The question of whether genetic polymorphisms of CYP2D6 can affect treatment outcome in patients with early postmenopausal breast cancer has been a matter of debate. With the recent negative results with regard to CYP2D6 genotyping from the Breast International Group (BIG) 1-98 and Arimidex, Tamoxifen, Alone or in Combination (ATAC) studies, the study investigators suggest that testing for CYP2D6 has no value in clinical practice.1,2 The authors of the accompanying editorial conclude that this matter can be likely laid to rest.3 However, pharmacogenetic experts demanded the retraction of the BIG 1-98 CYP2D6 study4 on the basis of massive departures from Hardy-Weinberg equilibrium (HWE; Table 1), possibly because of the bias that may result when the CYP2D6 genotype is obtained from the tumor (somatic) genome and not the host genome (germline DNA). Various authors in their letters attribute the highly distorted genotype frequencies to allelic imbalance associated with loss of heterozygosity (LOH) in breast tumor tissue,4,5 deviation from the standard genotyping assay protocol,5 or the presence of pseudogenes neighboring the CYP2D6 locus.6 Because the BIG 1-98 and ATAC pharmacogenetic studies may be considered sufficient to settle the issue of the value of CYP2D6 in decision making regarding endocrine therapy in postmenopausal women with breast cancer, we revisit the hypothesis, comment on the complementary evidence, and discuss pros and cons of the validity of CYP2D6 tamoxifen pharmacogenetics.
Table 1.
Characteristics of Selected† Studies That Have Evaluated the Association Between CYP2D6 and Tamoxifen Outcome
| Study | Total No. of Patients Receiving Tamoxifen | Patients Genotyped |
End Points | Tamoxifen Dose (mg) | % of Patients Receiving Dose | Sample Size Calculation | DNA Source | % of Patients | Alleles Genotyped | Violation of HWE‡ for CYP2D6*4 | Use of CYP2D6 Inhibitors | Tamoxifen Adherence | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | ||||||||||||
| Schroth et al9,19 | 1,580 (monotherapy) | 1,325 | 84 | TTR, EFS, DFS | 20 | 88 | Yes | PBMC | 44.5§ | *3, *4, *5, *10, *41 | No, for PBMC-derived DNA§ Yes, for tumor section–derived DNA, P = .015∥ |
Unknown | Unknown |
| > 20 | 12 | Tumor section | 55.5∥ | ||||||||||
| Rae et al2 | 3,116 (± chemotherapy) | 588 | 19 | TTDR | 20 | 100 | No | Tumor section | 100 | *3, *4, *6, *10, *17, *41 | Yes,7 P = .00002 | Provided | Not provided |
| Regan et al1 | 2,459 (monotherapy) | 1,243 | 48 | TTR | 20 | 100 | No | Tumor core biopsy | 100 | *3, *4, *6, *7, *10, *41 | Yes,6,7 P = 2.5 × 10−92 | Not provided | Not provided |
Abbreviations: DFS, disease-free survival; EFS, breast cancer event–free survival; HWE, Hardy-Weinberg equilibrium; PBMC, peripheral blood mononuclear cells; TTDR, time to distant recurrence; TTR, time to recurrence (local, regional, distant recurrence or contralateral breast cancer).
The studies for comparison were selected on the basis of the criteria for levels of evidence, including study size and quality of marker specification, as well as prospective-retrospective study design as described in Simon et al.8 Other studies are summarized in Hoskins et al20 and Brauch et al.21
HWE is used in population genetics to describe the consistency of allele and genotype frequencies across generations. Deviations from HWE indicate a change in population demographics, substructure, selection, etc. In association studies using genetic markers, the test for HWE is often used to confirm uniformity of the population under investigation. Strong deviations indicate errors during the process of genetic data acquisition.
DNA-derived blood samples were regenotyped by AmpliChip (33 CYP2D6 alleles), thereby independently confirming the results.
The average nontumor cell content was 51% per tissue section and sample; therefore, both corresponding germline alleles are represented. However, the moderate deviation from HWE points to the inherent problem of genotyping bias as a result of potential allelic imbalance in tumor.
What Level of Evidence Is Needed?
We agree with the statement7 that a number of reported studies have been confounded as a result of a variety of biases and do not provide the level of evidence that is needed to recommend CYP2D6 genotyping. In fact, all published pharmacogenetic studies, by virtue of their retrospective nature (including prospective-retrospective studies), are prone to limitations; therefore, it is a minimum requirement that sample size, population stratification, quality of genotyping, and allele coverage, as well as correct genotype-phenotype assignment are vigorously addressed. Thus, studies that are based solely on the availability of samples are of little value.8 However, in agreement with Simon et al,8 prospective clinical trials such as BIG 1-98 and ATAC for CYP2D6 pharmacogenetic analyses1,2 and studies with determined participant eligibility, sample size estimation, and marker cut point specification, such as CYP2D6 phenotype definition as in Schroth et al,9 should have the potential to provide evidence for (or against) the value in clinical management. Notably, the ATAC2 and BIG 1-981 studies yielded results that did not confirm those of our previous publication.9 To illustrate the crucial differences in study design and quality control, the characteristics of the three studies1,2,9 are given in Table 1. Among these, the study by Schroth et al9 included a large percentage (45%) of genotypes derived from the germline (ie, DNA extracted from peripheral blood lymphocytes), whereas the remaining genotypes were derived from DNA extracted from formalin-fixed, paraffin-embedded (FFPE) tumor specimens. We reanalyzed the latter for an estimation of the tumor and nontumor cell content per sample (Table 1) to account for potential putative LOH, an issue that will be discussed in more detail in this article. Because the effects of CYP2D6 metabolism could alter the proportion of patients who experience recurrence and die, we did not include another large epidemiologic breast cancer study6,10 that lacked recurrence end points and did not provide detailed information on menopausal status.
The pharmacogenetic work-up of the ATAC study2 included only patients from the United Kingdom, thereby limiting the analysis to less than 19% of the original participating patients who were randomly assigned to receive tamoxifen (588 of 3,116). This violates the key recommendation of Simon et al8 that samples from at least two thirds of the patients be available for biomarker studies. As expected, when such a small proportion of the overall population is studied, the clinical characteristics of the genotyped group differed significantly (P ≤ .005) in important clinical characteristics (eg, chemotherapy, radiation therapy, hormone receptor status), both compared with nongenotyped United Kingdom patients and patients in the rest of the world. Previously, sample size estimation by Schroth et al9 revealed that more than 1,200 patients in ATAC would be required to detect a hazard ratio of 1.85 between CYP2D6 poor metabolizer (PM) and extensive metabolizer (EM), with 90% power as originally described by Goetz et al,11 on the basis of the consideration of CYP2D6 variants accessible in archived material. Taken together, the population in the ATAC study by Rae et al2 was not representative of the entire study, lacked sufficient power, and like the BIG 1-98 study (see What Is Important in the Prediction of Phenotypes Derived From Genotypes? section) showed significant deviation from HWE,5 thereby not meeting the standards proposed by Simon et al8 for a proper prospective-retrospective study.
What Is Important in the Prediction of Phenotypes Derived From Genotypes?
More than 100 germline polymorphisms, including 20 PM alleles with variable prevalence in different ethnicities, predict the enzymatic function of CYP2D6 (phenotype), as indicated by the Human Cytochrome P450 (CYP) Allele Nomenclature Database12 and the PharmGKB database.13 It follows that an accurate assessment of endoxifen exposure requires an accurate assessment of CYP2D6 phenotype on the basis of comprehensive genotyping of impaired-function alleles. Because the CYP2D6 phenotype results from germline predisposition, it must be assessed from DNA that is derived from normal cells such as peripheral blood lymphocytes or from DNA that is derived from normal epithelium contained in surgical specimens. The BIG 1-98 study,1 on the basis of 48% of the original patients who received tamoxifen, showed inconsistencies at the level of allele frequencies for the most prevalent CYP2D6*4 variant. Given their observed *4 allele frequency (q = 0.188) within patients who were treated with tamoxifen and no chemotherapy,1 one would expect 30.5% of heterozygous *4 patients, but the reported frequency is 19.7%. Although moderate deviations from theoretical expectation may occur, this strong allelic imbalance (P = 2.5 × 10−92)5 raises concerns with respect to the DNA source and CYP2D6 genotyping accuracy. Similarly, the ATAC study demonstrated violations of HWE for the most important CYP2D6 allele *4 (Table 1) evident from the supplementary information2 and stressed by Stanton in his correspondence5 in Journal of the National Cancer Institute. As pointed out,4 somatic deletion at CYP2D6 chromosomal locus 22q13 is well established in breast cancer.14,15 Recently, 22q LOH events have been confirmed by single nucleotide polymorphism arrays for estrogen receptor (ER) –positive breast cancer at a frequency of greater than 25%.16 Thus, given the isolation of BIG 1-98 and ATAC DNA from tumor cores that were originally intended for tumor biomarker studies, it can be inferred that these contained insufficient numbers of normal epithelial cells for the detection of germline genotypes, as evident from the strong deficiency of patients who were CYP2D6*4 heterozygous, particularly in the BIG 1-98 study. Notably, in a follow-up analysis of our previously reported CYP2D6-tamoxifen outcome association study,9 we compared allelic frequencies of DNA samples extracted from tumor (n = 517) and blood (n = 586) and observed a modest HWE deviation restricted to tumors (Table 1). The average nontumor cell content was 51% per tissue section, from which we conclude that our genotyping of FFPE tumor tissue was not profoundly biased by tumor LOH. In their rebuttals to the Journal of the National Cancer Institute letters,4,5 Rae et al17 and Regan et al18 suggested the lack of CYP2D6*5 genotyping data as possible reasons for their observed HWE departure of *4 genotypes; however, this is extremely unlikely because their reported frequency of *4/*4 homozygotes by far exceeds the number of expected *4/*5 compound heterozygotes (0.6%), and the excess number of homozygotes1 far outweighs the proportion of expected *5 carriers (3%), as determined from germline AmpliChip (Roche Diagnostics, Pleasanton, CA) genotyping of patients of European descent.19
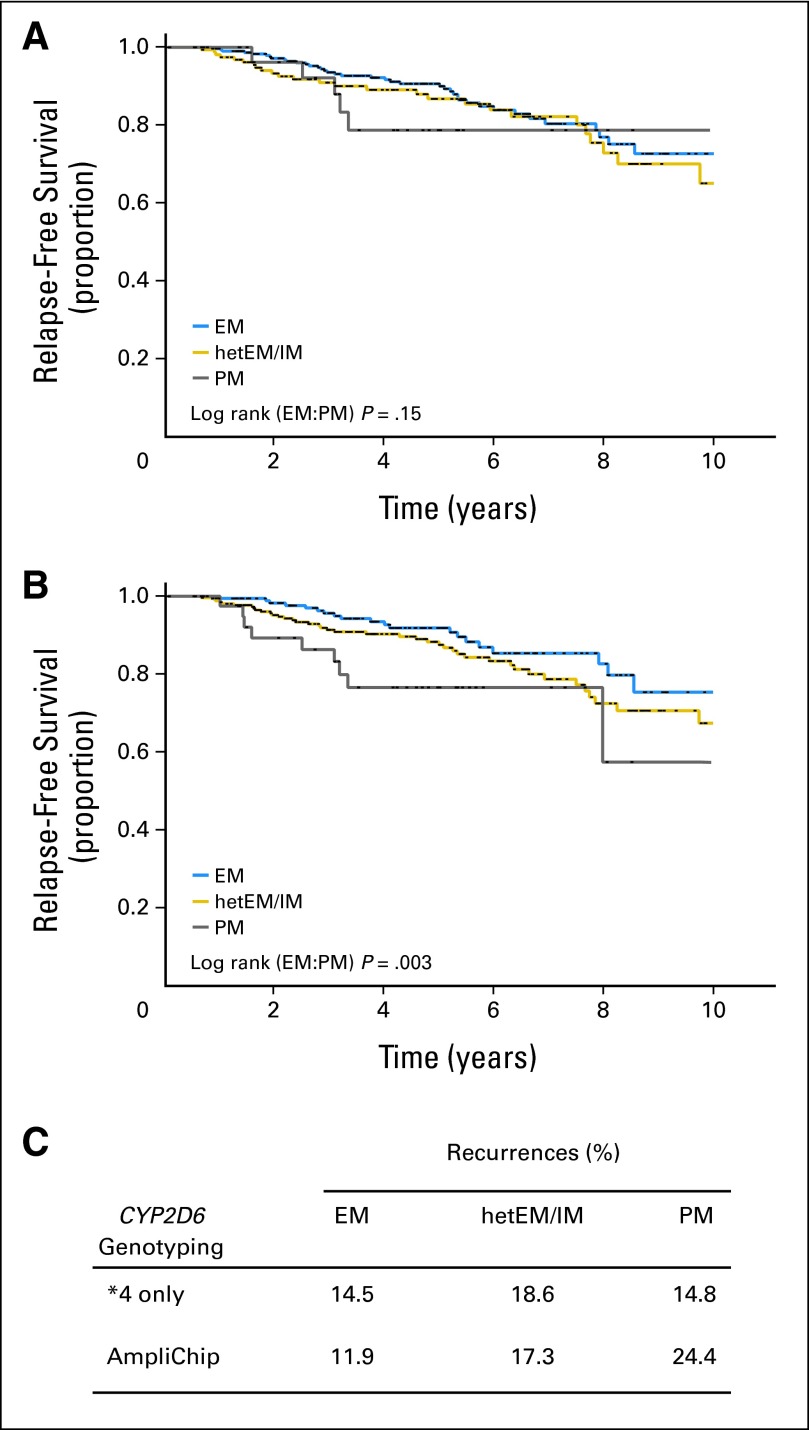
Limited genotyping of the multiple CYP2D6 alleles is a major cause of phenotype misclassification.19 Given that the DNA source in most retrospective studies is usually archival tissue with its inherent limitations, all previous studies have been challenged by the trade-off between sample size and allele coverage.20,21 On the basis of a hazard ratio of 1.5 (recalculated from a CYP2D6*4 PM prediction19), 3,258 to 7,133 patients are required to detect the risk with 90% power. In contrast, reanalysis of the same cohort (subgroup with blood-derived DNA) by comprehensive CYP2D6 allele coverage revealed a hazard ratio of 2.8,19 indicating that fewer than 860 patients are required to detect the PM-associated risk with 90% power. The dependency of CYP2D6 phenotype stratification from the number of variant alleles is shown in Figure A1 (online only). On the basis of a population of patients treated with tamoxifen (N = 492) with available AmpliChip data,19 it is evident that limited allele coverage (eg, *4 alone) can obscure clinical effects (Figs A1A and A1B). Notably, the recurrence frequencies of EM (14.5%) and PM (14.8%) patients are almost identical on the basis of the phenotype definition limited to CYP2D6*4; however, PM phenotypes show substantially higher recurrence rates (24.4%) when assessed from comprehensive AmpliChip genotyping (Fig A1C). We simulated the consequences of incomplete allele coverage by evaluating only those alleles that it is technically feasible to genotype from FFPE tissue versus a comprehensive approach that is standard using blood-derived DNA. This procedure results in substantial misclassification of the CYP2D6 phenotype, resulting in a smaller proportion of intermediate metabolizer at the expense of EM phenotypes.19 We therefore suspect that both BIG 1-98 and ATAC misclassified a large fraction of CYP2D6 phenotypes as a result of genotypes mistakenly obtained from the tumor and not the host. This is most evident in BIG 1-98, in which 62.7% of patients were classified as EM1 compared with an expected EM rate of 37.2% when determined in a similar population of patients with European descent using comprehensive (AmpliChip) genotyping of blood-derived DNA.19 It follows that comprehensive CYP2D6 genotyping using blood-derived DNA is mandatory for accurate phenotype assignment.
What Is the Pharmacologic Evidence That Tamoxifen Is a Prodrug and Endoxifen Is the Active Metabolite?
The available knowledge indicates that tamoxifen is a prodrug and that 4-hydroxy- tamoxifen (4-OH-tamoxifen) and endoxifen are the active metabolites.21–23 Endoxifen concentrations in CYP2D6 PM are up to 10-fold lower than CYP2D6 EM, and 4-OH-tamoxifen concentrations are even lower. However, the contribution of the hydroxyl-metabolites to tamoxifen's efficacy has been questioned in that the concentrations of tamoxifen and N-desmethyl-tamoxifen would saturate the ER by greater than 99.9%, thereby rendering the concentrations of hydroxyl-metabolites irrelevant.1,24,25 To counter this argument, an in vitro model was developed to simultaneously expose ER-positive breast cancer cells to fixed concentrations of tamoxifen and N-desmethyl-tamoxifen designed to saturate the ER, while varying only the concentrations of endoxifen. In this model, endoxifen effects on transcription and proliferation were concentration dependent, with minimal effects at low (< 20 nmol/L) concentrations (as in PM) but significantly greater effects at higher concentrations (40 to 60 nmol/L, as in intermediate metabolizer and EM).26 These data contradict the ER saturation argument and support the previous data that demonstrates that the hydroxylated metabolites exhibit a 40-fold– to 100-fold–higher affinity for the ER compared with tamoxifen27 (the ER-dissociation constant of tamoxifen is 4.5 nmol/L compared with 0.15 nmol/L for 4-OH tamoxifen28). Finally, it should be noted that ER occupancy calculation is based on total metabolite concentrations, not taking into account the high protein binding of tamoxifen of more than 99%.29 No protein binding data are available for endoxifen; therefore, the effects of protein-bound versus free metabolites at the ER are unknown.
Despite the extensive variability in the concentrations of tamoxifen and its metabolites, the optimal dose of tamoxifen is unknown. A presurgical window study evaluating three different doses of tamoxifen (1 mg, 5 mg, and 20 mg per day) demonstrated that dose did not significantly alter proliferation (Ki-67); however, dose dependency was observed with regard to ER targets such as insulin-like growth factor-1, with significantly greater reductions in plasma insulin-like growth factor-1 with increasing dose.30 The question of tamoxifen dose is therefore critical, given that recent prospective studies have demonstrated that increasing the dose of tamoxifen from 20 mg to 40 mg per day significantly increased endoxifen concentrations in PM but not EM.31 Finally, multiple studies evaluating novel formulations of endoxifen are ongoing, including a National Cancer Institute–sponsored study (Z-Endoxifen Hydrochloride in Treating Patients with Metastatic or Locally Recurrent Estrogen Receptor–Positive Breast Cancer) that is designed to bypass the limitations of cytochrome P450 metabolism through the direct administration of a novel formulation of endoxifen (endoxifen hydrochloride).
How Predictive Is the CYP2D6 Genotype for Endoxifen and 4-OH Tamoxifen Levels?
Clinical studies showed a strong CYP2D6 gene–dose effect (Ptrend = 10−16) with endoxifen concentrations highest in patients with ultrarapid metabolizer (77 nmol/L) and EM (36.9 nmol/L) but lowest in PM (9.9 nmol/L) phenotypes.32 These plasma concentrations refer to the (Z) isomer of the 4-hydroxymetabolite. Because another isomer, (Z)-4′-hydroxymetabolite, is inversely correlated with the number of CYP2D6 functional alleles,33 the separation of active (antiestrogenic) from nonactive stereoisomers is critical for the accurate prediction of endoxifen by CYP2D6 polymorphism.
CYP2D6 efficacy can be modulated by strong inhibitors given as comedication for the relief of tamoxifen-induced postmenopausal symptoms.34 However, other cytochrome P450 enzymes are involved in the formation of endoxifen, as demonstrated by our observation that polymorphic CYP2C9 contributes to the formation of 4-OH-tamoxifen, which is the source for 20% to 30% of total endoxifen.32,35
Synopsis
In essence, our commentary addresses the pitfalls of retrospective pharmacogenetic research, which, in the case of the CYP2D6-tamoxifen relationship, has produced conflicting results that have caused a delay in resolution of a clinical management question that affects two thirds of women who are diagnosed with breast cancer. This underscores the need to consult existing pharmacokinetic/dynamic/genetic studies that provide critical information on the pharmacology of the drug under investigation before large pharmacogenetic studies are conducted, a position that has been previously expressed.36 Given that alternatives to tamoxifen exist, the failure to resolve this question is particularly egregious in that the potential exists to deny women effective endocrine therapy. Given the inability of the published prospective-retrospective studies to resolve this issue to date, we conclude that a properly conducted prospective trial in the adjuvant setting is necessary to provide a definitive answer. However, we disagree with the comments of Rae et al17 and Regan et al18 that ongoing prospective clinical trials conducted in the metastatic setting (Eastern Cooperative Oncology Group E3108 [Tamoxifen Citrate in Treating Patients With Metastatic or Recurrent Breast Cancer] and European CYPTAMBRUT-2 [An Observational Study to Assess Response to Tamoxifen]) will provide an answer to the so-called CYP2D6 adjuvant question. This relates not only to their study design of enrolling patients with metastatic/advanced cancer, but the fact that patients with hormonally insensitive disease (aromatase-inhibitor refractory) are eligible for ECOG E3108, a population substantially different than that in the adjuvant setting. Therefore, until prospective, adjuvant trial data are available, it is our opinion that the current evidence is sufficient to accept the CYP2D6-tamoxifen pharmacogenetic relationship in postmenopausal women. However, it is clear that other host and tumor factors, besides CYP2D6, contribute to tamoxifen response/resistance. Researchers and clinicians should be encouraged to rigorously scrutinize the available pharmacogenetic, pharmacokinetic, and pharmacodynamic evidence to avoid drawing conclusions on the basis of potentially inaccurate data that could lead to the termination of research into a potentially promising biomarker.
Acknowledgment
Supported by the Bosch Foundation, Stuttgart, Germany; 7FP EU Marie Curie Initial Training Network, FightingDrugFailure (Grant No. GA 238132); and the US National Institutes of Health (Grant No. R01CA133049-01; M.P.G.).
Glossary Terms
- CYP2D6:
Cytochrome P450 2D6 is an isozyme of the cytochrome P-450 mixed-function oxidase system involved in the metabolism of xenobiotics including drugs. Several clinical important drugs are substrates for CYP2D6 including ß-blocker, anti-arrhythmics and anti-depressant drugs, codeine as well as tamoxifen.
- Extensive metabolizer (EM):
metabolic phenotype related to drug metabolizing enzymes which results in a normal metabolic ratio of a probe drug.
- Genotype-phenotype correlation:
the relationship between the presence of an individual genotype and the resulting physical trait e.g. biochemical reaction, morphology, behavior, pattern of abnormalities, drug response, etc.
- Hardy-Weinberg equilibrium:
A state in which genotype frequencies and ratios remain constant from generation to generation and in which genotype frequencies are a product of allele frequencies. A randomly mating population tends toward a Hardy- Weinberg equilibrium state if there are no mutations, migrations, or environmental factors favoring particular genotypes.
- LOH (loss of heterozygosity):
A situation where one chromosome has a normal allele of a gene and one chromosome has a mutant or deleted allele.
- Pharmacogenetics:
A branch of pharmacology dedicated to understanding the hereditary basis for drug responses that are idiosyncratic in nature. Although inborn errors of metabolism also have a genetic basis, pharmocogenetic disorders may never manifest if the drug is never introduced in the host.
- Polymorphism:
Genetic polymorphisms are natural variations in the genomic DNA sequence present in greater than 1% of the population, with SNP representing DNA variations in a single nucleotide. SNPs are being widely used to better understand disease processes, thereby paving the way for genetic-based diagnostics and therapeutics.
- Poor metabolizer (PM):
metabolic phenotype related to drug metabolizing enzymes which results in a slower metabolic ratio of a probe drug compared with an extensive metabolizer.
- Prodrug:
a drug that is given in an inactive form, and is bioactivated to a pharmacological drug by one or more metabolic processes.
Appendix
Fig A1.
Kaplan-Meier distribution and recurrence rates for time-to-breast cancer recurrence in 492 patients with breast cancer treated with tamoxifen. (A) Outcome stratification between CYP2D6 phenotypes extensive metabolizer (EM), heterozygous EM/intermediate metabolizer (hetEM/IM), and poor metabolizer (PM) by *4 only. (B) Outcome stratification between CYP2D6 phenotypes by comprehensive allele genotyping (AmpliChip; Roche Diagnostics, Pleasanton, CA). (C) Comparison of recurrence rates between CYP2D6 metabolizer subgroups indicating a shift to higher recurrence rates in PM on the basis of comprehensive genotyping for correct phenotype assessment.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Financial support: Hiltrud Brauch, Werner Schroth, Matthew P. Goetz, Matthias Schwab
Administrative support: Hiltrud Brauch, Matthias Schwab
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Regan MM, Leyland-Jones B, Bouzyk M, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The Breast International Group 1-98 trial. J Natl Cancer Inst. 2012;104:441–451. doi: 10.1093/jnci/djs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rae JM, Drury S, Hayes DF, et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104:452–460. doi: 10.1093/jnci/djs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly CM, Pritchard KI. CYP2D6 genotype as a marker for benefit of adjuvant tamoxifen in postmenopausal women: Lessons learned. J Natl Cancer Inst. 2012;104:427–428. doi: 10.1093/jnci/djs139. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura Y, Ratain MJ, Cox NJ, et al. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The Breast International Group 1-98 trial. J Natl Cancer Inst. 2012;104:1264. doi: 10.1093/jnci/djs304. [DOI] [PubMed] [Google Scholar]
- 5.Stanton V., Jr Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The Breast International Group 1-98 trial. J Natl Cancer Inst. 2012;104:1265–1266. doi: 10.1093/jnci/djs305. [DOI] [PubMed] [Google Scholar]
- 6.Pharoah PD, Abraham J, Caldas C. Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: The Breast International Group 1-98 trial and Re: CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104:1263–1264. doi: 10.1093/jnci/djs312. [DOI] [PubMed] [Google Scholar]
- 7.Rae JM. Personalized tamoxifen: What is the best way forward? J Clin Oncol. 2011;29:3206–3208. doi: 10.1200/JCO.2011.36.3895. [DOI] [PubMed] [Google Scholar]
- 8.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–1452. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham JE, Maranian MJ, Driver KE, et al. CYP2D6 gene variants: Association with breast cancer specific survival in a cohort of breast cancer patients from the United Kingdom treated with adjuvant tamoxifen. Breast Cancer Res. 2010;12:R64. doi: 10.1186/bcr2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 12.The Human Cytochrome P450 (CYP) Allele Nomenclature Committee: The human cytochrome P450 (CYP) allele nomenclature database. http://www.cypalleles.ki.se/
- 13.PharmGKB: Well-known pharmacogenomics associations. http://www.pharmgkb.org/search/knownPairs.action.
- 14.Castells A, Gusella JF, Ramesh V, et al. A region of deletion on chromosome 22q13 is common to human breast and colorectal cancers. Cancer Res. 2000;60:2836–2839. [PubMed] [Google Scholar]
- 15.Hirano A, Utada Y, Haga S, et al. Allelic losses as prognostic markers for breast cancers. Int J Clin Oncol. 2001;6:6–12. doi: 10.1007/pl00012082. [DOI] [PubMed] [Google Scholar]
- 16.Loo LW, Ton C, Wang YW, et al. Differential patterns of allelic loss in estrogen receptor-positive infiltrating lobular and ductal breast cancer. Genes Chromosomes Cancer. 2008;47:1049–1066. doi: 10.1002/gcc.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rae JM, Hayes DF, Cuzick J, et al. Response. J Natl Cancer Inst. [epub ahead of print on July 31, 2012] [Google Scholar]
- 18.Regan MM, Bouzyk M, Rae JM, et al. Response. J Natl Cancer Inst. [epub ahead of print on July 31, 2012] [Google Scholar]
- 19.Schroth W, Hamann U, Fasching PA, et al. CYP2D6 polymorphisms as predictors of outcome in breast cancer patients treated with tamoxifen: Expanded polymorphism coverage improves risk stratification. Clin Cancer Res. 2010;16:4468–4477. doi: 10.1158/1078-0432.CCR-10-0478. [DOI] [PubMed] [Google Scholar]
- 20.Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer. 2009;9:576–586. doi: 10.1038/nrc2683. [DOI] [PubMed] [Google Scholar]
- 21.Brauch H, Mürdter TE, Eichelbaum M, et al. Pharmacogenomics of tamoxifen therapy. Clin Chem. 2009;55:1770–1782. doi: 10.1373/clinchem.2008.121756. [DOI] [PubMed] [Google Scholar]
- 22.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 23.Brauch H, Jordan VC. Targeting of tamoxifen to enhance antitumour action for the treatment and prevention of breast cancer: The ‘personalised' approach? Eur J Cancer. 2009;45:2274–2283. doi: 10.1016/j.ejca.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Dowsett M, Haynes BP. Hormonal effects of aromatase inhibitors: Focus on premenopausal effects and interaction with tamoxifen. J Steroid Biochem Mol Biol. 2003;86:255–263. doi: 10.1016/s0960-0760(03)00365-0. [DOI] [PubMed] [Google Scholar]
- 25.Lash TL, Lien EA, Sørensen HT, et al. Genotype-guided tamoxifen therapy: Time to pause for reflection? Lancet Oncol. 2009;10:825–833. doi: 10.1016/S1470-2045(09)70030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Hawse JR, Subramaniam M, et al. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009;69:1722–1727. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 28.Ratliff B, Dietze EC, Bean GR, et al. Re: Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2004;96:883. doi: 10.1093/jnci/djh170. [DOI] [PubMed] [Google Scholar]
- 29.Kochansky CJ, McMasters DR, Lu P, et al. Impact of pH on plasma protein binding in equilibrium dialysis. Mol Pharm. 2008;5:438–448. doi: 10.1021/mp800004s. [DOI] [PubMed] [Google Scholar]
- 30.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–790. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 31.Irvin WJ, Jr, Walko CM, Weck KE, et al. Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: A multicenter study. J Clin Oncol. 2011;29:3232–3239. doi: 10.1200/JCO.2010.31.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mürdter TE, Schroth W, Bacchus-Gerybadze L, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89:708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 33.Barginear MF, Jaremko M, Peter I, et al. Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: Effect on active metabolite isomers and the antiestrogenic activity score. Clin Pharmacol Ther. 2011;90:605–611. doi: 10.1038/clpt.2011.153. [DOI] [PubMed] [Google Scholar]
- 34.Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Mauvais-Javis P, Baudot N, Castaigne D, et al. Trans-4-Hydroxytamoxifen concentration and metabolism after local percutaneous administration to human breast. Cancer Res. 1986;46:1521–1525. [PubMed] [Google Scholar]
- 36.McLeod HL, Isaacs KL. Preemptive pharmacogenetic testing: Insufficient data equal unsatisfactory guidance. Ann Intern Med. 2011;154:842–844. doi: 10.7326/0003-4819-154-12-201106210-00016. [DOI] [PubMed] [Google Scholar]