Abstract
Background:
Mechanotransduction plays a pivotal role in remodeling and repair of skeletal tissues. This mechanism has been widely used in bone tissue engineering especially under in vitro conditions. To date, various stem cells have been used for this purpose. The present study was the first to evaluate the effect of mechanical loading on differentiation of human endometrial stem cells (hESCs) to osteoblasts.
Materials and Methods:
Adhesion of endometrial stem cells after isolation and culture on a silicone membrane covered with collagen was evaluated under scanning electron microscope (SEM). Twenty-four hours after cell culture on the membrane and ensuring appropriate cell adhesion, a group of cells in a conventional culture medium received 3% static uniaxial strain. In the positive control group, cells cultured on the membrane were placed in an osteogenic medium without receiving any mechanical strain. The negative control group was placed in a regular medium and received no strain either. Two weeks later, cultured cells were evaluated for expression of osteogenic markers using immunofluorescence staining and real-time polymerase chain reaction (PCR). Data of real-time PCR was analyzed by ANOVA. P < 0.05 was considered statistically significant.
Results:
SEM analysis revealed adequate cell adhesion to the membrane after 24 h. Two weeks after loading, expression of markers in the positive control group was significantly higher compared to test group.
Conclusion:
We can conclude that static uniaxial strain exerted on hESCs results in their differentiation to osteoblasts. However, this magnitude of static strain in the tested time period cannot yield excellent differentiation when compared to the osteogenic medium.
Keywords: Bone, differentiation, endometrial stem cells, uniaxial strain
INTRODUCTION
During the past decades, great advancements have occurred in bone tissue engineering. At present, bone tissue engineering is a potentially better treatment option for replacement of injured bone in comparison to conventional treatments.[1] Osteogenic factors or mechanical signals can be used for bone tissue engineering. Numerous studies have demonstrated that mechanical forces can stimulate the synthesis of bone extracellular matrix. They may even enhance the mechanical properties of formed tissues. There is lots of evidence supporting the important role of mechanical forces in enhancing bone remodeling.[2] On the contrary, studies have shown that absence of load can result in tissue atrophy and bone loss.[3] Mechanical forces can also regulate fetal growth and development. Studies on completely paralyzed avian embryos have revealed specific growth defects in the mandible and large bones which indicate that muscle contraction and the resultant forces are necessary for skeletal development and correct morphogenesis of tissues.[4]
Mechanosensing is a process through which a biophysical mechanism converts the deformation into a biochemical response that can move the gene or alter its expression. Several studies have investigated the effect of mechanical forces on osteoblasts.[5] These studies raised the question whether mechanical loading is also effective on the differentiation of stem cells to osteoblasts. The answer to this question can be important in bone tissue engineering for reconstruction of bone loss due to injury or osteoporosis. In tissue engineering, transplantation of stem cells that have been cultured on a proper scaffold and differentiated into osteoblasts under the induction of mechanical signals can replace the use of autologous bone grafts which are associated with great patient discomfort.[6]
The response of bone marrow stem cells to the mechanical signals has been evaluated in many studies.[7,8,9] However, the process of isolation of these cells is usually painful and requires general anesthesia. Also, the heterogeneous cell population obtained from the bone marrow contains a mixture of differentiated and undifferentiated cells.
Endometrial stem cells are a new source of stem cells present in the uterine endometrium that probably play a role in cyclic endometrial regeneration.[10,11] Chan, et al., detected a population of stem cells in the human endometrium that had clonogenic activity.[12] Also, Gargett, et al., assessed the proliferation and differentiation potential of these cells.[13] Some other researchers evaluated the ability of endometrial stem cells to differentiate into three cell lineages and confirmed their mesenchymal origin.[14] Since no study has investigated the effect of mechanical signals on endometrial stem cells, this study aimed at assessing the effect of uniaxial static mechanical strain on differentiation of endometrial stem cells to osteoblasts and comparing it with the effect of osteogenic factors.
MATERIALS AND METHODS
All cell culture chemicals and supplies were purchased from Sigma (NY, USA) and Gibco-BRL (Grand Island, NY, USA) unless otherwise noted.
Isolation and culture of endometrial stem cells
A biopsy sample was obtained from the uterine endometrial tissue of women in the age range of 20 to 50 years presenting to the Gynecology Department of Shariati Hospital because of infertility problems, using a biopsy device. These patients were in days 19 to 24 of their menstrual cycle, did not have endometriosis, fibroma or any other uterine conditions, did not have intrauterine device (IUD) and had not used any hormonal medications in the past three months prior to biopsy. All procedures were confirmed by the Ethics Committee of Shahid Beheshti University of Medical Science and written informed consent was obtained from all patients. Biopsy specimens were transferred to the reference laboratory of cell and molecular oral biology (Shahid Beheshti University of Medical Science, Tehran, Iran) under sterile conditions. Isolation steps were carried out according to the published protocols.[15] In summary, endometrial tissue was chopped by a scalpel and then subjected to collagenase type IA (2 mg/ml) digestion for 2 h at 37°C. After tissue digestion, epithelial and stromal cells were separated by passing through 70-μm and 45-μm filters. After passing through the filters, cells were centrifuged at 1000 g for 15 min and purified using Ficoll. The cells were then washed with phosphate-buffered saline (PBS) for several times. The obtained cell sediment was suspended in 5 ml conventional culture medium containing Dulbecco's Modified Eagle's Medium (DMEM), 1% antibiotic 100 and 10% fetal bovine serum (FBS) and after transferring to the flask was incubated at 37°C, 5% CO2 and 95% moisture. Culture medium was refreshed every three days. After proliferation and covering approximately 70% of the flask surface, the cells were passaged using trypsin. For characterization, they were analyzed by flow cytometry and they were directionally differentiated towards adipogenic, and osteogenic lineages. Passage 4-5 cells were used for this study.
Membrane preparation
Medical-grade silicone membrane was used in this study. In order to enhance cell adhesion, the membrane surface was covered with 0.5 mg/ml Type I collagen (Sigma) in 0.2% citric acid. Cell suspension in an amount of 1 × 106 cells in 100-microliter culture medium was transferred to the membrane and placed in an incubator for 24 h.
SEM preparation
After 24 h, cells were fixed in 2.5% glutaraldehyde for 2 h in a refrigerator. They were washed with buffer and dehydrated using alcohol of different percentages. The samples were then air-dried. Cell-containing membranes were evaluated under scanning electron microscope at 24 kV (KYKY-EM3200, China).
Static uniaxial strain
In order to apply mechanical strain to the human stromal stem cells cultured on a silicone membrane, a uniaxial strain device designed in the National Cell Bank of Iran[16] was used [Figure 1]. The exerted strain was static, uniaxial and with a magnitude of 3% and conventional culture medium (DMEM, 10%FBS, 1%antibiotic/antimycotic) was used in the test group. Positive and negative control groups did not receive any strain and were placed on silicone membrane in cell culture plate containing respectively, osteogenic medium (containing dexamethasone, ascorbic acid and β-glycerophosphate) or conventional medium (DMEM/10%FBS/antibiotic). The medium used for cells cultured on the scaffold was refreshed after 24 h or the cells were transferred to the device.
Figure 1.

Picture of the device used for application of uniaxial tensile strain
Immunofluorescence
Two weeks after culturing the cells on the scaffold, expression of osteocalcin in the cells was evaluated using immunofluorescence in order to determine the induction of osteogenic markers in response to tensile strain.[17] Cells in the three groups of test, positive and negative control were washed with PBS and fixed with 4% paraformaldehyde at 4°C for 30 min. Nonspecific antibodies were blocked with 0.5% goat serum for an hour and the samples were incubated with osteocalcin primary antibodies (polyclonal rabbit anti-bovine with cross-reactivity to human, 1:50 dilution, Chemi-Con, Temecula, CA) overnight at 4°C. Incubation with secondary antibodies (anti-mouse IgG-FITC 1:160 dilution; Sigma, St. Louis, MO) was done for 3 h. In the final step, cells were washed with PBS and photographed using florescent microscope.
Real-time PCR
Real time PCR was used for quantitative analysis of osteocalcin and alkaline phosphatase (ALPL) expression. RNA extraction from the cells was done using RNeasy Plus Mini Kit (QIAGEN, MD, USA) followed by cDNA synthesis using QuantiTect Rev. Transcription Kit (QIAGEN, MD, USA). In this way, RNA extraction was done according to the manufacturer protocol, with minor modifications. The important advantage of this kit is elimination of genomic DNA. The concentration and purity of all extracted RNAs were measured. So RNA quality was determined utilizing spectrophotometry (Nanophotometer™, Implen, Germany). After checking the quality of RNAs, high-quality RNA samples (A260/280 ≥ 1.8) were selected for further experiments and kept at −80°C until the suitable time for cDNA synthesis. QuantiTect kit was chosen for two reasons: 1 - This kit could provide DNA from 10 pg to 1 μg RNA amounts; 2 - RT buffer of the kit was compatible with SYBR Green Real-Time PCR buffer. These two properties make the reagents of the kit appropriate for Real-Time PCR.
In the next step an electrophoresis was done to verify the integrity of the cDNA (data not shown).
Two genes were selected as early and late osteoblastic target genes and RPL-13A (ribosomal protein large subunit-13a) was selected as housekeeping gene. Alkaline phosphatase (ALPL) is expressed in the early stage of osteoblastic differentiation and osteocalcin (OCN) is expressed in the late stage of differentiation.
Primers were designed using Primer Express v3.0 software. All primers which were recommended by Primer Express were checked with gene runner to analysis of oligos.
The sequence of primers for RPL13A, osteocalcin (OCN) and ALPL was as follows:
RPL13A: Forward primer = 5′- CCTGGAGGAGAAGAGGAAAGAGA -3′, Reverse primer = 5′-TTGAGGACCTCTGTGTATTTGTCAA-3′. OCN: Forward primer = 5′- AACGCCGACCAAGGAAAACT-3′, Reverse primer = 5′- GGCCACAGCATCTGGGTATT-3′. ALPL: Forward primer = 5′- CCTGGACCTCGTTGACACCT-3′, Reverse primer = 5′- GTCCCCTGGCTCGAAGAGA-3′. The Real-Time PCR experiments were performed on ABI StepOne system using StepOne v2.1 software. Each reaction mixture contained 5 μl cDNA, 5 pmol of each primer (Forward and Reverse), 10 μl of SYBR Green PCR master mix (Applied Biosystems, Foster City, USA) and 4 μl of double distilled water (D.D.W).
The following thermal cycling profile was used: holding stage set at 95°C (10 min); cycling stage set at temperatures varying from 95°C (15 sec) and 60°C (1 min) (40 cycles); and melt curve stage set at 95°C (15 sec), 60°C (1 min) and 95°C (15 sec). The assay experiments were performed in triplicate and mean CT of triplicate reactions was applied in data analysis. mRNA expression of target genes was normalized to RPL-13A gene expression. Relative gene expression was achieved using this formula:
ΔΔCT = [min CTTargets − min CTRPL-13A] Test sample − [min CTTargets − mCTRPL-13A] Normal sample
Relative gene expression = 2−ΔΔCT
Statistical analysis
SPSS version 16.0 software was used for statistical analysis. All samples were averaged and the means and standard deviation (SD) calculated for each group and was compared using one-way analysis of variance (ANOVA). Values were presented as mean ± standard deviation for the individual groups. P < 0.05 was considered statistically significant.
RESULTS
As demonstrated in Figure 2, 24 h after cell culture on the membrane, SEM indicated adequate adhesion of cells to the collagen-covered membrane.
Figure 2.
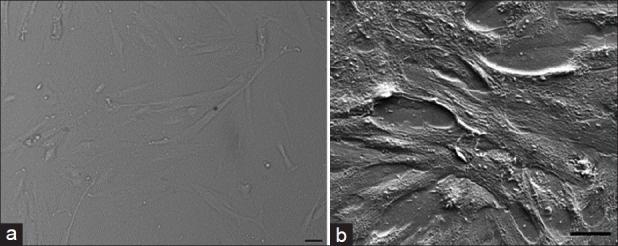
Picture of cells under inverted microscope (a) and SEM (b)
Using immunofluorescence study, osteocalcin expression was observed after two weeks in both groups under mechanical and chemical signals while osteocalcin expression was not detected in the negative control group [Figure 3].
Figure 3.
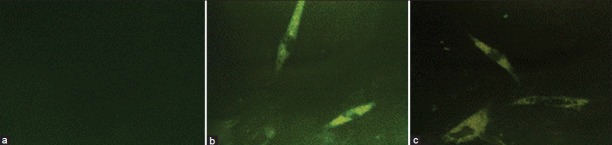
Immunofluorescence staining of the osteocalcin marker in the negative control group (a), positive control group (b) and test group under mechanical signals (c)
The mRNA expression level of ALPL and OCN relative to RPL-13A was determined by Real-time PCR. As presented below [Figure 4] chemical induction has greater effects on endometrial stem cells to direct osteogenic differentiation as compared to static uniaxial mechanical stretch.
Figure 4.
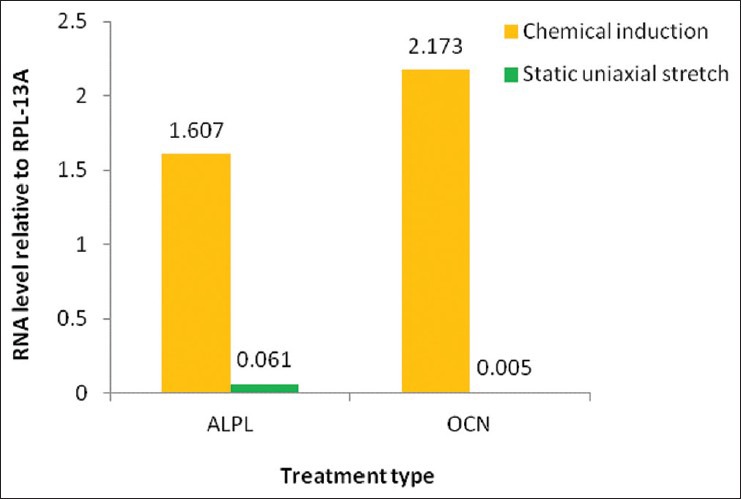
The comparison between the positive control group: chemical induction and the test group: Uniaxial stretch. The mRNA levels were normalized relative to RPL-13A as reference gene
DISCUSSION
The role of mechanical forces in the differentiation of stem cells has been the focus of attention during the recent years. Although differentiation of endometrial stem cells in the presence of osteogenic factors has been demonstrated in the literature, it has not been specified how these cells respond to mechanical forces. In this study, we sought to assess the effect of mechanical strain on these cells without using any biochemical reagent.
All cells are mechanosensitive; but the main question is which cells can play a more prominent role in this respect? Many researchers have assessed the effect of tensile stimuli on bone marrow stem cells.[18,19,20] However, to the best of our knowledge, the effect of such stimuli on endometrial stem cells has not been investigated. Considering the optimal properties of these cells and the possibility of angiogenesis,[15] which is an important factor in bone tissue engineering, the present study used endometrial stem cells as the cell source.
Since the first interaction between cells and the scaffold is done through cell adhesion, surface characteristics of the substrate play a key role in success of tissue engineering.[21] Cell adhesion results in attachment of cells to the substrate and provides signals that induce cell differentiation.[22] Some researchers have demonstrated that covering the substrate surface with extracellular matrix molecules such as collagen, fibronectin, or laminin improves efficient cell seeding and enhances expansion of cells on the substrate.[23,24] In this study, Type I collagen was used for covering the surface of silicone membrane. SEM images obtained 24 h after the transfer of cells on the scaffold demonstrated adequate adhesion of cells to the substrate.
In general, chemical induction is the most commonly used method for stem cell differentiation. However, at present it has been revealed that tissue engineering in tissues that are under strain requires mechanical stimuli as well.[25] Use of uniaxial tensile strain is a mechanical stimulation technique for successful induction of bone remodeling.[26] In the present study, the test group receiving mechanical strain was placed in a conventional medium and was compared after two weeks with cells placed in an osteogenic medium with no mechanical force application. The results demonstrated that the level of differentiation in the positive control groups was more than the test group. Sumanasinghe, et al., in their study reported that even in the absence of osteogenic medium, 10% uniaxial tensile strain can differentiate bone marrow stem cells after one week.[9] Our study demonstrated that mechanical stimuli alone can initiate osteogenic differentiation in endometrial stem cells. The type of strain used in this study was static strain. Although dynamic forces can better mimic normal in vivo conditions, several studies have also demonstrated the positive effects of static forces.[27]
In the present study, 3% strain was applied for evaluation of the effect of mechanical strain on differentiation of endometrial stem cells. Chen, et al., studied the effect of 3% and 10% strains on bone marrow stem cells and demonstrated that only 3% strain results in increased expression of osteoblast-specific genes; while 10% strain results in differentiation of stem cells to ligament/tendon.[18] This study indicated that the magnitude of strain can determine into what type of cells the stem cells will transform. Duration of strain has been different in various studies ranging from a few hours to several days or weeks. For instance, Simmons, et al., reported the effect of 3% strain on differentiation of stem cells after nine days.[28] We chose a two-week period in the present study in order to make a precise comparison with the effect of osteogenic medium.
For evaluation of the osteogenic differentiation response to mechanical strain, expression of proteins or osteogenic marker genes such as osteocalcin[29] and ALPL[30] is usually assessed. Tong, et al., investigated the expression of bone sialoprotein and indicated that uniaxial tensile strain can induce bone formation.[31] In the present study, expression of osteocalcin was evaluated by immunofluorescence and real-time PCR. Osteocalcin is a late marker for osteogenic differentiation. In our study, the level of expression of osteocalcin was significantly more in the positive control group than in the test group receiving mechanical strain.
Expression of ALPL was also evaluated by real-time PCR. This marker is usually expressed during the early phases of osteoblastic differentiation[8] and in our study its expression was significantly lower in the test group than in the positive control group. Researchers have demonstrated that bone remodeling can be facilitated by application of cyclic forces.[32] Future studies can focus on the effect of cyclic forces and use of three-dimensional scaffolds. It should be mentioned that the magnitude of exerted strain, its duration and type of stem cells may be responsible for the controversial results about the effects of mechanical strain on stem cell differentiation.
CONCLUSION
To the best of our knowledge, the present study is the first to evaluate the effect of mechanical stimuli on endometrial stem cells. In summary, this study showed that application of 3% static tensile strain for two weeks could not cause a high-quality osteogenic differentiation in endometrial stem cells compared to cells placed in an osteogenic medium. It seems that longer or cyclic application of strain is necessary for induction of proper differentiation. Therefore, chemically-induced differentiation is still the gold standard for osteogenic differentiation of endometrial stem cells.
ACKNOWLEDGMENT
This study is a revised version of a thesis for the degree of Professional Doctorate in Dentistry, supported by the Faculty of Dentistry, Shahid Beheshti University of Medical Science (Project No. 89-01-92-7036).
Footnotes
Source of Support: Faculty of Dentistry, Shahid Beheshti University of Medical Science (Project No. 89-01-92-7036)
Conflict of Interest: None declared
REFERENCES
- 1.Yang Y, El Haj A. Ashammakhi N, Reis RL, editors. Enhancement of mechanical signals for tissue engineering bone. Topics in Tissue Engineering. 2005. pp. 1–21. Website: www.oulu.fi/spareparts/ebook_topics_in_t_e_vol2 .
- 2.Jones D. Influence of Mechanical Effects on Cells. Biophysical Basis. In: Meyer U, editor. Fundamentals of Tissue Engineering and Regenerative Medicine. Berlin: Springer; 2009. pp. 83–8. [Google Scholar]
- 3.Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5:843–50. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 4.Ingber DE. Mechanical control of tissue morphogenesis during embryological development. Int J Dev Biol. 2006;50:255–66. doi: 10.1387/ijdb.052044di. [DOI] [PubMed] [Google Scholar]
- 5.Kaspar D, Seidl W, Neidlinger-Wilke C, Claes L. In vitro effects of dynamic strain on the proliferative and metabolic activity of human osteoblasts. J Musculoskelet Neuronal Interact. 2000;1:161–4. [PubMed] [Google Scholar]
- 6.van Griensven M, Diederichs S, Kasper C. Ashammakhi N, Reis RL, editors. Mechanical strain of bone marrow stromal cells induces proliferation and differentiation into osteoblast-like cells. Topics in Tissue Engineering. 2005. pp. 1–21. Website: www.oulu.fi/spareparts/ebook_topics_in_t_e_vol2 .
- 7.Ignatius A, Blessing H, Liedert A, Schmidt C, Neidlinger-Wilke C, Kaspar D, et al. Tissue engineering of bone: Effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials. 2005;26:311–8. doi: 10.1016/j.biomaterials.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Lee SK, Lee CY, Kook YA, Kim EC. Mechanical stress promotes odontoblastic differentiation via the heme oxygenase-1 pathway in human dental pulp cell line. Life Sci. 2010;86:107–14. doi: 10.1016/j.lfs.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Sumanasinghe RD, Bernacki SH, Loboa EG. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: Effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006;12:3459–65. doi: 10.1089/ten.2006.12.3459. [DOI] [PubMed] [Google Scholar]
- 10.Gargett CE, Chan RW, Schwab KE. Endometrial stem cells. Curr Opin Obstet Gynecol. 2007;19:377–83. doi: 10.1097/GCO.0b013e328235a5c6. [DOI] [PubMed] [Google Scholar]
- 11.Schwab KE, Chan RW, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84:1124–30. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 12.Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–50. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 13.Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–45. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cervelló I, Gil-Sanchis C, Mas A, Delgado-Rosas F, Martínez-Conejero JA, Galán A, et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5:e10964. doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabatabaei FS, Ai J, Jafarzadeh Kashi TS, Khazaei M, Kajbafzadeh A-M, Ghanbari Z. Effect of dentine matrix proteins on human endometrial adult stem-like cells: In vitro regeneration of odontoblasts cells. Arch Oral Biol. 2013;58:871–9. doi: 10.1016/j.archoralbio.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Haghighipour N, Tafazzoli-Shadpour M, Shokrgozar MA, Amini S, Amanzadeh A, Khorasani MT. Topological remodeling of cultured endothelial cells by characterized cyclic strains. Mol Cell Biomech. 2007;4:189–99. [PubMed] [Google Scholar]
- 17.Kearney EM, Farrell E, Prendergast PJ, Campbell VA. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann Biomed Eng. 2010;38:1767–79. doi: 10.1007/s10439-010-9979-4. [DOI] [PubMed] [Google Scholar]
- 18.Chen YJ, Huang CH, Lee IC, Lee YT, Chen MH, Young TH. Effects of cyclic mechanical stretching on the mRNA expression of tendon/ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connect Tissue Res. 2008;49:7–14. doi: 10.1080/03008200701818561. [DOI] [PubMed] [Google Scholar]
- 19.Nieponice A, Maul TM, Cumer JM, Soletti L, Vorp DA. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J Biomed Mater Res A. 2007;81:523–30. doi: 10.1002/jbm.a.31041. [DOI] [PubMed] [Google Scholar]
- 20.Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359–68. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 21.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 22.Schakenraad JM, Busscher HJ, Wildevuur CR, Arends J. The influence of substratum surface free energy on growth and spreading of human fibroblasts in the presence and absence of serum proteins. J Biomed Mater Res. 1986;20:773–84. doi: 10.1002/jbm.820200609. [DOI] [PubMed] [Google Scholar]
- 23.Ye Q, Zund G, Jockenhoevel S, Schoeberlein A, Hoerstrup SP, Grunenfelder J, et al. Scaffold precoating with human autologous extracellular matrix for improved cell attachment in cardiovascular tissue engineering. ASAIO J. 2000;46:730–3. doi: 10.1097/00002480-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Schneider A, Schwalb H, Vlodavsky I, Uretzky G. An improved method of endothelial seeding on small caliber prosthetic vascular grafts coated with natural extracellular matrix. Clin Mater. 1993;13:51–5. doi: 10.1016/0267-6605(93)90090-t. [DOI] [PubMed] [Google Scholar]
- 25.Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50–9. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loboa EG, Fang TD, Warren SM, Lindsey DP, Fong KD, Longaker MT, et al. Mechanobiology of mandibular distraction osteogenesis: Experimental analyses with a rat model. Bone. 2004;34:336–43. doi: 10.1016/j.bone.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto E, Iwanaga W, Miyazaki H, Hayashi K. Effects of static stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon. J Biomech Eng. 2002;124:85–93. doi: 10.1115/1.1427924. [DOI] [PubMed] [Google Scholar]
- 28.Simmons CA, Matlis S, Thornton AJ, Chen S, Wang CY, Mooney DJ. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36:1087–96. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 29.Jagodzinski M, Drescher M, Zeichen J, Hankemeier S, Krettek C, Bosch U, et al. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004;16:35–41. doi: 10.22203/ecm.v007a04. [DOI] [PubMed] [Google Scholar]
- 30.Wozniak M, Fausto A, Carron CP, Meyer DM, Hruska KA. Mechanically strained cells soft of the osteoblastlineage organize their extracellular matrixthrough unique sites of alphavbeta3-integrin expression. J Bone Miner Res. 2000;15:1731–45. doi: 10.1359/jbmr.2000.15.9.1731. [DOI] [PubMed] [Google Scholar]
- 31.Tong L, Buchman SR, Ignelzi MA, Jr, Rhee S, Goldstein SA. Focal adhesion kinase expression during mandibular distraction osteogenesis: Evidence for mechanotransduction. Plast Reconstr Surg. 2003;111:211–22. doi: 10.1097/01.PRS.0000033180.01581.9A. [DOI] [PubMed] [Google Scholar]
- 32.Brown TD. Techniques for mechanical stimulation of cells in vitro: A review. J Biomech. 2000;33:3–14. doi: 10.1016/s0021-9290(99)00177-3. [DOI] [PubMed] [Google Scholar]


