Abstract
Background:
Recently, there has been interest in non-mammalian collagen sources such as fish collagen in the development of biomatrices and scaffolds for periodontal regeneration. In the present study, a novel collagen barrier membrane of fish origin was assessed in the treatment of periodontal intra-bony defects.
Materials and Methods:
Ten systemically healthy chronic periodontitis patients having an osseous defect in the mandibular posterior teeth were selected and following the open flap debridement, a collagen membrane was placed over the defect and the flap was sutured with interrupted sutures. Clinical parameters such as Plaque Index, Gingival Bleeding Index, probing pocket depth (PPD), relative attachment level (RAL), and recession (R) were recorded at baseline, 6 and 9 months, whereas radiographic evaluation was done to assess alveolar crestal bone level and defect depth fill at 6 and 9 months using Auto-computer aided design (ACAD) 2007 software. Statistical significance was set at 5% level of significance.
Results:
There was statistical significant differences with respect to periodontal clinical parameters such as Plaque Index, Gingival Bleeding Index, PPD, RAL, and gingival recession assessed at baseline, at 6 and 9 months respectively (P < 0.05), and radiographic evaluation showed a defect fill of 58.62 median % at 9 months.
Conclusion:
This preliminary study has shown predictable results in using fish collagen membrane, for treating periodontal intra-bony defects. Further, long-term clinical trials are needed to validate the effectiveness of this membrane.
Keywords: Barriers, collagen, guided-tissue regeneration, intrabony defects, membranes
INTRODUCTION
Periodontal therapy involves controlling of periodontal infection and aims at regeneration of the lost periodontium.[1] The regeneration of the periodontium with new connective fiber insertion, new cementum, and new bone formation constitutes ideal healing, which is based on a theory (Melcher AH, 1976) that only the periodontal ligament cells derived from the remaining viable periodontium are capable of differentiating into new fibroblasts, cementoblasts, and osteoblasts.[2] The two techniques with the most successful documentation of periodontal regeneration are osseous grafting and guided-tissue regeneration (GTR).[3] Nyman, et al., suggested the placement of a physical barrier between the flap and the root surface to exclude gingival connective tissue and epithelium from the healing process, giving the periodontal ligament cells, the opportunity to repopulate the coagulum on the root surface. This technique was named as guided tissue regeneration (GTR).[2,4]
The barriers recommended for the use in GTR, regardless of the material used, must be safe, biocompatible, and non-toxic, not induce any inflammatory response, and be designed for clinical applicability based on the morphology of the osseous defects.[5] The barrier used are either non-resorbable or bioresorbable membranes. Resorbable membranes are preferred as non-resorbable membranes require second surgical procedures to remove it. Furthermore, gingival recession (R), device exposure, infection, and inflammation are frequently experienced with non-resorbable membranes.[6]
Among resorbable barriers, investigators have examined type-I collagen for the use in GTR procedures as it is a major extracellular macromolecule of the periodontal connective tissue. Also collagen is known to be a weak immunogen, has a chemotactic property for fibroblasts, and acts as a barrier for migrating epithelial cells in vitro.[7] Collagen from mammalian sources, primarily bovine skin has been utilized in foods, cosmetics, and biomaterials and has the advantage of biodegradability and low toxicity. Therefore, there have been various attempts to use mammalian collagen in the medical field as a scaffold for developing artificial organs. However, the use of bovine collagen has been reconsidered, as some reports have shown the risk of transmission of bovine spongiform encephalopathy to human beings. Recently, there has been interest in non-mammalian collagen sources, primarily in fish collagen such as that of shark and salmon as it is known to have low risk for transmission of infectious disease to humans than bovine collagen.[8]
Hence, an attempt was made to evaluate a bio-resorbable collagen membrane of fish origin (Periocol™, Eucare pharmaceuticals Private limited, Chennai, India.) in the treatment of periodontal intra-bony defects by assessing the clinical parameters like probing pocket depth (PPD), relative attachment level (RAL) and Recession (R), and radiographic parameters such as alveolar crestal bone level (ABL) and defect fill (DF).
MATERIALS AND METHODS
A total of 10 patients (six males and four females) aged between 21 years and 50 years attending the Department of Periodontics, V. S. Dental College and Hospital, Bangalore, Karnataka, India, having isolated intra-osseous defects in the mandibular posterior teeth were selected based on the following inclusion and exclusion criteria.
Inclusion criteria: (a) Patients of either sex having chronic adult periodontitis, (b) Patients who were systemically healthy with no contraindication to periodontal surgery, (c) non-smokers, (d) presence of an isolated 2/3 walled intra-bony defect in the mandibular posterior teeth with residual PPD of ≥ 5 mm and with radiographic evidence of the affected site, (e) patients having 2-3 mm band of keratinized tissue to allow surgical manipulation and suturing, and (f) patients who were cooperative and able to come for regular follow-up. Exclusion criteria: (a) Patients allergic or sensitive to any medication or any ingredient of the test material, (b) patients showing unacceptable oral hygiene compliance during or after phase I therapy, and (c) pregnant and lactating mothers.
Following selection of subjects based on inclusion criteria, patients were given an explanation of the study and an informed consent was obtained. The study was analyzed and approved by the Institutional Review Board and the Ethical Clearance Committee of the institution. All patients underwent initial therapy consisting of oral hygiene instructions and scaling and root planing. Following phase-1 periodontal therapy, patients were re-evaluated after a period of 6 weeks. Only those patients who were not responding to phase-1 therapy such as persistence of PPD, bleeding on probing were considered for the study. Baseline parameters were measured after initial phase-1 periodontal therapy, prior to periodontal flap surgery. All the surgeries were performed by the same operator and the measurements were recorded by a blinded examiner.
Clinical parameters assessment
Plaque Index[9] (Silness and Loe, 1964), Gingival Bleeding Index[10] (Ainamo and Bay, 1975), RAL, PPD, and R were recorded from baseline, 6 months, and at 9 months using University of North Carolina-15 probe. A customized acrylic occlusal stent with a vertical groove was made for proper guidance and orientation of periodontal probe in the same plane. The stents were preserved on the study casts to minimize distortion for follow-up measurements.[11] RAL was measured from the reference point (RP) on the stent to the base of the pocket (BOP). R was measured from RP to the free gingival margin (FGM). PPD was recorded by noting the difference between measurements from RP to FGM and RP to the BOP [Figure 1].
Figure 1.
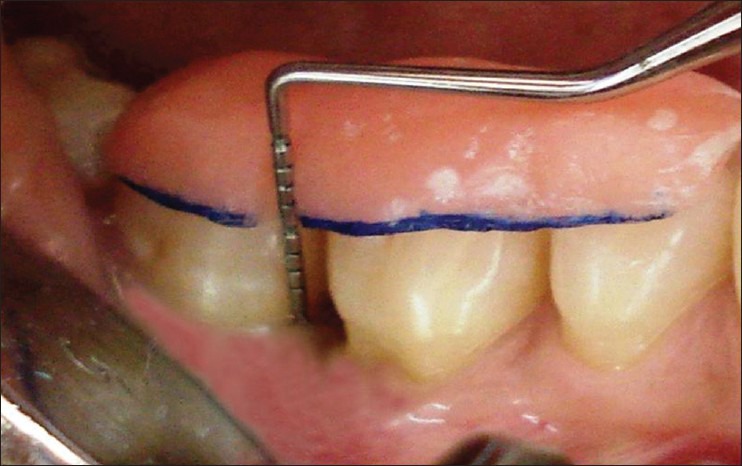
Baseline clinical parameters were assessed using University of North Carolina-15 probe on a custom made acrylic stent in relation to 46
Radiographic parameters assessment
Radiographs were taken at baseline, 6 and 9 months postoperatively. Intra-oral periapical radiographs were taken using long cone/extension cone paralleling technique with the positioning device (Rinn® XCP, Dentsply, IL, USA.) and a size 2E speed intra-oral periapical radiographic film (Kodak X-ray film, USA.) was used in a roentgen machine operating at 70 Kilo Voltage Power (KVP), 0.6 milli amperes (mA’s). Bite registration was done using rubber base impression material and stored for follow-up assessment. Radiographs were scanned using a digital scanner at an input of 300 dpi and 100% scale. The scanned images were then analyzed with the help of Auto-computer aided design (ACAD) 2007 software. Radiographic parameters were recorded as follows: (1) Distance from cemento-enamel junction to base of the defect (CEJ to BD) = A, (2) distance from CEJ to alveolar crest (CEJ to AC) = B, (3) Defect depth (DD) = A-B, (4). Defect fill (DF) was measured as difference in DD from baseline to 6 and 9 months.[12]
Surgical procedure
Patients were anesthized with 2% lignocaine solution at the surgical site. Intra-crevicular incisions were given both buccally and lingually and a full thickness mucoperiosteal flaps were elevated. After the reflection of the flap and exposure of osseous defect, a thorough surgical debridement was carried out to remove sub-gingival plaque, calculus, diseased granulation tissue, and pocket epithelium [Figure 2]. The surgical sites were irrigated with sterile saline and care was taken to keep the area free of saliva. The GTR membrane was removed from the sterile package and hydrated in normal saline for few seconds before placement on to the defect to improve adhesion properties and malleability. The membrane design was custom prepared chair side to receive for an intra-bony defect and then carefully tweezed through the interproximal contact area [Figure 3] and extended 2-3 mm beyond the bony margin, so as to provide a broad base during placement. Prior to closure of mucoperiosteal flaps, decortication of the osseous defect was done to induce bleeding. Surgical flaps were repositioned to the pre-surgical level and sutured with 3-0 silk suture (Mersilk-Ethicon, Division of Johnson and Johnson Ltd., Aurangabad, India.) utilizing an interdental direct loop suturing technique thereby achieving primary closure [Figure 4]. Care was taken not to displace the GTR membrane during suturing. A non-eugenol periodontal dressing (Coe-pack-GC America INC. ALSIP, IL, USA) was placed over the surgical area following which the post-operative instructions were given.
Figure 2.
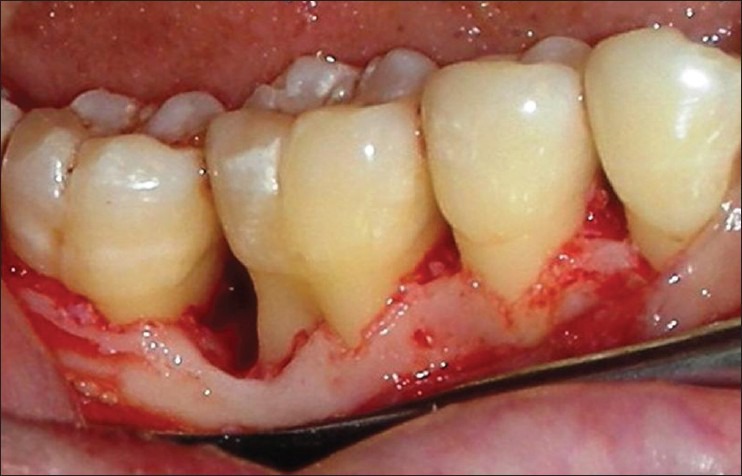
Following incision, a full thickness mucoperiosteal flap was reflected and the defect debridement was done
Figure 3.
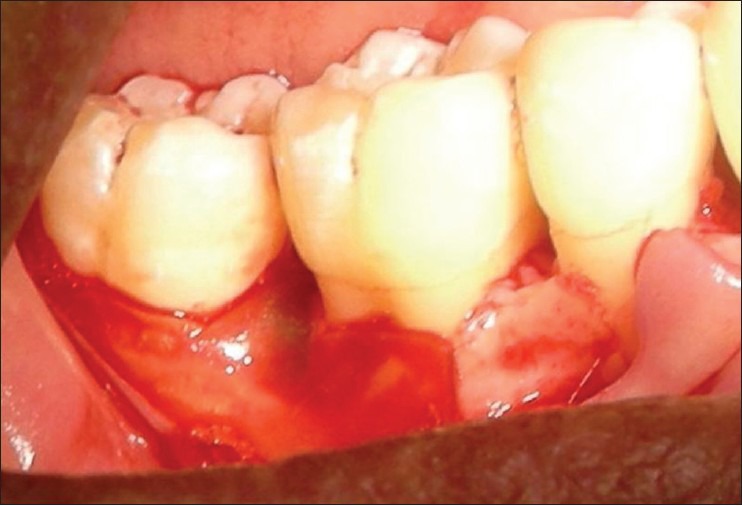
The collagen barrier membrane was trimmed to the required size and shape and placed over the interproximal vertical defect
Figure 4.
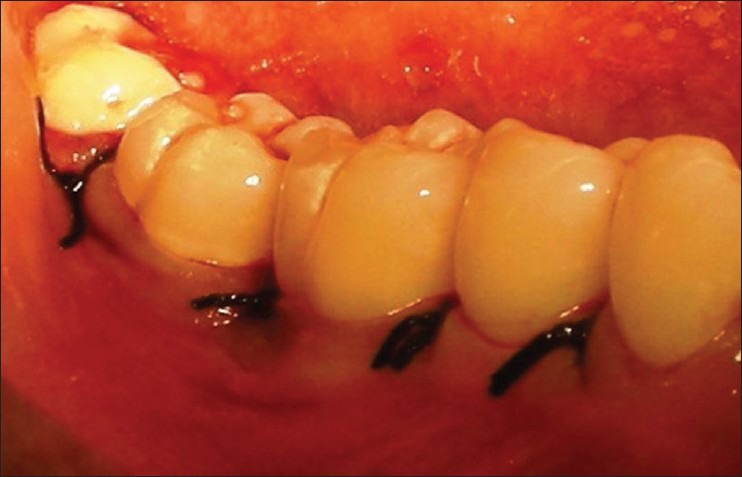
Suturing was done using 3-0 silk with simple interrupted technique
Post-operative medication included amoxicillin (Novamox® Cipla Pharmaceuticals Limited, India) 500 mg thrice daily for 5 days, a non-steroidal anti-inflammatory agent thrice daily for 5 days and 0.2% chlorhexidine gluconate (Hexidine®-ICPA Health Products Ltd, Mumbai, India.) mouth rinse for 3-4 weeks. Periodontal dressing and sutures were removed 1 week post-surgery. All patients were recalled at 1-month interval initially to monitor wound healing and to reinforce oral hygiene instructions. Later, they were followed up at 6 months and at 9 months to assess clinical and radiographic parameters postoperatively [Figures 5–8].
Figure 5.
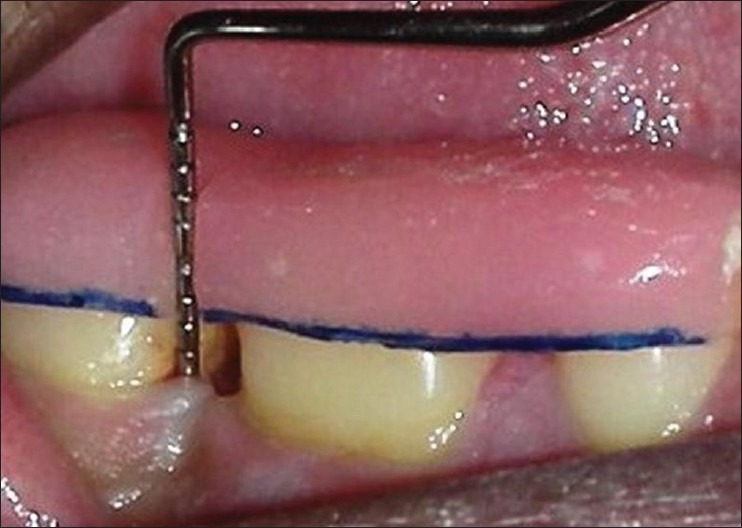
Clinical post-operative follow-up at 9 months
Figure 8.
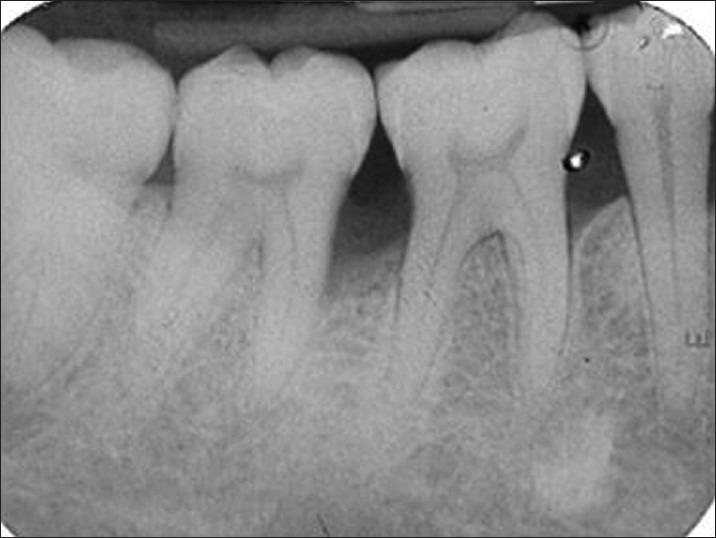
Radiograph showing defect depth fill at 9 months
Figure 7.
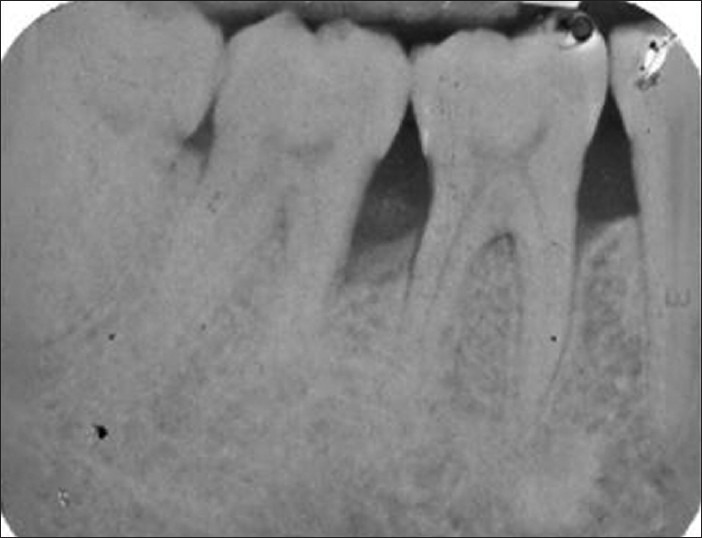
Radiograph showing defect depth fill at 6 months
Statistical analysis
The statistical software SPSS (Statistical Package for the Social Sciences) 15.0, Stata 8.0, MedCalc 9.0.1 and Systat 11.0 were used for the analysis of the data. Student's t-test was used to find the significance of study parameters on continuous scale. Significance was set at 5% level of significance. Wilcoxon signed rank test was used to find the significance in defect fill (DF).
RESULTS
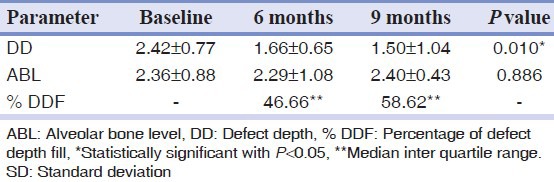
Of the 10 patients, one patient failed to return for the 9 months recall and nine patients completed the follow-up in the study. There was no membrane exposure or dehiscence or infectious episodes, or any other adverse complications in treated sites. There were statistically significant differences with respect to periodontal clinical parameters such as Plaque Index, Gingival Bleeding Index, PPD, RAL, and R assessed at baseline, 6 and 9 months respectively [Table 1]. Radiographic evaluation showed a defect fill of 46.66 median % at 6 months and 58.62 median % at 9 months respectively [Table 2].
Table 1.
Clinical parameters assessed (mean±SD) at baseline, 6 months and at 9 months
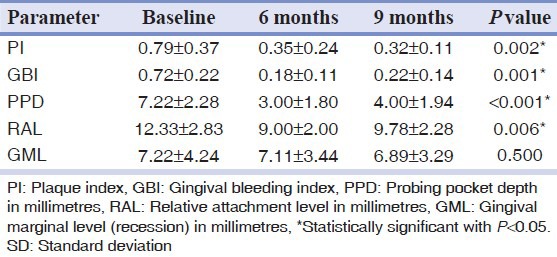
Table 2.
Radiographic parameters assessed (mean±SD) at baseline, 6 months and at 9 months
DISCUSSION
The regeneration of the periodontium is the result of elective cellular events that are facilitated by tissue exclusion using bio-absorbable or non-resorbable barriers.[1] The results of our study demonstrated that there were significant differences both clinically and radiographically. The clinical methods used to evaluate therapeutic end points include various assessments of gingival inflammation, periodontal probing, radiographs, and re-entry procedures. The true endpoint is determined by histology. However, due to ethical reasons and patient concerns, re-entry procedures and histologic analysis are not possible in routine clinical trials. Hence, clinical criteria that provide surrogate evidence of periodontal regeneration were considered.[13]
The plaque and gingival bleeding indices were assessed to monitor patient's oral hygiene and its effect on soft tissues. There was a reduction in Mean Plaque Index and Gingival Bleeding Index in the treated sites, indicating that there was a good maintenance of oral hygiene throughout the study. Cortellini and Pini-Prato et al. (1994) have reported the clinical effect of plaque control and the influence of increased bacterial contamination on the outcomes to GTR.[14]
The clinical attachment level or RAL has become widely accepted as the primary clinical endpoint of regenerative attempts around natural teeth. Significant loss in clinical attachment levels is reflected in histologic loss of the tooth's attachment apparatus.[15] The mean RAL reduced from 12.33 ± 2.83 mm to 9.78 ± 2.28 mm at 9 months (P < 0.05) [Table 1]. Cortellini, et al.,[16] and Hanne Falk, et al.,[17] have reported greater gain in the clinical attachment level after GTR; however, these authors were dealing with deeper initial intra-bony defects. The defects in this study were smaller when compared with other studies. This may suggest that clinical attachment level after GTR is dependent on the initial DD.
During the surgical procedures, efforts were made to preserve the interproximal soft tissues. There was decrease of 0.3 mm of gingival recession from baseline to 9 months in GTR-treated sites. The possible explanation could be due to non-exposure of the barrier membrane following placement in any of the test sites. This would have prevented undue exposure of the barrier to oral environment, thereby preventing infection or soft-tissue inflammation which would lead to faster resorption of membrane and Cause recession. In contrast, studies by Cortellini et al.,[16] and Becker, et al.,[18] have shown that sites treated with GTR have increased amount of recession between 1.8 and 2 mm. Gottlow, et al.,[19] has postulated that greater the degree of gingival recession, shorter the root surface area that is provided for the repopulation of the periodontal ligament cells thereby negatively influencing new attachment formation.
New bone formation is frequently used as a primary outcome variable in regenerative therapy. Radiographic monitoring of alveolar bone changes following regenerative procedures is a non-invasive painless alternate to direct bone measurement. The radiographic variables assessed were the DD, ABL, and extent of DF. Crestal bone resorption is a characteristic feature after the flap procedures. Most of the alveolar bone changes following regenerative therapy of intra-bony defects occur in the intra-bony component whereas crestal resorption may be minimal or may not occur at all.[13] In our study, no significant changes in the level of the AC were seen. This was in accordance with a similar study done by Becker and Burton[20] where 0.33 mm of crestal resorption was noted in GTR-treated sites. There was a significant difference in reduction of DD from baseline to 9 months with 58.62% of defect fill at 9 months [Table 2, Figures 6–8]. This observation is in accordance to the findings by Gottlow, et al.,[19] Becker and Burton,[20] and Micheal, et al.[21] In our study, conventional radiography with image analysis software (Auto CAD 2007) was used to assess radiographic parameters as applicability and reliability of image analysis system in alveolar bone measurement has been studied previously by Verdonschot, et al.,[22] Micheal,[23] and Hausmann, et al.[24] However, advanced radiographic techniques like subtraction digital radiography remains the method of choice if subtle changes in mineralization of alveolar bone need to be detected.[22]
Figure 6.
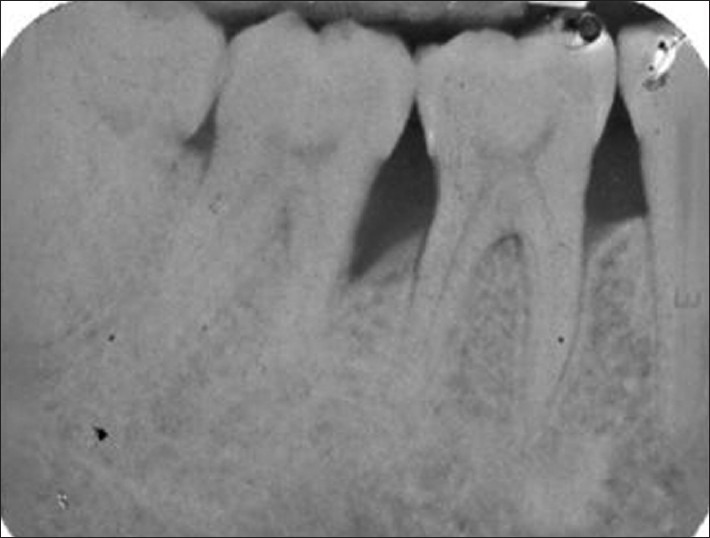
Radiograph at baseline
The GTR material utilized in this study is an orange brown color type-I fish collagen. It is available in dimensions of 1 × 1 cm and 1 × 2 cm and can be easily manipulated and adapted to the root surface. It has a resorption time of 6 weeks. In fish, the largest concentration of collagen is found in the skeleton, fins, skin, and air bladder. Use of this barrier in our study did not show any case reporting with any forms of allergy or hypersensitivity reaction.
The results of the study is difficult to compare due to a number of possible differences like small sample size, uncontrolled study design, patient/defect selection, percentage of 1/2/3 wall defect components, baseline depth of defects, probing forces, and evaluation method. Hence, the regenerative potential and beneficial effects of this GTR membrane should be further evaluated with larger sample size, longer follow ups, use of advanced radiographic aids (like cone beam computed tomography etc.), and with combination therapy involving bone grafts.
CONCLUSION
Within the limits of the study, it can be inferred that the use of collagen barrier membrane of fish origin has clinically and radiographically shown predictable results in the treatment of periodontal intra-bony defects. Further long-term clinical trials are needed to validate the effectiveness of this membrane.
ACKNOWLEDGMENT
The authors thank Dr. Suresh K. P., PhD, Scientist (Biostatistian, National Institute of Animal Nutrition and Physiology, Bangalore, Karnataka, India), for carrying statistical work of our study. The study was self-funded and the authors report no conflicts of interest related to the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Joly JC, Palioto DB, de Lima AF, Mota LF, Caffesse R. Clinical and radiographic evaluation of periodontal intrabony defects treated with guided tissue regeneration. A pilot study. J Periodontol. 2002;73:353–9. doi: 10.1902/jop.2002.73.4.353. [DOI] [PubMed] [Google Scholar]
- 2.Nyman S, Gottlow J, Karring T, Lindhe J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol. 1982;9:257–65. doi: 10.1111/j.1600-051x.1982.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang HL, Cooke J. Periodontal regeneration techniques for treatment of periodontal diseases. Dent Clin North Am. 2005;49:637–59. doi: 10.1016/j.cden.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9:290–6. doi: 10.1111/j.1600-051x.1982.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 5.Gottlow J. Guided tissue regeneration using bioresorbable and non-resorbable devices: Initial healing and long-term results. J Periodontol. 1993;64:1157–65. doi: 10.1902/jop.1993.64.11s.1157. [DOI] [PubMed] [Google Scholar]
- 6.Laurell L, Falk H, Fornell J, Johard G, Gottlow J. Clinical use of a bioresorbable matrix barrier in guided tissue regeneration therapy. Case series. J Periodontol. 1994;65:967–75. doi: 10.1902/jop.1994.65.10.967. [DOI] [PubMed] [Google Scholar]
- 7.Wang HL, MacNeil RL. Guided tissue regeneration. Absorbable barriers. Dent Clin North Am. 1998;42:505–22. [PubMed] [Google Scholar]
- 8.Nagai N, Yunoki S, Suzuki T, Sakata M, Tajima K, Munekata M. Application of cross-linked salmon atelocollagen to the scaffold of human periodontal ligament cells. J Biosci Bioeng. 2004;97:389–94. doi: 10.1016/S1389-1723(04)70224-8. [DOI] [PubMed] [Google Scholar]
- 9.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 10.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 11.Isidor F, Karring T, Attström R. Reproducibility of pocket depth and attachment level measurements when using a flexible splint. J Clin Periodontol. 1984;11:662–8. doi: 10.1111/j.1600-051x.1984.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 12.Zybutz M, Rapoport D, Laurell L, Persson GR. Comparisons of clinical and radiographic measurements of inter-proximal vertical defects before and 1 year after surgical treatments. J Clin Periodontol. 2000;27:179–86. doi: 10.1034/j.1600-051x.2000.027003179.x. [DOI] [PubMed] [Google Scholar]
- 13.Machtei EE. Outcome variable for the study of periodontal regeneration. Ann Periodontol. 1997;2:229–39. doi: 10.1902/annals.1997.2.1.229. [DOI] [PubMed] [Google Scholar]
- 14.Cortellini P, Pini-Prato G, Tonetti M. Periodontal regeneration of human infrabony defects (V). Effect of oral hygiene on long-term stability. J Clin Periodontol. 1994;21:606–10. doi: 10.1111/j.1600-051x.1994.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 15.Garrett S. Specific issues in clinical trials on the use of barrier membranes in periodontal regeneration. Ann Periodontol. 1997;2:240–58. doi: 10.1902/annals.1997.2.1.240. [DOI] [PubMed] [Google Scholar]
- 16.Cortellini P, Pini Prato G, Tonetti MS. Periodontal regeneration of human intrabony defects with bioresorbable membranes. A controlled clinical trial. J Periodontol. 1996;67:217–23. doi: 10.1902/jop.1996.67.3.217. [DOI] [PubMed] [Google Scholar]
- 17.Falk H, Laurell L, Ravald N, Teiwik A, Persson R. Guided tissue regeneration therapy of 203 consecutively treated intrabony defects using a bioabsorbable matrix barrier. Clinical and radiographic findings. J Periodontol. 1997;68:571–81. doi: 10.1902/jop.1997.68.6.571. [DOI] [PubMed] [Google Scholar]
- 18.Becker W, Becker BE, Mellonig J, Caffesse RG, Warrer K, Caton JG, et al. A prospective multi-center study evaluating periodontal regeneration for Class II furcation invasions and intrabony defects after treatment with a bioabsorbable barrier membrane: 1-year results. J Periodontol. 1996;67:641–9. doi: 10.1902/jop.1996.67.7.641. [DOI] [PubMed] [Google Scholar]
- 19.Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J Clin Periodontol. 1986;13:604–16. doi: 10.1111/j.1600-051x.1986.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 20.Becker W, Becker BE. Treatment of mandibular 3-wall intrabony defects by flap debridement and expanded polytetrafluoroethylene barrier membranes. Long-term evaluation of 32 treated patients. J Periodontol. 1993;64:1138–44. doi: 10.1902/jop.1993.64.11s.1138. [DOI] [PubMed] [Google Scholar]
- 21.Zybutz MD, Laurell L, Rapoport DA, Persson GR. Treatment of intrabony defects with resorbable materials, non-resorbable materials and flap debridement. J Clin Periodontol. 2000;27:169–78. doi: 10.1034/j.1600-051x.2000.027003169.x. [DOI] [PubMed] [Google Scholar]
- 22.Verdonschot EH, Sanders AJ, Plasschaert AJ. Applicability of an image analysis system in alveolar bone loss measurement. J Clin Periodontol. 1991;18:30–6. doi: 10.1111/j.1600-051x.1991.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 23.Reddy MS. Radiographic methods in the evaluation of periodontal therapy. J Periodontol. 1992;63:1078–84. doi: 10.1902/jop.1992.63.12s.1078. [DOI] [PubMed] [Google Scholar]
- 24.Hausmann E, Allen K, Carpio L, Christersson LA, Clerehugh V. Computerized methodology for detection of alveolar crestal bone loss from serial intraoral radiographs. J Periodontol. 1992;63:657–62. doi: 10.1902/jop.1992.63.8.657. [DOI] [PubMed] [Google Scholar]


