Abstract
Background
Immunoassays for 1α,25-dihydroxyvitamin D [1α,25(OH)2D] lack specificity. We aimed to characterize the cross-reactivity of an anti-1α,25(OH)2D antibody using purified vitamin D metabolites and to use these data to map the chemical features of 1α,25(OH)2D that are important for antibody binding. Additionally, we hypothesized that when combined with isotope dilution-liquid chromatography-tandem mass spectrometry (LC-MS/MS), antibody cross-reactivity could be used to semi-selectively enrich for structurally similar metabolites of vitamin D in a multiplexed assay.
Methods
Sample preparation consisted of immunoaffinity enrichment with a solid-phase anti-1α,25(OH)2D antibody and derivatization. Analytes were quantified using LC-MS/MS. Spike-recovery studies were performed using eleven vitamin D metabolites. A novel method for quantifying 25(OH)D2, 25(OH)D3, 24,25(OH)2D3, 1α,25(OH)2D2 and 1α,25(OH)2D3 simultaneously was developed and evaluated, which included deuterated internal standards for each analyte.
Results
The important chemical features of vitamin D metabolites for binding to the antibody were (1) native orientation of the hydroxyl group on carbon C3 in the A-ring, (2) the lack of substitution at carbon C4 in the A-ring, and (3) the overall polarity of the vitamin D metabolite. The new multiplexed method had lower limits of quantification (20% CV) of 0.2 ng/mL, 1.0 ng/mL, 0.06 ng/mL, 3.4 pg/mL and 2.8 pg/mL for 25(OH)D2, 25(OH)D3, 24,25(OH)2D3, 1α,25(OH)2D2 and 1α,25(OH)2D3, respectively. Method comparisons to three other LC-MS/MS methods were acceptable (r2>0.9, intercept<lower limit of quantification, slope statistically indistinguishable from 1.0).
Conclusions
LC-MS/MS can be used to characterize antibody cross-reactivity. We developed and evaluated a multiplexed assay for five vitamin D metabolites using immunoenrichment in a targeted metabolomic assay.
Keywords: Liquid chromatography-tandem mass spectrometry, immunoaffinity enrichment, multiplexed assay, specificity, hapten mapping, vitamin D metabolism
Vitamin D is present in blood in two chemically distinct forms, vitamin D2 and vitamin D3. While both are sterol prohormones, only vitamin D3 is produced in vivo. Vitamin D2 is instead obtained through diet and supplementation in the thermally stable form of ergocalciferol. Vitamin D3 is synthesized in the skin by the photolysis of 7-dehydrocholesterol to an unstable previtamin D3 which is then converted by isomerization to thermally stable cholecalciferol.(1) Vitamin D is then transported to the liver where it undergoes hydroxylation by CYP2R1 and other cytochrome P450 enzymes to 25-hydroxyvitamin D [25(OH)D],(2,3) the most abundant vitamin D metabolite in blood with a half-life of approximately 1 month.(4) As such, 25(OH)D is the most commonly measured metabolite of vitamin D to assess vitamin D stores.(1) The prohormone 25(OH)D undergoes further hydroxylation by CYP27B1 and CYP24A1 in the kidney into two different dihydroxyvitamin D metabolites, 1α,25(OH)2D and 24,25(OH)2D, respectively.(5–8) It is well-established that 1α,25(OH)2D plays an important role in calcium metabolism. Studies on the function of 24,25(OH)2D are more inconsistent, some suggesting that the metabolite is simply a catabolic breakdown product of 25(OH)D and others demonstrating a putative role in bone formation.(7) Other vitamin D metabolites have been described, including 25,26(OH)2D, which may have a regulatory role in intestinal calcium transport,(9) a metabolite hydroxylated at carbon 4 by CYP3A4, which may explain certain drug-induced disorders of bone,(10) and an epimer at carbon C3 in the A-ring, whose concentration appears to be relatively constant throughout the human lifespan but has unclear biological function after 1α-hydroxylation.(11–13)
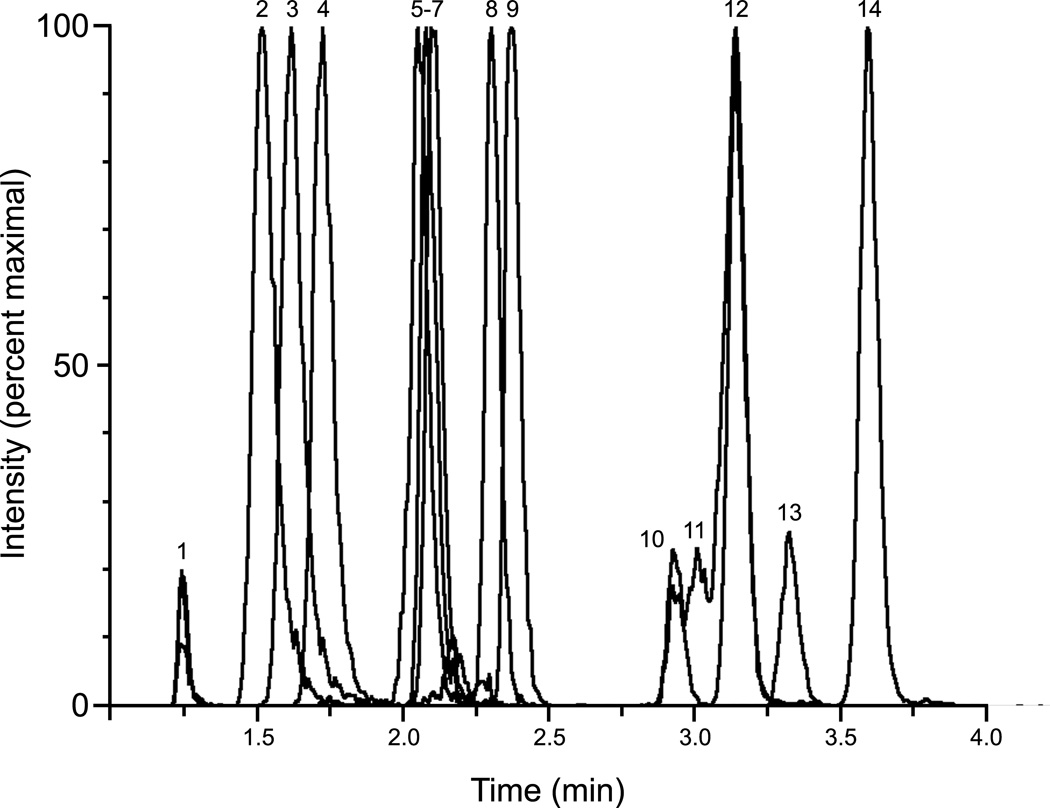
The concentrations of vitamin D metabolites in plasma span more than 3 orders of magnitude. For example, the concentration of the most active vitamin D metabolite, 1α,25(OH)2D is 1,000-fold lower than the prohormone 25(OH)D. Previous experiments in our laboratory demonstrated significant cross-reactivity of an antibody used in a commercially available (IDS, Scottsdale, AZ) competitive RIA for 1α,25(OH)2D,(14) which might raise concern regarding the utility of previous clinical and epidemiologic studies that have investigated 1α,25(OH)2D as a biomarker in human disease. Using LC-MS/MS to resolve potential interfering analytes and directly detect the analytes of interest, we previously demonstrated that it is possible to greatly improve the specificity of the antibody-based assay.(14) Since the publication of that assay, we have confirmed the results of another study that demonstrated the utility of a different solid-phase antibody (ALPCO, Salem, NH) to immunopurify analyte prior to LC-MS/MS,(15) which does not need sample preparation prior to immunoaffinity enrichment (data not shown). The simpler workflow and preliminary data demonstrating saturation of the IDS solid-phase reagent with moderate concentrations of spiked 25(OH)D (data not shown) led us to transfer our clinical workflow to the new solid-phase reagent from ALPCO. The chromatographic gradient was similar to that described,(14) with the exception of the starting conditions being revised to 56% mobile phase A [Optima water (Fisher, Pittsburg, PA)/0.1% formic acid (VWR, Randor, PA)/0.5mM methylamine (Sigma-Aldrich, St. Louis, MO)], 44% mobile phase B [Acetonitrile (Fisher)/0.1% formic acid/0.5mM methylamine]. The chromatographic separation of multiple vitamin D analytes is shown in Figure 1.
Figure 1. Composite chromatogram.
Analytes were individually added to an immunoaffinity extract of stripped serum, derivatized, and chromatographically resolved as described for the multiplexed assay. The MRM transition for peaks 1, 2, 3, 4 and 5 is 623.5>298.2; peaks 6, 7, and 8 is 623.5 > 314.2; peak 9 is 635.5 > 314.2; peak 10, 11 and 12 is 607.6 > 298.2; peak 13 and 14 is 619.6 > 298.2. Peak 1: Minor 24,25(OH)2D3 PTAD isomer, peak 2: 23(S),25(OH)2D3, peak 3: 25,26(OH)2D3, peak 4: 24,25(OH)2D3, peak 5: 23(R),25(OH)2D3, peak 6: 4β,25(OH)2D3, peak 7: 1α25(OH)2D3, peak 8: 3-epi-1α,25(OH)2D3, peak 9: 1α,25(OH)2D2, peak 10: Minor 25(OH)D3 PTAD isomer, peak 11: 3-epi-25(OH)D3, peak 12: 25(OH)D3, peak 13: Minor 25(OH)D2 PTAD isomer, peak 14: 25(OH)D2. A chromatogram of unsupplemented immunoaffinity extract of a stripped serum sample is shown in Supplemental Figure 1. The derivatization efficiency of 25(OH)D2 and 25(OH)D3 is 99.4% and 99.8%, respectively.
To characterize the cross-reactivity of the solid-phase antibody, we determined the extraction efficiency (analytical recovery) of the antibody for different vitamin D metabolites. Each analyte (see Table 1 for a complete list of the metabolites and their structures and Supplemental Table 1 for the source of each analyte) was individually added to a vitamin D-depleted human serum matrix (MSG-4000, Golden West Biologicals, Temecula, CA), extracted using the immunoaffinity reagent from ALPCO, derivatized, and quantified using LC-MS/MS. The chromatographic peak areas obtained from the extraction were compared with the peak area observed after adding each analyte individually to the resulting extract of a non-spiked MSG-4000 sample (Table 1). There were three important features of the vitamin D metabolites that seemed to determine their affinity for the antibody. First, the extraction efficiency of 25(OH)D2 and 25(OH)D3 was significantly lower than the majority of the dihydroxyvitamin D metabolites, suggesting that overall polarity was important for binding. Second, vitamin D metabolites with an epimeric orientation of the C3 hydroxyl group of the A-ring had significantly reduced affinity for the antibody, suggesting that this was an important part of the molecule for specific binding. Third, 4β,25(OH)2D3 had very little affinity, suggesting that this part of the A-ring was also important and further highlighting the importance of the A-ring and the region near the C3 carbon for antibody affinity. To further support these observations, we determined the apparent dissociation constant (Kd) in serum for several of the analytes using standard Scatchard analysis (Kd equals the negative slope of [bound]/[free] vs. [bound] analyte). The apparent Kd of 1α,25(OH)2D3 was 0.10 µM. 1α,25(OH)2D2 was approximately 4-fold lower (0.41 µM), which was similar to that observed for the dihydroxylated 24,25(OH)2D3 metabolite (0.39 µM). The monohydroxylated 25(OH)D3 metabolite had a lower affinity (14 µM) and the affinity of the C3 epimer of 25(OH)D3 could not be determined exactly but was noted to be >140 µM. Taken together, these data suggest that specific A-ring substitutions and overall molecular polarity are important for hapten binding.
Table 1.
Summary data of the new multiplexed vitamin D metabolite method.
| Compound | % Recovery (SD)d | Structure | Regressiona | r2 a | Conc | Intra-assayb %CV |
Totalb %CV |
LLOQc |
|---|---|---|---|---|---|---|---|---|
| 25(OH)D3 | 43.3 (2.1) | 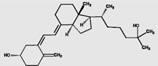 |
Y=1.04x+0.08 | 0.955 | 12.3 ng/mL | 3.0 | 3.7 | 1.0 |
| 25(OH)D2 | 32.2 (3.3) | 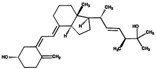 |
y=0.94x−1.00 | 0.981 | 10.6 ng/mL | 4.7 | 10.2 | 0.2 |
| 24,25(OH)2D3 | 70.8 (9.8) | 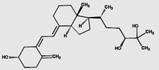 |
Y=0.96x−0.23 | 0.922 | 1.6 ng/mL | 2.6 | 6.4 | 0.06 |
| 1α,25(OH)2D3 | 79.4 (3.5) | 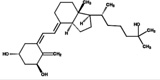 |
y=0.96x−2.97 | 0.901 | 14.6 pg/mL | 10.0 | 15.6 | 3.4 |
| 1α,25(OH)2D2 | 78.2 (12.4) | 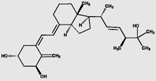 |
y=0.89x−0.54 | 0.976 | 12.8 pg/mL | 10.9 | 17.1 | 2.8 |
| 23(S),25(OH)2D3 | 64.0 (2.1) | 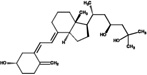 |
||||||
| 23(R),25(OH)2D3 | 67.0 (3.2) | 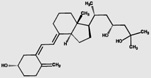 |
||||||
| 25,26(OH)2D3 | 69.2 (4.0) | 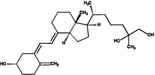 |
||||||
| 3-epi-25(OH)D3 | 3.2 (1.0) | 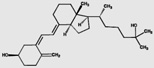 |
||||||
| 4β,25(OH)2D3 | 3.0 (0.01) | 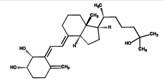 |
||||||
| 3-epi-1α,25(OH)2D3 | 15.0 (0.4) | 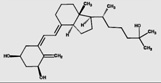 |
The equation of the Deming regression (y and x are the new and reference method, respectively) and Pearson correlation coefficient are presented.
Intra-assay (N=10) and total-assay CV (sqrt[(intra-assay CV)2 + (between-day CV)2]) at the concentrations listed.
Five replicates of linear dilutions were analyzed and the lowest dilution at which CV≤20% is listed. For 25(OH)D2, 25(OH)D3, and 24,25(OH)2D3 units are ng/mL. For 1α,25(OH)2D2 and 1α,25(OH)2D3 units are pg/mL.
Analytical recovery was calculated as the analyte peak area when spiked before divided by the analyte peak area spiked after extraction.
Our chemical characterization of the hapten complementarity of the antibody has two important implications. First, the C3-epimer of 25(OH)D3 is not well-recognized by the antibody. Because the epimer is not easily resolved from the native 25(OH)D3 in rapid chromatographic methods, the immunoextraction step could lead to shortened LC-MS/MS methods without interference from the epimer.(13) Similarly, 4β,25(OH)2D3, which is present at similar concentrations to 1α,25(OH)2D3, is not well-recognized by the antibody. It is difficult to resolve these two analytes in short chromatographic methods (16,17) and as a result, methods to quantify 1α,25(OH)2D3 without immunoaffinity extraction need to be carefully evaluated for interference from 4β,25(OH)2D3.(18)
Given the favorable affinities of many vitamin D metabolites, we decided to evaluate the possibility of using the immunoextraction of vitamin D metabolites as a step in a multiplexed assay of 25(OH)D2, 25(OH)D3, 1α,25(OH)2D2, 1α,25(OH)2D3, and 24,25(OH)2D3. Such an assay could simultaneously evaluate vitamin D stores, production levels of active metabolite, and inactivation levels of metabolites. The multiplexed assay used 400 µL of calibrators, controls, or patient sample, 20 µL internal standard mixture in methanol [containing 500 ng/mL each of 25(OH)D2-d3, 25(OH)D3-d6, 24,25(OH)2D3-d6 and 4 ng/mL each of 1α,25(OH)2D3-d6 and 1α,25(OH)2D2-d6], and 100 µL immunoaffinity beads (the commercial sources of the deuterated internal standards are listed in Supplemental Table 1). The plate was then covered and incubated for 2 h at 45°C while shaking at 800 rpm in a Thermomixer (Eppendorf, Hauppague, NY). After immuno-extraction, the beads were quantitatively transferred to a 2 mL filter plate (Strata Impact, Phenomenex, Torrance, CA) and the beads were washed ten times with 1 mL Optima grade water (Fisher, Pittsburg, PA). The analytes were eluted from the beads with 0.25 mL of acetonitrile into a 1 mL 96 deep-well collection plate (Waters, Milford, MA) and the eluate was evaporated in a Turbovap concentrator (Biotage, Charlotte, NC) at 30°C under nitrogen (20 ft3/hr). The residue was reconstituted in 50 µL of acetonitrile containing 0.7 mg/mL 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD, Sigma-Aldrich, St. Louis, MO). Following a 30-minute incubation at ambient temperature, the excess PTAD was quenched with 70 µL of water (Optima) and 40 µL was injected onto a Waters Acquity LC system coupled to a Waters Xevo TQ MS tandem mass spectrometer. Relevant mass spectrometer parameters are listed in Supplemental table 2. The experiments described spanned five lots of affinity beads.
The new multiplexed assay had lower limits of quantitation (LLOQ, 20% CV) of 1.0 ng/mL, 0.2 ng/mL, 0.06 ng/mL, 3.4 pg/mL and 2.8 pg/mL for 25(OH)D3, 25(OH)D2, 24,25(OH)2D3, 1α,25(OH)2D3, and 1α,25(OH)2D2, respectively. Total assay imprecision for each analyte is listed in Table 1 and was ≤17.1% for all analytes at low concentrations (at or below clinical decision points). We compared the new multiplexed method with liquid-liquid extraction methods optimized for 25(OH)D2, 25(OH)D3, and 24,25(OH)2D3, which have been previously described (19) or are described in Supplemental Material/Supplemental Table 3, respectively. We also compared the new multiplexed method with the previously described immunoaffinity assay for 1α,25(OH)2D2 and 1α,25(OH)2D3 that uses IDS solid-phase antibody.(14) Descriptive data for the method comparisons are presented in Table 1 and the data are plotted in Supplemental Figures 2–4. The methods compared acceptably (defined as r2>0.9, intercept<lower limit of quantification for each analyte, and slope statistically indistinguishable from 1.0). In terms of identifying low total 25(OH)D or low total 1α,25(OH)2D concentrations in plasma (<20 ng/mL or <17 pg/mL, respectively), the new method reclassified 3.3% and 1.9% of people compared with the existing methods, respectively. We also compared the new method with a liquid-liquid extraction method for 1α,25(OH)2D3 that has been previously described.(17) The correlation was relatively poor between the two methods, which may be due to the improved specificity of the immunoaffinity purification approach (Supplemental Fig. 5). Ion suppression for the analytes ranged −1.2%—26.3%. We did not perform an extensive mapping experiment with the IDS solid-phase antibody, but the peak areas from a single patient sample were similar with both reagents (Supplemental Fig. 6). Although there is an increased cost of the new assay compared with standard liquid-liquid extraction, there are important advantages to the immunoaffinity approach: (1) interferences from 4β,25(OH)2D3 and epi-C3-25(OH)D3 are eliminated, which can permit shorter chromatographic runs (16) and (2) the LLOQ for 1α,25(OH)2D3 is lower,(17) which permits the use of smaller volumes of plasma in the assay.
Competitive assays rely on antibody specificity or in the case of dihydroxyvitamin D analytes on extensive sample preparation prior to analysis (e.g. protein precipitation and chromatographic resolution of interfering substances).(8,20) The thermodynamics of the interference of related compounds with reagent antibodies are most often characterized by equilibrium inhibition studies. In this study we have used equilibrium binding studies in a human serum matrix to map the chemical features of the vitamin D hapten that most strongly dictate binding to the reagent antibody from a commercially available reagent antibody. This work complements competitive binding studies that have been performed in the past and the many epitope mapping experiments performed with overlapping synthetic peptides.
The obvious lack of specificity of reagent antibodies in small molecule immunoassays can be an advantage in multiplexed mass spectrometric assays, simplifying specimen preparation and greatly enriching for target analytes. It should be noted that the success of this multiplexed method relies on the large differences in concentration between each of the analytes being quantified. For example, if the plasma concentration of 25(OH)D3 were similar to 1α,25(OH)2D3, we might not be able to quantify 25(OH)D3 using this approach due to the 140-fold lower affinity of the antibody for 25(OH)D3 compared with 1α,25(OH)2D3. In addition, the antibodies for the same analyte used in immunoaffinity enrichment steps prior to mass spectrometry could differ in significant ways between manufacturers and as a result, competition with similar analytes could differentially affect recovery of the analytes of interest. While this did not appear to be a significant problem between ALPCO and IDS, it could be important in other systems. Projecting forward from our findings in this study, it will be interesting to see if combining antibodies in a single assay will permit the quantification of different classes of analytes simultaneously.
Supplementary Material
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- 24,25(OH)2D
24,25-dihydroxyvitamin D
- 1,25(OH)2D
1,25-dihydroxyvitamin D
Footnotes
Publisher's Disclaimer: This is an un-copyedited authored manuscript copyrighted by the American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of 'Fair Use of Copyrighted Materials' (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.
References
- 1.Holick MF. The use and interpretation of assays for vitamin D and its metabolites. J Nutr. 1990;120(Suppl 11):1464–1469. doi: 10.1093/jn/120.suppl_11.1464. [DOI] [PubMed] [Google Scholar]
- 2.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A. 2004;101:7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J, Deluca HF. Vitamin D 25-hydroxylase - Four decades of searching, are we there yet? Arch Biochem Biophys. 2012 doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Clements MR, Davies M, Hayes ME, Hickey CD, Lumb GA, Mawer EB, Adams PH. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin Endocrinol (Oxf) 1992;37:17–27. doi: 10.1111/j.1365-2265.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA. Vitamin D metabolism and its clinical application. J Bone Joint Surg Br. 1982;64:542–560. doi: 10.1302/0301-620X.64B5.6754741. [DOI] [PubMed] [Google Scholar]
- 6.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 7.St-Arnaud R. Targeted inactivation of vitamin D hydroxylases in mice. Bone. 1999;25:127–129. doi: 10.1016/s8756-3282(99)00118-0. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y, DeLuca HF. Measurement of mammalian 25-hydroxyvitamin D3 24R-and 1 alpha-hydroxylase. Proc Natl Acad Sci U S A. 1981;78:196–199. doi: 10.1073/pnas.78.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLuca HF, Suda T, Schnoes HK, Tanaka Y, Holick MF. 25,26-dihydroxycholecalciferol, a metabolite of vitamin D3 with intestinal calcium transport activity. Biochemistry. 1970;9:4776–4780. doi: 10.1021/bi00826a022. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M, et al. An Inducible Cytochrome P450 3A4-dependent Vitamin D Catabolic Pathway. Mol Pharmacol. 2011 doi: 10.1124/mol.111.076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AJ, Ritter C, Slatopolsky E, Muralidharan KR, Okamura WH, Reddy GS. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem. 1999;73:106–113. [PubMed] [Google Scholar]
- 12.Fleet JC, Bradley J, Reddy GS, Ray R, Wood RJ. 1 alpha,25-(OH)2-vitamin D3 analogs with minimal in vivo calcemic activity can stimulate significant transepithelial calcium transport and mRNA expression in vitro. Archives of biochemistry and biophysics. 1996;329:228–234. doi: 10.1006/abbi.1996.0213. [DOI] [PubMed] [Google Scholar]
- 13.Strathmann FG, Sadilkova K, Laha TJ, LeSourd SE, Bornhorst JA, Hoofnagle AN, Jack R. 3-epi-25 hydroxyvitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chim Acta. 2012;413:203–206. doi: 10.1016/j.cca.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1alpha,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57:1279–1285. doi: 10.1373/clinchem.2010.161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan C, Kosewick J, He X, Kozak M, Wang S. Sensitive measurement of serum 1alpha,25-dihydroxyvitamin D by liquid chromatography/tandem mass spectrometry after removing interference with immunoaffinity extraction. Rapid Commun Mass Spectrom. 2011;25:1241–1249. doi: 10.1002/rcm.4988. [DOI] [PubMed] [Google Scholar]
- 16.Aronov PA, Hall LM, Dettmer K, Stephensen CB, Hammock BD. Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2008;391:1917–1930. doi: 10.1007/s00216-008-2095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Senn T, Kalhorn T, Zheng XE, Zheng S, Davis CL, et al. Simultaneous measurement of plasma vitamin D(3) metabolites, including 4beta,25-dihydroxyvitamin D(3), using liquid chromatography-tandem mass spectrometry. Anal Biochem. 2011;418:126–133. doi: 10.1016/j.ab.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding S, Schoenmakers I, Jones K, Koulman A, Prentice A, Volmer DA. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Anal Bioanal Chem. 2010;398:779–789. doi: 10.1007/s00216-010-3993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoofnagle AN, Laha TJ, Donaldson TF. A rubber transfer gasket to improve the throughput of liquid-liquid extraction in 96-well plates: application to vitamin D testing. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1639–1642. doi: 10.1016/j.jchromb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray TK, McAdoo T, Pool D, Lester GE, Williams ME, Jones G. A modified radioimmunoassay for 1,25-dihydroxycholecalciferol. Clin Chem. 1981;27:458–463. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.