DR, Mendoza TR, et al. A new measure of symptom burden in chronic graft-versus-host disease [abstract]. Biol Blood Marrow Transplant. 2010;16(2 suppl 2):S177. Abstract 60. [Google Scholar]
- 24.Nikitina T, Fedorenko DA, Kurbatova KA, et al. The new instrument for comprehensive symptom assessment in patients with chronic myeloid leukemia [abstract]. Qual Life Res. 2012;21(suppl 1):69. Abstract 1080. [Google Scholar]
- 25.Efficace F, Breccia M, Saussele S, et al. International Development of An EORTC measure to assess Patient-reported quality of life (QoL) and symptoms in chronic myeloid leukemia (CML) [abstract]. Blood. 2011;118(21) Abstract 3132. [Google Scholar]
- 26.Stalfelt AM, Zettervall O. Quality of life in young patients with chronic myelocytic leukaemia during intensive treatment including interferon. Leuk Res. 1997;21(8):775–783. doi: 10.1016/s0145-2126(97)00026-x. [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. FDA website. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071975.pdf. Accessed January 6, 2013. [DOI] [PMC free article] [PubMed]
- 28.Sloan JA, Loprinzi CL, Kuross SA, et al. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J Clin Oncol. 1998;16(11):3662–3673. doi: 10.1200/JCO.1998.16.11.3662. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer WO, Dobson AJ, Hall J, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis. 1981;34(12):585–597. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- 30.Parse RR, Coyne AB, Smith MJ. Bowie, MD: Brady Communications; 1985. Nursing Research: Qualitative Methods. [Google Scholar]
- 31.Nunnally JC, Bernstein IH. New York, NY: McGraw-Hill; 1994. Psychometric Theory. [Google Scholar]
- 32.Cohen J. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 33.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 34.Harman HH. Chicago, IL: University of Chicago Press; 1967. Modern Factor Analysis. [Google Scholar]
- 35.Sloan JA, Vargas-Chanes D, Kamath CC, Sargent DJ, Novotny PJ, Atherton P. Detecting worms, ducks and elephants: a simple approach for defining clinically relevant effects in qualty-of-life measures. J Cancer Integr Med. 2003;1(1):41–47. [Google Scholar]