Abstract
Converging evidence suggests too few activation-ready N-methyl-d-aspartic acid (NMDA) receptor complexes in the postsynaptic density in schizophrenia. Postsynaptic density protein 95 (PSD95), Synaptic GTPase-activating protein (SynGAP), and Multiple PDZ domain protein (MUPP1) are integral components of the NMDA receptor signaling complex, and help facilitate signaling, trafficking, and stabilization. We hypothesized that deficits involving these molecules may contribute to the pathophysiology of schizophrenia. To test our hypothesis, we measured protein expression of PSD95, SynGAP, and MUPP1 in the anterior cingulate cortex and dorsolateral prefrontal cortex. We found decreased PSD95 expression in the anterior cingulate cortex. Antipsychotic medication analyses showed decreased SynGAP expression in the anterior cingulate cortex in patients off medication when analyzed against our comparison group. These data suggest that NMDA receptor complex formation, localization, and downstream signaling may be abnormal in schizophrenia.
Keywords: MUPP1, N-methyl-d-aspartic acid receptor, PSD95, schizophrenia, SynGAP
Introduction
Converging evidence implicates N-methyl-d-aspartic acid (NMDA) receptor dysfunction in schizophrenia [1]. These data suggest too few activation-ready NMDA receptor complexes localized in the postsynaptic density, leading to a deficit in downstream signaling pathways. Decreased localization and stabilization of the NMDA receptor could lead to altered neocortical circuit development and synaptic plasticity, contributing to the pathophysiology of this illness.
The NMDA receptor signaling complex includes molecules that facilitate signaling, trafficking, and stabilization of the complex [2]. Postsynaptic density protein 95 (PSD95) is concentrated in the postsynaptic density, and is part of a family of membrane-associated guanylate kinase proteins, which includes SAP97, SAP102, and PSD93 [3]. PSD95 binds to the C-terminus of NR2A and NR2B NMDA receptor subunits through specific N-terminal PDZ domains. PSD95 is believed to play a pivotal role by stabilizing and providing scaffolding connections with other molecules for downstream signaling, such as Synaptic GTPase-activating protein (SynGAP) and Multiple PDZ domain protein (MUPP1) [4]. PSD95, SynGAP, and MUPP1 form a biochemical complex that is regulated by NMDA receptor activation, and SynGAP–MUPP1 interactions are involved in the modulation of Ras-like GTPases and mitogen-activated protein kinase signaling [5]. It has been shown that these proteins localize to the postsynaptic density, forming a structural unit that facilitates placement and proper signaling of the NMDA receptor complex [6].
SynGAP is an attractive molecule to regulate signaling downstream of NMDA receptors, as it is an abundant synaptic protein that also contains a RasGAP signaling domain. SynGAP binds the C-terminus of PSD95 and interacts with CaMKII and MUPP1 in a complex of proteins important for phosphorylation and activation of many downstream targets [5,7–9]. SynGAP is heavily enriched in the postsynaptic density and coprecipitates with PSD95 and NMDA receptor subunits [7,10–12]. Functionally, SynGAP is a potent modulator of alpha-amino-3- hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor trafficking, synaptic function and spine morphogenesis [5,10–12]. SynGAP mutant mice have various cognitive and social deficits, and their behavioral endophenotype is nearly identical to that of mice with reduced expression or function of NMDA receptors [13,14]. It has been hypothesized that one function of SynGAP is to transduce synaptic NMDA receptor function into biochemical signals necessary for proper circuit function and behavior [13]. Therefore, we hypothesized that this protein, and the SynGAP-interacting proteins PSD95 and MUPP1 may be abnormal in patients with schizophrenia.
In this study, we performed western blot analyses using a large sample of post-mortem brain tissue from elderly patients with schizophrenia and a comparison group to determine whether there are alterations of NMDA receptor complex-associated molecules in schizophrenia.
Materials and methods
Tissue acquisition and preparation
Patients (Table 1) from the Mount Sinai Medical Center Brain bank were recruited prospectively and underwent extensive ante-mortem diagnostic and clinical assessments. Patients were excluded for a history of alcoholism, substance abuse, death by suicide, or coma for more than 6 h before death. Next of kin consent was obtained for each patient. At time of autopsy, anterior cingulate cortex and dorsolateral prefrontal cortex were dissected and samples were stored at − 80°C. Tissue was pulverized into a powder, adding small amounts of liquid nitrogen as necessary, and stored at − 80°C. Tissue was reconstituted in 5mM Tris–HCl pH 7.4, 0.32M sucrose, and a protease inhibitor tablet (Complete Mini, Roche Diagnostics, Mannheim, Germany). A Power Gen 125 homogenizer (Thermo Fisher Scientific, Rockford, Illinois, USA) was used at speed 5 for 60 s to homogenize the tissue. The homogenate was assayed for protein concentration using a BCA protein assay kit (Thermo Scientific, Rockford, Illinois, USA), and stored at − 80°C.
Table 1.
Patients’ characteristics
| Comparison group | Schizophrenia | |||
|---|---|---|---|---|
| Region | ACC | DLPFC | ACC | DLPFC |
| N | 33 | 31 | 36 | 35 |
| Sex (M/F) | 14/19 | 12/19 | 24/12 | 23/12 |
| Tissue pH | 6.4± 0.2 | 6.4± 0.2 | 6.4± 0.3 | 6.4 ±0.3 |
| PMI (h) | 8.3± 6.8 | 8.1± 6.9 | 13.2 ± 8.0 | 12.5 ±6.6 |
| Age (years) | 78± 14 | 78± 14 | 74± 11 | 74 ±12 |
| On/off Rx | 0/33 | 0/31 | 25/11 | 24/11 |
Values are presented as mean ± standard deviation.
ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; F, female; M, male; PMI, post-mortem interval; Rx, antipsychotic medication.
Western blot analysis
Commercially available antisera were used for the western blot analyses, including SynGAP (rabbit anti-SynGAP 1 : 5000 dilution; Affinity Bioreagents, Golden, Colorado, USA), PSD95 (mouse anti-PSD95 1 : 100 000 dilution; Upstate, Lake Placid, New York, USA), MUPP1 (mouse anti-MUPP1 1 : 500 dilution; BD Transduction, San Jose, California, USA), and β-tubulin (1 : 10 000 dilution; Upstate). IR-Dye labeled secondary antibodies (1 : 15 000 dilution; LiCor, Lincoln, Nebraska, USA) were used to detect primary antibodies.
Samples for the western blot analysis were placed in reducing buffer containing β-mercaptoethanol and heated at 70°C for 10 min. Samples were then run in duplicate by SDS–polyacrylamide gel electrophoresis on Invitrogen (Carlsbad, California, USA) 4–12% gradient gels, and then transferred to polyvinylidene fluoride membrane using BioRad semi-dry transblotter (Hercules, California, USA). The membranes were blocked in LiCor blocking buffer for 1 h at room temperature, and probed with primary antibody in 0.1% Tween LiCor blocking buffer overnight at 4°C. Samples for PSD95 analysis were incubated with primary antibody at room temperature for 1 h.
The membranes were washed twice for 10 min each in 0.01% Tween phosphate buffer solution then probed with IR-Dye labeled secondary antibodies in 0.1% Tween, 0.01% SDS LiCor blocking buffer for 1 h at room temperature. Washes were repeated after secondary labeling, washing twice for 10 min in Tween phosphate buffer solution and then placed in water. The blots were scanned using the LiCor laser-based image detection method.
Data analysis
The near-infrared fluorescent signals obtained from the LiCor Odyssey scanner were expressed as raw intensity with intralane background subtraction done using Odyssey 2.1 analytical software (LiCor, Lincoln, Nebraska, USA). Duplicate lanes of PSD95, SynGAP, and MUPP1 protein expression were normalized to β-tubulin and then averaged for each patient. We used β-tubulin as a loading control, as it was unchanged in patients with schizophrenia in an earlier study [15].
Data were analyzed using Statistica (Statsoft, Tulsa, Oklahoma, USA). Correlation analyses were performed to probe for associations between the dependent variables and pH, age, and post-mortem interval. One-way analysis of covariance was used to analyze the data when significant correlations were found, otherwise one-way analysis of variance was used. Analysis of variance was performed to assess the effects of antipsychotic medication in patients with schizophrenia.
Results
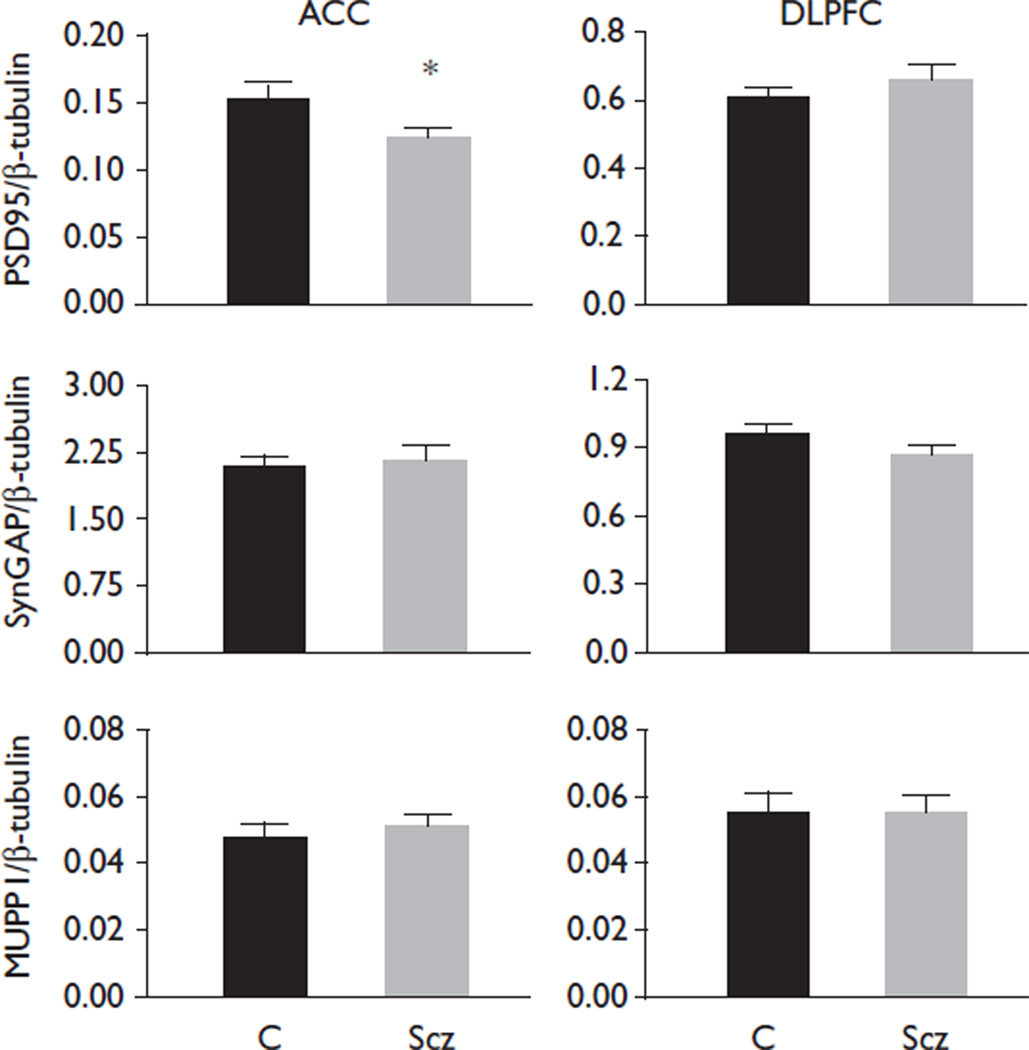
In patients with schizophrenia, we found decreased PSD95 in the anterior cingulate cortex [F(1,68)=6.336, P=0.014], but no change in dorsolateral prefrontal cortex (Fig. 1). In the anterior cingulate cortex, patients with schizophrenia off antipsychotics for 6 weeks or more at the time of death had decreased SynGAP expression compared with patients with schizophrenia on antipsychotics [F(1,34)=4.66, P=0.038] (Fig. 2). We also found decreased SynGAP in schizophrenia patients off medication, compared with the comparison group in the anterior cingulate cortex [F(1,42)=4.27, P=0.045] (Fig. 2). There were no antipsychotic effects in the dorsolateral prefrontal cortex for either group. No significant correlation was found between pH, post-mortem interval or age and any of our dependent measures. We did not detect changes in β-tubulin protein expression in schizophrenia or in patients on versus off antipsychotic medication.
Fig. 1.
N-methyl-d-aspartic acid receptor-associated protein expression in the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC). Data are presented as a ratio of signal intensity for the protein of interest normalized to β-tubulin. Data are expressed as mean±standard error. Postsynaptic density protein 95 (PSD95) was decreased (P=0.014) in the anterior cingulate cortex with no change in the dorsolateral prefrontal cortex. No changes were observed for Synaptic GTPase-activating protein (SynGAP) or Multiple PDZ domain protein (MUPP1). C, comparison group; Scz, schizophrenia. *P<0.05.
Fig. 2.
Analysis of antipsychotic effects in patients with schizophrenia. Data are presented as a ratio of signal intensity of Synaptic GTPase-activating protein (SynGAP) normalized to β-tubulin. SynGAP was decreased in the anterior cingulate cortex (ACC) in patients with schizophrenia off antipsychotic medication ≥6 weeks compared with the comparison group (P=0.045). SynGAP was also decreased in the ACC in patients with schizophrenia off antipsychotic medication ≥6 weeks compared with patients with schizophrenia on antipsychotic medication (P=0.038). Off, ≥6 weeks off medication; On, <6 weeks off medication. *P<0.05.
Discussion
In this study, we evaluated expression of molecules that facilitate localization and mediate downstream signaling of the NMDA receptor in anterior cingulate and dorsolateral prefrontal cortex from elderly patients with schizophrenia and a comparison group. Our finding of decreased PSD95 in the anterior cingulate cortex in patients with schizophrenia is consistent with an earlier finding from our lab [16], although this study used a larger sample from the same brain collection (n=20 vs. n=69). This finding supports the hypothesis of altered NMDA receptor complex localization in schizophrenia.
A decrease in PSD95 may be a compensatory mechanism whereby local circuits try to enhance NMDA receptor function through the recruitment of D1 dopamine receptors. Increased PSD95 has been shown to negatively regulate D1/NR1 subunit interaction [17]. Further, activation of the D1 receptor potentiates NMDA receptor-mediated cytosolic increases in calcium through a protein kinase A-dependent mechanism [18]. Taken together, these data support the hypothesis that regional decreases in PSD95 may increase D1 receptor colocalization with NMDA receptors to increase glutamate neurotransmission.
We also evaluated SynGAP and MUPP1 expression, owing to their direct interaction with the NMDA receptor complex and downstream actions on the mitogen-activated protein kinase (MAPK) signaling cascades [7,8,10,11,19,20]. Our finding of decreased SynGAP expression in the anterior cingulate cortex in the off medication group suggests that SynGAP expression is decreased in this illness and that medication may normalize SynGAP protein expression. This finding suggests that patients with schizophrenia may have decreased activation of the MAPK/extracellular signal-regulated kinase (ERK) pathway through NR2B-containing NMDA receptors [11,12,14].
We did not find changes in expression of MUPP1. Although, total MUPP1 protein levels are unchanged, there may still be abnormalities associated with MUPP1. For example, protein–protein interactions within the complex may be dysregulated, hindering the proper connections between these molecules and the NMDA receptor complex. There may also be impaired trafficking or alterations in phosphorylation of MUPP1. Improper trafficking to the postsynaptic density would result in a localized deficit of the NMDA receptor complex. Altered subcellular localization of MUPP1, with no change in total protein levels, could also lead to abnormal NMDA receptor complex function and localization.
In summary, our data support the hypothesis that NMDA receptor complex trafficking, localization, and down-stream signaling may be abnormal in schizophrenia. In addition, these data support the hypothesis that functional glutamate neurotransmission is diminished in a region-specific manner in schizophrenia, and suggests that NMDA receptor dysfunction may arise from disorganized biochemical signaling pathways downstream of channel activation.
Acknowledgements
This study was supported by MH53327 (J.M.W.), MH0 64673 and MH066392 (V.H.), and MH074016 (R.E.M.).
References
- 1.Belsham B. Glutamate and its role in psychiatric illness. Hum Psychopharmacol. 2001;16:139–146. doi: 10.1002/hup.279. [DOI] [PubMed] [Google Scholar]
- 2.Husi H, Grant S. Proteomics of the nervous system. Trends Neurosci. 2001;24:259–266. doi: 10.1016/s0166-2236(00)01792-6. [DOI] [PubMed] [Google Scholar]
- 3.Boeckers T. The postsynaptic density. Cell Tissue Res. 2006;326:409–422. doi: 10.1007/s00441-006-0274-5. [DOI] [PubMed] [Google Scholar]
- 4.Elias G, Nicoll R. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham D. SynGAPMUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Ross C, Margolis R, Reading S, Pletnikov M, Coyle J. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Liao D, Lau L, Huganir R. SynGAP: a synaptic RasGAP that associates with the PSD-95;/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe H, Vinade L, Dosemeci A. Identification of novel phosphorylation sites on postsynaptic density proteins. Biochem Biophys Res Commun. 2004;321:210–218. doi: 10.1016/j.bbrc.2004.06.122. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Lee H, Takamiya K, Huganir R. The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J Neurosci. 2003;23:1119–1124. doi: 10.1523/JNEUROSCI.23-04-01119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vazquez L, Chen H, Sokolova I, Knuesel I, Kennedy M. SynGAP regulates spine formation. J Neurosci. 2004;24:8862–8872. doi: 10.1523/JNEUROSCI.3213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rumbaugh G, Adams J, Kim J, Huganir R. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci U S A. 2006;103:4344–4351. doi: 10.1073/pnas.0600084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim M, Dunah A, Wang Y, Sheng M. Differential roles of NR2A- and NR2 B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Hamilton P, Reish N, Sweatt J, Miller C, Rumbaugh G. Reduced expression of the NMDA receptor-interacting protein SynGAP causes behavioral abnormalities that model symptoms of schizophrenia. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2008.223. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komiyama N, Watabe A, Carlisle H, Porter K, Charlesworth P, Monti J, et al. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22:9721–9732. doi: 10.1523/JNEUROSCI.22-22-09721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer D, Haroutunian V, McCullumsmith R, Meador-Woodruff J. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009;116:487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristiansen L, Beneyto M, Haroutunian V, Meador-Woodruff J. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11:737–747. 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Xu T, Hallett P, Watanabe M, Grant S, Isacson O, et al. PSD-95 uncouples dopamine-glutamate interaction in the D1/PSD-95/NMDA receptor complex. J Neurosci. 2009;29:2948–2960. doi: 10.1523/JNEUROSCI.4424-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruse M, Prémont J, Krebs M, Jay T. Interaction of dopamine D1 with NMDA NR1 receptors in rat prefrontal cortex. Eur Neuropsychopharmacol. 2009;19:296–304. doi: 10.1016/j.euroneuro.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Huang C, Yang C, Lai M, Chen W, Wang C, et al. Impaired SynGAP expression and long-term spatial learning and memory in hippocampal CA1 area from rats previously exposed to perinatal hypoxia-induced insults: beneficial effects of A68930. Neurosci Lett. 2004;371:73–78. doi: 10.1016/j.neulet.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Song B, Yan X, Zhang G. PSD-95 promotes CaMKII-catalyzed serine phosphorylation of the synaptic RAS-GTPase activating protein SynGAP after transient brain ischemia in rat hippocampus. Brain Res. 2004;1005:44–50. doi: 10.1016/j.brainres.2004.01.032. [DOI] [PubMed] [Google Scholar]