Abstract
Purpose
Survivors of childhood acute lymphoblastic leukemia (ALL) are at increased risk for neurocognitive problems, with significant interindividual variability in outcome. This study examined genetic polymorphisms associated with variability in neurocognitive outcome.
Patients and Methods
Neurocognitive outcomes were evaluated at the end of therapy in 243 survivors treated on an institutional protocol featuring risk-adapted chemotherapy without prophylactic cranial irradiation. Polymorphisms in genes related to pharmacokinetics or pharmacodynamics of antileukemic agents, drug metabolism, oxidative stress, and attention problems in noncancer populations were examined as predictors of outcome, using multiple general linear models and controlling for age at diagnosis, sex, race, and treatment intensity.
Results
Compared with national norms, the cohort demonstrated significantly higher rates of problems on direct assessment of sustained attention (P = .01) and on parent ratings of attention problems (P = .02). Children with the A2756G polymorphism in methionine synthase (MS) were more likely to demonstrate deficits in attentiveness (P = .03) and response speed (P = .02), whereas those with various polymorphisms in glutathione S-transferase demonstrated increased performance variability (P = .01) and reduced attentiveness (P = .003). Polymorphisms in monoamine oxidase (T1460CA) were associated with increased attention variability (P = .03). Parent-reported attention problems were more common in children with the Cys112Arg polymorphism in apoliopoprotein E4 (P = .01).
Conclusion
These results are consistent with our previous report of association between attention problems and MS in an independent cohort of long-term survivors of childhood ALL treated with chemotherapy only. The results also raise the possibility of an impact from genetic predispositions related to oxidative stress and CNS integrity.
INTRODUCTION
Survivors of childhood acute lymphoblastic leukemia (ALL) are at risk for neurocognitive problems, generally characterized by reduced attention and processing speed.1 Although neurocognitive problems are clearly linked to cranial radiation therapy, they can also be associated with chemotherapy.2 We recently demonstrated increased frequency of attention problems in survivors of childhood ALL who were treated with chemotherapy only.3 Neurocognitive problems in survivors of childhood ALL have been associated with increased treatment intensity, younger age at treatment exposure, and female sex,4,5 factors which account for only a proportion of the variability in outcome, suggesting additional risk factors.
One potential source of outcome variability is genetic polymorphisms that affect key enzyme pathways associated with pharmacokinetics or pharmacodynamics of antileukemic agents, particularly antifolates (eg, lower folate availability, higher homocysteine). We recently reported a link between polymorphisms associated with the folate pathway and parent-reported attention problems and neurocognitive measures in long-term survivors of childhood ALL.6,7 Among these survivors treated with chemotherapy only, those who had germline polymorphisms of 10-methylenetetrahydrofolate reductase (MTHFR) demonstrated elevated ratings on parent-reported attention problems, whereas children with polymorphisms in either MTHFR or the methionine synthase (MS) gene demonstrated reduced performance on direct measures of attention and processing speed.
Other potential genetic polymorphisms affecting neurocognitive outcome in ALL survivors include those associated with glucocorticoids (eg, glucocorticoid receptor gene, nuclear receptor subfamily 3 [NR3C1]),8 metabolism of additional chemotherapeutic agents (eg, cytochrome P450 family 3 [CYP3A]),9 and/or regulators of oxidative stress generated by chemotherapy (eg, glutathione S-transferases [GSTs]).10 Although polymorphisms in many of these genes have been examined for their contribution to genetic risk for leukemia and survival,11,12 their association with neurocognitive functional outcomes has not been reported.
Specific germline polymorphisms have been linked to problems with attention in noncancer populations. Polymorphisms in dopamine receptor and monoamine oxidase A (MAOA) genes have been associated with developmental attention deficits,13 whereas polymorphisms in the catechol-O-methyltransferase gene (COMT) have been linked to the development of impaired attention regulation.14,15 Variants in apoliopoprotein E (APOE) have been implicated in mediating early-onset dementia,16 neurocognitive outcome after traumatic brain injury17 (presumably through oxidative stress and CNS response to injury18), and neurocognitive outcome in survivors of breast cancer and adult-onset lymphoma.19 The impact of these polymorphisms in mediating neurocognitive impairment in survivors of childhood cancer exposed to potentially neurotoxic chemotherapy has not been well explored.
The aim of our study was to examine, in a large cohort of leukemia survivors, the association between neurocognitive outcome and genetic polymorphisms related to antifolate and glucocorticoid chemotherapy and oxidative stress, as well as those commonly associated with attention problems in noncancer populations. Consistent with our previous reports,6,7 we hypothesized that polymorphisms in the folate pathway would be associated with attention problems.
PATIENTS AND METHODS
Participants
All participants were treated on the Total XV therapeutic protocol for childhood ALL, which has been previously reported.20 Briefly, on the basis of presenting features and response to initial remission induction therapy, patients were assigned to either low-risk or standard-/high-risk treatment arms.20 Children in the low-risk arm received 13 to 18 intrathecal (IT) treatments with methotrexate (MTX), hydrocortisone, and cytarabine; high-dose (HD) intravenous (IV) MTX at 2.5 gm/m2 per dose for four doses; and dexamethasone pulses at 8 mg/m2 per day for 5 days, in addition to other chemotherapeutic agents. Children in the standard-/high-risk arm received 16 to 25 IT injections, HD IV MTX at 5.0 gm/m2 per dose for four doses, and dexamethasone pulses at 12 mg/m2 per day for 5 days. Prophylactic cranial irradiation was not administered to any patient, regardless of presenting features, including the presence of CNS leukemia at diagnosis. None of the patients had received therapeutic cranial radiation therapy for CNS relapse before the collection of outcome measures. Patients were excluded from analyses if they had been diagnosed with a neurodevelopmental disorder (eg, Down syndrome), if their primary language was not English, or if they had developed CNS relapse before the neurocognitive testing. This study was approved by the institutional review board at St Jude Children's Research Hospital. Informed consent was obtained from the parent or guardian, and assent was obtained from the patient when appropriate.
The Total XV protocol enrolled 408 patients at St Jude Children's Research Hospital. Of these, 345 (84.6%) participated in a neurocognitive assessment at least once during the course of therapy, and 243 of these had a single assessment (70.4%) 2 years after completion of consolidation therapy (ie, approximately 2.3 years from diagnosis). No differences were apparent in demographic, disease, or treatment characteristics among survivors who completed the assessments versus those who did not (Appendix Table A1, online only). This time point was used in the current analyses because it represented the latest point of assessment for the majority of the group and thus the point most likely to be associated with chronic effects of treatment. DNA was collected from peripheral blood cells during remission and was available for 346 of the patients who completed the therapeutic protocol (84.8%), and 210 of the patients who were evaluated at the end of therapy (86.4%).
Genotyping
DNA was provided by the laboratory of Mary Relling, PharmD. Forty-two single nucleotide polymorphisms (SNPs) were a priori selected based on their know contribution to: one, the folate pathway; two, steroid receptors, general drug metabolism, or oxidative stress; or three, attention deficits in the general population. Whole-genome amplification was conducted with GenomePlex WGA kits (Sigma-Aldrich, St Louis, MO). Purified genomic DNA was plated into 96-well polymerase chain reaction (PCR) plates. Silicon-based multiplexing was performed to divide SNPs into groups. PCR was carried out in multiplex on an MJ Research DNA engine (St Bruno, Quebec, Canada). Reactions were carried out in multiplex using the SNaPshot Multiplex kit (Applied Biosystems, Foster City, CA). The reaction included the extension of an oligonucleotide probe designed to lie adjacent to the SNP of interest by one of four fluorescently labeled dideoxynucleotides complementary to the base found at the SNP site. Oligonucleotide probes were designed to be of different lengths by the addition of neutral sequence. After the SNaPshot reaction, the mixture was treated with 1.0 unit of shrimp alkaline phosphatase to remove the 5′-phosphoryl groups. After digestion, 1.0 μL of PCR product was added to a mixture of 8.5 μL of formamide (Invitrogen, Carlsbad, CA) and 0.5 μL of fluorescently labeled standard-size LIZ120 (Applied Biosystems). The fluorescently extended oligonucleotides were separated on an ABI 3730xl capillary electrophoresis system (Applied Biosystems). PCR fragment-size analysis using flurorescently labeled primer pairs was conducted for variations in DHFR and TYMS (RS70991108 and RS34489327, respectively). PCR was performed with 50 ng of genomic DNA and the Takara Bio Ex Taq Hot Start PCR system (Otsu, Japan) in a 25-μL reaction on an MJ Research DNA engine. Thermocycling conditions were adjusted specifically for the gene to be amplified. After amplification, 1.0 μL of PCR product was added to a mixture of 8.5 μL of formamide (Invitrogen) and 0.5 μL of fluorescently labeled standard-size ROX 400HD (Applied Biosystems). The samples were denatured for 5 minutes at 95°C and then loaded onto an ABI 3730xl DNA analyzer (Applied Biosystems). Allele determination and fragment-size analysis were performed using GeneMapper 4.0 software (Applied Biosystems). SNP genotypes with questionable call rates (< 90%) were repeated using additional DNA. Forward and reserve primers for all genes are listed in Appendix Table A2 (online only).
Neurocognitive Evaluations
All participants completed a standard neurocognitive battery 2 years after completion of consolidation therapy. Specific neurocognitive domains evaluated (and corresponding age-appropriate tests) included general intelligence (Wechsler Preschool and Primary Scale of Intelligence–Revised,21 Wechsler Intelligence Scale for Children–Third Edition [WISC-III],22 or Wechsler Adult Intelligence Scale–Third Edition [WAIS-III]23); processing speed (processing speed index from WISC-III22 or WAIS-III23); working memory (freedom from distractibility index from WISC-III22 or WAIS-III23); sustained attention (beta [response speed], D-prime [attentiveness], and SE of reaction time [variability] indices from Conners' Continuous Performance Test [CPT]24); and parent-reported attention problems (Conners' Parent Rating Scale25). These indices of attention represent related although separate constructs26 and are associated with a vast network of neural activation.27 Age-adjusted standard scores were calculated for each measure according to standard procedures outlined in the test manuals.
Covariates
Patient demographic and clinical treatment variables were considered as covariates. Patient variables included age at diagnosis (dichotomized into < 5 v ≥ 5 years, based on previous research demonstrating increased sensitivity at < 5 years3) and sex (male v female). The impact of race and ethnicity was considered, given their potential association to differences in allele frequency. Hispanic origin was identified in only nine patients, an insufficient number for analysis of this ethnicity. Race was included as a covariate, with 39 black patients identified. IT and HD IV MTX have been previously identified as potential treatment factors associated with neurocognitive problems. However, the intensity and frequency of these treatments are determined by risk stratum. Furthermore, risk stratum was identified as a more consistent predictor of neurocognitive outcome in our previous report.28 For these reasons, we used risk stratum in lieu of specific treatment doses to capture the combined effect of treatment intensity.
Statistical Analyses
Descriptive statistics were generated for patient and treatment characteristics as well as for neurocognitive outcome measures. Impairment on neurocognitive outcome was defined as attaining a standard score falling in the lowest 15% of the normal distribution. Rates of impairment within the sample were compared with the expected 15% in the general population. The Hochberg and Benjamini29 adaptive step-down Bonferroni method was used to adjust for multiple comparisons. Only those neurocognitive measures with impairment rates significantly higher than expected were included in linkage analyses. Common and rare allele frequencies were determined for each of the SNPs of interest. SNPs with limited frequency of rare alleles were dropped from additional analyses. Univariate associations were examined among each of the remaining SNPs and relevant neurocognitive outcomes. Unless otherwise specified, SNPs significantly associated with a neurocognitive outcome at the P < .10 level in the univariate analyses were included in a multiple general linear model, along with risk, sex, age at diagnosis, and race. Separate multivariate models were generated for each neurocognitive outcome with an elevated impairment rate. All analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC).
RESULTS
Demographic and treatment characteristics of participants are listed in Table 1. Performance levels and rates of impairment on neurocognitive outcome measures are summarized in Table 2. Survivors demonstrated significantly elevated rates of impairment on measures of sustained attention, including inattentiveness, slow response speed, and high variability (all P < .01) on the Conners' CPT. Parents reported elevated rates of attention problems (P = .02). On the basis of these results, a phenotype of impaired attention, defined by CPT performance and parent-reported attention problems, was used to explore associations with genetic polymorphisms.
Table 1.
Survivor Demographic and Clinical Characteristics
| Characteristic | Survivors |
|
|---|---|---|
| No. | % | |
| Sex | ||
| Male | 131 | 53.9 |
| Female | 112 | 46.1 |
| Race | ||
| White | 194 | 79.8 |
| Black | 39 | 16.1 |
| Other | 10 | 4.1 |
| Risk arm | ||
| Low | 126 | 51.9 |
| Standard/high* | 117 | 48.1 |
| Characteristic | Survivors |
|||
|---|---|---|---|---|
| No. | Mean | SD | Range | |
| Age at diagnosis, years | 243 | 6.6 | 4.39 | 1.0-18.7 |
| HD IV MTX dose, gm/m2† | ||||
| Low risk | 126 | 11.7 | 2.14 | 3.6-18.0 |
| Standard/high risk | 117 | 18.6 | 3.64 | 7.4-29.3 |
| IT MHA dose, ml‡ | ||||
| Low risk | 126 | 150.1 | 69.75 | 94.0-856.0 |
| Standard/high risk | 117 | 204.6 | 50.12 | 80.0-375.0 |
| Dexamethasone, mg/m2† | ||||
| Low risk | 126 | 1,008.2 | 177.96 | 175.5-1,302.6 |
| Standard/high risk | 117 | 1,212.6 | 374.11 | 60.3-1,690.1 |
Abbreviations: HD IV MTX, high-dose intravenous methotrexate; IT MHA, intrathecal injection of MTX plus hydrocortisone plus cytarabine; SD, standard deviation.
Children identified as standard and high risk were treated in same therapeutic arm.
Cumulative doses listed.
Cumulative doses listed; 1 mL contains MTX 1 mg, hydrocortisone 2 mg, and cytarabine 3 mg.
Table 2.
Performance and Rates of Impairment on Outcome Measures
| Neurocognitive Outcome | Population |
Subset |
95% CI | Impaired (%)* | P | Adjusted P† | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Wechsler scales‡ | ||||||||
| Intelligence | 100 | 15 | 96.0 | 15.78 | 93.9 to 98.0 | 22.9 | .058687696 | .176063 |
| Working memory | 100 | 15 | 96.0 | 14.46 | 93.5 to 98.4 | 24.3 | .093919976 | .18784 |
| Processing speed | 100 | 15 | 99.8 | 17.00 | 96.9 to 102.7 | 17.2 | 1.00 | 1.00 |
| Conners' CPT§ | ||||||||
| Attentiveness | 50 | 10 | 60.0 | 10.43 | 58.3 to 61.6 | 44.9 | < .001 | < .001 |
| Response speed | 50 | 10 | 71.7 | 19.17 | 68.7 to 74.8 | 63.5 | < .001 | < .001 |
| Variability | 50 | 10 | 58.7 | 13.61 | 56.6 to 60.9 | 46.2 | < .001 | < .001 |
| Conners' parent report∥ | ||||||||
| Attention problems | 50 | 10 | 53.2 | 14.19 | 51.4 to 55.1 | 24.8 | .024548342 | .073645 |
| Impulsivity/hyperactivity | 50 | 10 | 50.0 | 10.79 | 48.6 to 51.5 | 16.2 | 1.00 | 1.00 |
| Hyperactivity index | 50 | 10 | 50.2 | 11.65 | 48.7 to 51.8 | 15.8 | 1.00 | 1.00 |
Abbreviations: CPT, Continuous Performance Test; SD, standard deviation.
Age-appropriate version of Wechsler intelligence scales (ie, Wechsler Preschool and Primary Scale of Intelligence–Revised,21 Wechsler Intelligence Scale for Children–Third Edition,22 or Wechsler Adult Intelligence Scale–Third Edition23); lower scores reflect worse performance.
Conners' CPT24: variability, SE of reaction time; attentiveness, D-prime; slow response speed, beta. Higher scores reflect worse performance. Low scores on beta can also reflect problem behavior, specifically impulsive response style; however, such abnormally fast responses were infrequent and not considered in impairment classification.
Conners' Parent Rating Scale24; higher scores reflect more problems.
Impairment defined as score falling > one SD below population mean.
P values adjusted using Holm-Bonferroni step-down method to account for multiple comparisons and reduce risk of type I error.29
Frequency results for the 42 polymorphisms identified as potential mediators of neurocognitive outcome are listed in Table 3. Limited or no variation was observed in dihydrofolate reductase, CYP3A, and MAOA. The remaining 39 genomic variations were used in univariate linkage analysis with the four attention outcomes. Allele frequency differed by race for the following genes: CYP3A5, DBH, DRD2, FPGS, GSTP1, GSTT1, MDR1, MTHFD1, and TYMS. Given these multiple differences by race and the relatively small sample of nonwhite participants (ie, 20.2% of 243 patients), race was used as an independent variable in the multiple regression analyses. Because three of the attention outcomes (ie, attentiveness, response speed, and variability) were derived from a common test (ie, Conners' CPT) and are correlated with one another, 500 multiple permutations were conducted to examine the reliability of associations with these three measures. Polymorphisms associated with an average P < .05 across the 500 permutations were included in multivariate analyses. These polymorphisms included MS, MAOA, and GST. Polymorphisms associated with parent-reported attention problems in univariate analyses included GST, APOE, CYP3A, and immunophilin protein.
Table 3.
Frequency of Targeted Pathway Polymorphisms Examined As Mediators of Neurocognitive Outcomes
| Gene Description | Gene Symbol | Genomic Variation | RSID | Detection Method | Wild Type* |
Heterozygous* |
Homozygous* |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||||
| Folate pathway polymorphisms | ||||||||||
| Dihydrofolate reductase | DHFR | Intron-1 19bp del | 70991108 | PCR fragment-size analysis | 334 | 100 | 1 | < 1 | ||
| Folylpolyglutamate synthase | FPGS | G144A | 10760502 | SNaPshot | 187 | 55 | 117 | 35 | 33 | 10 |
| A1994G | 10106 | SNaPshot | 87 | 30 | 153 | 52 | 54 | 18 | ||
| Gamma-glutamyl hydrolase | GGH | C452T | 11545078 | SNaPshot | 281 | 84 | 47 | 14 | 5 | 2 |
| Methionine synthase | MS | A2756G | 1805087 | SNaPshot | 202 | 63 | 105 | 33 | 12 | 4 |
| Methionine synthase reductase | MTRR | A66G | 1801394 | SNaPshot | 136 | 44 | 86 | 28 | 85 | 28 |
| Methylenetetrahydrofolate dehydrogenase | MTHFD1 | G1958A | 2236225 | SNaPshot | 209 | 67 | 88 | 28 | 15 | 5 |
| T401C | 1950902 | SNaPshot | 116 | 38 | 142 | 46 | 50 | 16 | ||
| Methylenetetrahydrofolate reductase | MTHFR | C677T | 1801133 | SNaPshot | 137 | 43 | 140 | 44 | 39 | 12 |
| A1298C | 1801131 | SNaPshot | 135 | 47 | 122 | 42 | 31 | 11 | ||
| Reduced folate carrier | SLC19A1 | G80A | 1051266 | SNaPshot | 72 | 24 | 184 | 62 | 41 | 14 |
| Serine hydroxymethyltransferase | SHMT | C1420T | 1979277 | SNaPshot | 149 | 45 | 149 | 45 | 30 | 9 |
| Thymidylate synthase | TYMS | 1494del6 | 34489327 | PCR fragment-size analysis | 258 | 91 | 25 | 9 | 1 | < 1 |
| Polymorphisms related to steroid receptors, drug metabolism, or oxidative stress | ||||||||||
| Cytochrome P450 family 3 | CYP3A4 | CYP3A4*1B | 3091339 | SNaPshot | 325 | 100 | ||||
| CYP3A5 | CYP3A5*3 | 776746 | SNaPshot | 133 | 42 | 136 | 43 | 47 | 15 | |
| Glucocorticoid receptor | NR3C1 | G1088A | 56149945 | SNaPshot | 294 | 95 | 14 | 5 | ||
| NR3C1 | Asn363Ser | 6195 | SNaPshot | 320 | 95 | 14 | 4 | 3 | 1 | |
| Glutathione S-transferase | GSTP1 | G313A | 1695 | SNaPshot | 109 | 39 | 125 | 45 | 45 | 16 |
| C341T | 1138272 | SNaPshot | 239 | 81 | 45 | 15 | 10 | 3 | ||
| GSTM1 | GSTM1*0 | 2071487 | SNaPshot | 185 | 56 | 142 | 43 | |||
| GSTT1 | GSTT1*0 | 2266637 | SNaPshot | 312 | 97 | 6 | 2 | 3 | 1 | |
| Multidrug resistance protein 1 | MDR1 | T3435C | 1045642 | SNaPshot | 88 | 29 | 151 | 50 | 62 | 21 |
| A2677G | 2032582 | SNaPshot | 128 | 40 | 135 | 43 | 54 | 17 | ||
| Immunophilin protein | FKBP5 | AC | 3800373 | SNaPshot | 156 | 48 | 136 | 41 | 35 | 11 |
| GA | 9296158 | SNaPshot | 263 | 86 | 33 | 11 | 9 | 3 | ||
| CT | 1360780 | SNaPshot | 148 | 45 | 147 | 45 | 31 | 10 | ||
| CT | 9470080 | SNaPshot | 122 | 39 | 151 | 48 | 41 | 13 | ||
| Vitamin D receptor | VDR | Intron 8 G→A | 1544410 | SNaPshot | 114 | 37 | 152 | 49 | 44 | 14 |
| VDR foklstartsite T→C | 2228570 | SNaPshot | 115 | 36 | 181 | 57 | 23 | 7 | ||
| Polymorphisms related to attention deficit phenotype | ||||||||||
| Apoliopoprotein E | APOE4 | Cys112Arg | 429358 | SNaPshot | 235 | 73 | 80 | 25 | 7 | 2 |
| Brain-derived neurotrophic factor | BDNF | Val66Met | 6265 | SNaPshot | 215 | 68 | 87 | 28 | 12 | 4 |
| Catechol-O-methyltransferase | COMT | Val158Met | 4680 | SNaPshot | 99 | 32 | 168 | 53 | 47 | 15 |
| Dopamine beta hydroxylase | DBH | C-1021T | 1611115 | SNaPshot | 197 | 74 | 66 | 25 | 2 | 1 |
| Taq1 (intron 5) | 2519152 | SNaPshot | 152 | 45 | 134 | 40 | 50 | 15 | ||
| Dopamine receptor | DRD2 | A241G | 6277 | SNaPshot | 107 | 35 | 147 | 48 | 50 | 17 |
| Taq1 A | 1800496 | SNaPshot | 313 | 94 | 19 | 6 | 1 | < 1 | ||
| DRD4 | Val194Gly | 1800443 | SNaPshot | 189 | 97 | 5 | 3 | |||
| Monoamine oxidase A | MAOA | G941T | 1799835 | SNaPshot | 281 | 100 | ||||
| T1460C | 1137070 | SNaPshot | 156 | 57 | 72 | 26 | 45 | 17 | ||
| Serotonin receptor | HTR1B | G861C | 6296 | SNaPshot | 224 | 73 | 71 | 23 | 12 | 4 |
| Synaptosomal-associated protein 25 | SNAP25 | T1065G | 3746544 | SNaPshot | 129 | 39 | 107 | 32 | 94 | 29 |
| T1069C | 10513112 | SNaPshot | 199 | 65 | 75 | 24 | 33 | 11 | ||
Abbreviations: PCR, polymerase chain reaction; RSID, rapid stain identification series for Homo sapiens.
Allele frequency differed by race for following genes: CYP3A5, DBH, DRD2, FPGS, GSTP1, GSTT1, MDR1, MTHFD1, and TYMS. Given these multiple differences by race and relatively small sample of nonwhite participants (20.2% of 243 patients), race was used as independent variable in multiple regression analyses.
Table 4 summarizes the results of multivariate general linear models for each of the four attention outcomes. Controlling for risk, sex, age at diagnosis, and race, problems with attentiveness were demonstrated in children with polymorphisms in MS (P = .03) and GST (P = .003 and P = .002 for GSTT1*0 and GSTP1, respectively). Slower response speed was demonstrated in children with polymorphism in MS (P = .02), whereas increased variability was demonstrated in children with polymorphisms in GST (P = .01) and MAOA (P = .03). With regard to parent-reported symptoms, increased attention problems were identified in children with polymorphism in APOE (P = .01). Table 4 also lists estimates of the effect size associated with each polymorphism, expressed in standard score units on an age-adjusted scale (mean, 50; standard deviation, 10). Positive values ≥ 10 points (ie, one standard deviation) would generally be considered clinically significant.
Table 4.
Multiple Regression Models* Predicting Attention Deficits in Survivors of Childhood ALL
| Parameter | Attentiveness† |
Response Speed† |
Variability† |
Attention Problems‡ |
||||
|---|---|---|---|---|---|---|---|---|
| Estimate§ | P | Estimate§ | P | Estimate§ | P | Estimate§ | P | |
| Risk (low v standard/high) | −0.28 | .90 | −3.30 | .42 | −0.69 | .82 | 6.34 | .005 |
| Sex (female v male) | −0.34 | .88 | −0.09 | .98 | 0.01 | .99 | −0.59 | .78 |
| Age at diagnosis (< 5 v ≥ 5 years) | 0.38 | .88 | −5.94 | .21 | 2.43 | .49 | 3.25 | .14 |
| Race (black v white) | −1.75 | .60 | 13.31 | .02 | 0.19 | .96 | −3.25 | .29 |
| Methionine synthase (A2756G) | 3.93 | .03 | 7.00 | .02 | 1.42 | .54 | ||
| Monoamine oxidase A (T1460C) | 1.31 | .35 | 1.54 | .53 | 3.95 | .03 | ||
| Glutathione S-transferase (GSTT1*0) | 21.75 | .003 | 23.10 | .07 | 24.44 | .01 | ||
| Glutathione S-transferase (GSTP1) | 8.09 | .002 | 8.73 | .06 | 1.22 | .72 | ||
| Glutathione S-transferase (GSTM1*0) | 2.99 | .16 | ||||||
| Apoliopoprotein E (Cys112Arg) | 4.92 | .01 | ||||||
| Cytochrome P450 family 3 (CYP3A5*3) | 1.45 | .36 | ||||||
| Immunophilin protein (FKBP5 GA) | 1.68 | .50 | ||||||
NOTE. Bold font indicates significance.
Abbreviation: ALL, acute lymphoblastic leukemia.
Separate multiple regression models were conducted on each outcome (ie, attentiveness, response speed, variability, attention problems) using multiple independent variables identified under each column.
Conners' Continuous Performance Test24: variability, SE of reaction time; attentiveness, D-prime; response speed, beta.
Conners' Parent Rating Scale.25
Estimate represents unit change in age-adjusted standard score (mean, 50; standard deviation, 10) associated with each parameter.
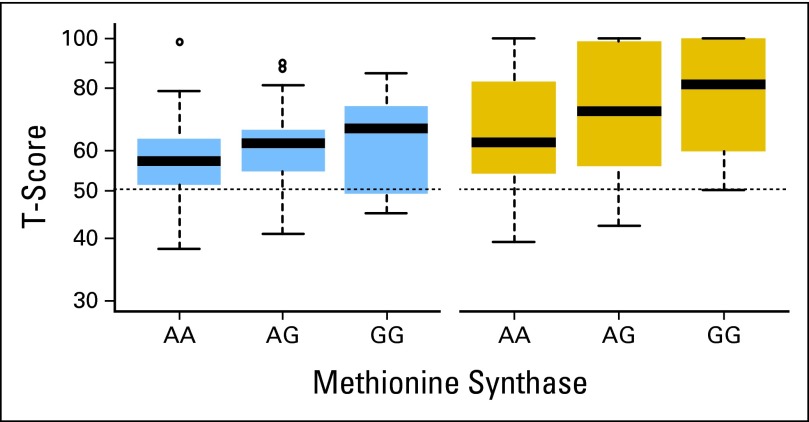
Children with the variant G allele in MS demonstrated a progressive increase in problems with attentiveness and response speed (Fig 1). Mean performance on the attention measures and parent reports are listed in Appendix Table A3 (online only) according to allele pattern.
Fig 1.
Box plots of age-adjusted standard scores on measures of attentiveness (left) and response speed (right) by genomic variation in methionine synthase. Dashed line represents mean T score on Conners' Continuous Performance Test24 for normative sample.
DISCUSSION
This study demonstrates a common phenotype of attention problems in survivors of childhood ALL, manifested through direct assessment of sustained attention (ie, inattentiveness, slow response speed, and increased variability) and parent-reported behavior. Although the onset of these problems may occur earlier in cancer therapy, they are present in a significant percentage of survivors 2 years after completion of consolidation therapy. The attention problems seem to be mediated by multiple factors, including treatment intensity as well as polymorphisms in genes related to antifolate chemotherapy, oxidative stress, and CNS integrity. Treatment intensity was related to parent-reported attention problems only and not to direct assessment, likely because the latter is more sensitive and negatively affected, even at lower-intensity therapies.
Polymorphisms in MS were associated with decreased attentiveness and slowed response speed. We recently demonstrated an association between attention and processing-speed problems in long-term survivors of childhood ALL and polymorphisms in the MS gene.7 Although the current attention test differed from that used in the previous study, and the current cohort was tested earlier in the survival process, the identified association between MS and attention outcome is consistent with the initial discovery in the previous report. MS is involved in the conversion of homocysteine to methionine, and polymorphisms in MS are associated with hyperhomocysteinemia.30 Excess homocysteine increases risk for vascular abnormalities, including CNS stroke.31,32 Survivors with MS polymorphisms who were treated with MTX, which could increase homocysteine levels, may be at increased risk for these abnormalities.
Specific attention problems were also associated with polymorphisms in additional genes. GSTs are enzymes involved in sequestering reactive oxygen species,33 among other things, and polymorphisms in the GST gene may interfere with the ability to respond to oxidative stress. MAOA is involved in serotonin and norepinephrine catabolism,34,35 and low activity of this gene has been associated with increased norepinephrine and overactivation of the sympathetic nervous system.36 This process may result in increased anxiety and/or physiologic stress, which have also been associated with attention problems.37,38 The association with polymorphisms in MAOA may suggest a predisposition for attention problems in a subset of survivors, one that may not be related to specific chemotherapy agents. However, given the discovery nature of these associations, validation is required.
APOE-4 was associated with parent report of attention problems. APOE is involved in lipoprotein metabolism,39,40 and the E4 polymorphism is a risk factor for dementia.41 APOE-4 has been associated with age-related myelin breakdown42 and risk of neurocognitive impairment after traumatic brain injury,17 HIV infection,43 and obstructive sleep apnea in children.44 The association between APOE-4 and attention problems in survivors of childhood ALL would also suggest a predisposition that may be independent of specific chemotherapy agents, although this too would require validation.
The finding that direct assessment of attention and parent report of attention problems were related to different polymorphisms suggests that these measures assess different aspects of a complex phenotype. This phenomenon is not unexpected; previous studies have reported limited correspondence between direct assessment and behavioral ratings.45 However, both sources of assessment are relevant, as evidenced by their independent correspondence to magnetic resonance imaging of brain integrity.46 This specificity reinforces the need for comprehensive assessment, including direct measures and patient-reported outcomes, to fully capture the complex phenotype of attention problems.
This study is not without limitation. Although the neurocognitive assessment occurred at the same point in treatment for all survivors, an advantage over most other reports in the literature, the survivorship phase was slightly earlier than those of the other reports and may not reflect patterns seen in long-term survivors (ie, > 5 years after diagnosis). Still, attention problems are a common long-term outcome, and these deficits at the end of therapy are likely to continue. A second limitation is the inability to confirm associations for all of the significant polymorphisms. Our previous study focused only on polymorphisms in the folate pathway, and our current study confirmed the association between MS and attention problems. Recruitment of an independent cohort will be necessary to validate associations with GST, MAOA, and APOE-4.
Despite these limitations, the current results support the conclusion that a phenotype of attention problems is a common outcome in survivors of childhood ALL and that the etiology of this phenotype is multifactorial. Risk for this outcome is associated with treatment intensity and polymorphisms in the folate pathway and may also be increased by predispositions that influence response to physiologic stress and CNS integrity. Given the potential influence of pre-existing factors, early cognitive intervention focused on enhancing attention networks to prevent post-therapy decline seems warranted. We recently described a pilot study of such a preventative approach47 and recommend further research along these lines. Knowledge of specific polymorphisms contributing to neurocognitive outcome may also assist in development of personalized interventions. For example, contributions from polymorphisms in GST may warrant preventive antioxidant trials targeting those affected.
Appendix
Table A1.
Clinical Features Between Tested Versus Nontested Patient Cases
| Characteristic | Tested (%) | Nontested (%) | P* |
|---|---|---|---|
| Sex | 1.00 | ||
| Male | 55.36 | 55.56 | |
| Female | 44.64 | 44.44 | |
| Race | .32 | ||
| White | 77.68 | 84.13 | |
| Black | 18.55 | 11.11 | |
| Other | 3.77 | 4.76 | |
| Risk | .27 | ||
| Low | 49.28 | 41.27 | |
| Standard/high | 50.72 | 58.73 | |
| Age group, years | .28 | ||
| < 5 | 47.25 | 39.68 | |
| ≥ 5 | 52.75 | 60.32 |
Exact χ2 test with two-sided comparison.
Table A2.
Forward and Reverse Primers Used for Sequencing Genomic Variation
| Gene Description | Gene Symbol | Genomic Variation | RSID | Forward Primer | Reverse Primer |
|---|---|---|---|---|---|
| Apoliopoprotein E | APOE4 | Cys112Arg | 429358 | TGTCCAAGGAGCTGCAGGCG | TCATCGGCATCGCGGAGGAG |
| Brain-derived neurotrophic factor | BDNF | Val66Met | 6265 | AGGCTTGACATCATTGGCTGACAC | AGGCTCCAAAGGCACTTGACTACT |
| Catechol-O-methyltransferase | COMT | Val158Met | 4680 | ATCACCCAGCGGATGGTGGATTT | GGGCCTGGTGATAGTGGGTTT |
| Cytochrome P450 family 3 | CYP3A4 | CYP3A4*1B | 3091339 | TGAAGACTTGAGTGGCTCCTGTGT | TGCTTACCCTCCGGTTTGTGAAGA |
| CYP3A5 | CYP3A5*3 | 776746 | CCAACTGCCCTTGCAGCATTTAGT | AGGGTAATGTGGTCCAAACAGGGA | |
| Dopamine beta hydroxylase | DBH | C-1021T | 1611115 | AGCTGGAGGGATCAAGCAGAATGT | TGAATTTGAAGCCTCTCAGGGCAG |
| Taq1 (intron 5) | 2519152 | GCATCTGGCAGCTTCCCTTATGAA | GCTGTCCATCTTCCATGGCTGT | ||
| Dihydrofolate reductase | DHFR | Intron-1 19bp del | 70991108 | AATCCGGGCAGAAATCAGCAACTG | AGAACATGGGCATCGGCAAGAA |
| Dopamine receptor | DRD2 | Taq1 A | 1800496 | TGTGGTGTTTGCAGGAGTCTTCAG | GTGGTCTTTGGCATGCCCATTCTT |
| A241G | 6277 | AGGAGCTGGAGATGGAGATGCT | ATGCCCATTCTTCTCTGGTTTGGC | ||
| DRD4 | Val194Gly | 1800443 | TACTGTGCGGCCTCAACGA | TAGGAAGAAGGAGCACACGGACGA | |
| Immunophilin protein | FKBP5 | CT | 1360780 | GCCCTTATTCTATAGCTGCAAGTCCC | TCTCTTGTGCCAGCAGTAGCAAGT |
| GA | 9296158 | CGTTCTGTTATACTCATTCCATGCCC | CCCTAGTGTCTACACCATTTCTGT | ||
| AC | 3800373 | ACACAGTACTTCCTCCCAGCATTG | AGAACAGAGAAGCTTGACAGGGCA | ||
| CT | 9470080 | GAACAGTACCTTATTCTACAGATACGGAG | TTGGCCTCCCAGAGTGTTAGGATT | ||
| Folylpolyglutamate synthase | FPGS | A1994G | 10,106 | AATCTACCACCCAGCACATGGCAA | CCCATGAACTTACATACTAGGTGCC |
| G144A | 10760502 | ACGCTGCGCTGATTGGCT | GCCTGATACCTGGTACTCCATGCT | ||
| Gamma-glutamyl hydrolase | GGH | C452T | 11545078 | GGGCACATGCCTTGGATTTGAAGA | TGCTACTTACTAATCCTGCCCAGC |
| Glutathione S-transferase | GSTM1 | GSTM1*0 | 2071487 | TGGAGGTTCCAGCCCACATATTCT | TGGGCTCAAATATACGGTGGAGGT |
| GSTP1 | G313A | 1695 | AGTGACTGTGTGTTGATCAGGCG | AACCCTGGTGCAGATGCTCACATA | |
| C341T | 1138272 | GATGATACATGGTGGTGTCTGGCA | TCTCCCACAATGAAGGTCTTGCCT | ||
| GSTT1 | GSTT1*0 | 2266637 | AGAGTTGGATGTGACCCTGCAGTT | AAGCAGGACTTCAGCAACTAGCCA | |
| Serotonin receptor | HTR1B | G861C | 6296 | AGCGAATCCGGATCTCCTGTGTAT | AGCCAACACACAATAAAGGCTCCC |
| Monoamine oxidase A | MAOA | T1460C | 1137070 | GCTAGCAGGGCCTTGAATCTGTAGAA | TGCCCAGAGTCACCAAACTTACCT |
| G941T | 1799835 | TCCGACCTTGACTGCCAAGATTCA | TAGCAGCCTACCCTTCTTCTTCCA | ||
| Multidrug resistance | MDR1 | T3435C | 1045642 | ACATTGCCTATGGAGACAACAGCC | CGATGAAGGCATGTATGTTGGCCT |
| A2677G | 2032582 | CCCATCATTGCAATAGCAGGAGTTG | AGAGCATAGTAAGCAGTAGGGAGT | ||
| Methionine synthase | MS | A2756G | 1805087 | GGGAGAAGAAATGAAGTTAAGGAAGCC | CTACCACTTACCTTGAGAGACTCAT |
| Methylenetetrahydrofolate dehydrogenase | MTHFD1 | T401C | 1950902 | TCCAAGCATCCCTTAGGCGTACAA | AGTGTGGCTTGGCTTCCTATGTCT |
| G1958A | 2236225 | TCCAAATCCTGCTTCCGTCACTGT | AACATCGCACATGGCAATTCCTCC | ||
| Methylenetetrahydrofolate reductase | MTHFR | A1298C | 1801131 | GGGCAGAATTTACAGGAATGGCCT | CCAGCATCACTCACTTTGTGACCA |
| C677T | 1801133 | TCTCTTCATCCCTCGCCTTGAACA | ATGTCGGTGCATGCCTTCACAAAG | ||
| Methionine synthase reductase | MTRR | A66G | 1801394 | ACATGCCTTGAAGTGATGAGGAGG | CGGCTCTAACCTTATCGGATTCAC |
| Glucocorticoid receptor | NR3C1 | G1088A | 56149945 | AGCATCCCTTTCTCAACAGCAGGA | TGTTCGACCAGGGAAGTTCAGAGT |
| Asn363Ser | 6195 | AGCATCCCTTTCTCAACAGCAGGA | TGTTCGACCAGGGAAGTTCAGAGT | ||
| Serine hydroxymethyltransferase | SHMT | C1420T | 1979277 | TTGGAGCAGCTCATCCATCTCTCA | TGTCAGAGCCACCCTGAAAGAGTT |
| Reduced folate carrier | SLC19A1 | G80A | 1051266 | AGACCATCTTCCAAGGTGCCCTGA | AGCCGTAGAAGCAAAGGTAGCACA |
| Synaptosomal-associated protein 25 | SNAP25 | T1065G | 3746544 | AAAGGACCGTGGCAGTAACTCTGT | TGGTCATTTGGTGGCTCTAACTCC |
| T1069C | 10513112 | TGGTCATTTGGTGGCTCTAACTCC | AAAGGACCGTGGCAGTAACTCTGT | ||
| Thymidylate synthase | TYMS | 1494del6 | 34489327 | GGAGCTGAGTAACACCATCGATCA | AGGAACTGAGCAGATAAGTGGCAG |
| Vitamin D receptor | VDR | VDR foklstartsite T→C | 2228570 | AGCCAGCTATGTAGGGCGAATCAT | TGAAGAAGCCTTTGCAGCCTTCAC |
| Intron 8 G→A | 1544410 | AGAGGTCAAGGGTCACTGCACATT | AACTAGATAAGCAGGGTTCCTGGG |
Abbreviation: RSID, rapid stain identification series for Homo sapiens.
Table A3.
Age-Adjusted Standard Scores* on Measures of Attention by Genotype
| SNP | Type | No. | Attentiveness† |
Response Speed† |
Variability† |
Attention Problems‡ |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| Apoliopoprotein E (Cys112Arg) | CC | 97 | 60 | 58 to 62 | 71 | 67 to 75 | 59 | 56 to 62 | 51 | 49 to 53 |
| CT | 30 | 58 | 55 to 62 | 69 | 63 to 75 | 56 | 51 to 61 | 58 | 53 to 62 | |
| TT | 5 | 58 | 53 to 63 | 69 | 52 to 85 | 61 | 45 to 76 | 61 | 43 to 78 | |
| Cytochrome P450 family 3 (CYP3A5*3) | GG | 50 | 59 | 56 to 62 | 70 | 64 to 75 | 56 | 52 to 59 | 54 | 51 to 57 |
| AG | 53 | 60 | 57 to 63 | 71 | 66 to 76 | 59 | 55 to 63 | 53 | 49 to 56 | |
| AA | 25 | 59 | 56 to 63 | 74 | 66 to 82 | 62 | 57 to 67 | 48 | 44 to 51 | |
| Immunophilin protein (FKBP5 GA) | GG | 102 | 59 | 57 to 61 | 70 | 66 to 74 | 58 | 55 to 61 | 52 | 50 to 55 |
| AG | 18 | 61 | 54 to 67 | 71 | 63 to 79 | 61 | 56 to 67 | 53 | 49 to 58 | |
| AA | 2 | 55 | −89 to 199 | 74 | −50 to 197 | 58 | −116 to 233 | 68 | 46 to 90 | |
| Glutathione S-transferase (GSTM1*0) | CC | 72 | 60 | 57 to 63 | 71 | 66 to 75 | 59 | 56 to 63 | 54 | 51 to 57 |
| CT | 55 | 58 | 56 to 61 | 69 | 64 to 75 | 57 | 54 to 61 | 50 | 48 to 53 | |
| Glutathione S-transferase (GSTP1) | CC | 94 | 58 | 56 to 60 | 69 | 65 to 72 | 58 | 55 to 61 | 52 | 49 to 54 |
| CT | 19 | 65 | 58 to 71 | 78 | 68 to 87 | 60 | 54 to 65 | 55 | 48 to 61 | |
| TT | 5 | 61 | 55 to 67 | 85 | 59 to 111 | 62 | 47 to 78 | 58 | 46 to 70 | |
| Glutathione S-transferase (GSTT1*0) | CC | 121 | 59 | 58 to 61 | 71 | 67 to 74 | 58 | 56 to 60 | 53 | 51 to 55 |
| CT | 3 | 70 | 29 to 110 | 84 | 13 to 154 | 77 | 52 to 103 | 52 | 31 to 73 | |
| Monoamine oxidase A (T1460C) | CC | 56 | 58 | 56 to 60 | 67 | 62 to 71 | 55 | 52 to 58 | 54 | 50 to 58 |
| CT | 29 | 58 | 53 to 63 | 71 | 63 to 78 | 62 | 56 to 68 | 52 | 48 to 55 | |
| TT | 20 | 64 | 59 to 69 | 78 | 69 to 87 | 62 | 56 to 68 | 50 | 45 to 55 | |
| Methionine synthase (A2756G) | AA | 69 | 58 | 55 to 60 | 68 | 63 to 72 | 57 | 54 to 60 | 51 | 49 to 54 |
| AG | 53 | 62 | 59 to 64 | 74 | 69 to 80 | 61 | 57 to 65 | 54 | 50 to 58 | |
| GG | 5 | 64 | 43 to 85 | 78 | 50 to 107 | 55 | 34 to 76 | 57 | 29 to 85 | |
NOTE. Bold font identifies scores in survivors with minor alleles that fall outside 95% CI for survivors with wild-type allele.
Standard score scale results in expected mean of 50 and standard deviation of 10 in general population.
Conners' Continuous Performance Test24: variability, SE of reaction time; attentiveness, D-prime; slow response speed, beta; lower scores reflect better performance.
Conners' Parent Rating Scale25; lower scores reflect fewer problems.
Footnotes
Supported by Cancer Center Support (CORE) Grant No. CA21765 and Grant No. CA90246 (W.E.R.) from the National Cancer Institute; by Grant No. MH085849 (K.R.K.) from the National Institute of Mental Health; and by the American Lebanese Syrian Associated Charities (ALSAC).
Presented in part at the 6th Biennial Cancer Survivorship Research Conference of the National Cancer Institute, Arlington, VA, June 14-16, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kevin R. Krull, Deepa Bhojwani, Ching-Hon Pui
Provision of study materials or patients: Ching-Hon Pui
Collection and assembly of data: Kevin R. Krull, Heather M. Conklin, Deqing Pei, John T. Sandlund, Ching-Hon Pui
Data analysis and interpretation: Kevin R. Krull, Deepa Bhojwani, Heather M. Conklin, Deqing Pei, Cheng Cheng, Wilburn E. Reddick, Ching-Hon Pui
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Krull KR, Okcu MF, Potter B, et al. Screening for neurocognitive impairment in pediatric cancer long-term survivors. J Clin Oncol. 2008;26:4138–4143. doi: 10.1200/JCO.2008.16.8864. [DOI] [PubMed] [Google Scholar]
- 2.Kadan-Lottick NS, Brouwers P, Breiger D, et al. Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:5986–5992. doi: 10.1200/JCO.2009.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conklin HM, Krull KR, Reddick WE, et al. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104:1386–1395. doi: 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain N, Brouwers P, Okcu MF, et al. Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer. 2009;115:4238–4245. doi: 10.1002/cncr.24464. [DOI] [PubMed] [Google Scholar]
- 5.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102:881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krull KR, Brouwers P, Jain N, et al. Folate pathway genetic polymorphisms are related to attention disorders in childhood leukemia survivors. J Pediatr. 2008;152:101–105. doi: 10.1016/j.jpeds.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Kamdar KY, Krull KR, El-Zein RA, et al. Folate pathway polymorphisms predict deficits in attention and processing speed after childhood leukemia therapy. Pediatr Blood Cancer. 2011;57:454–460. doi: 10.1002/pbc.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rossum EF, Roks PH, de Jong FH, et al. Characterization of a promoter polymorphism in the glucocorticoid receptor gene and its relationship to three other polymorphisms. Clin Endocrinol (Oxf) 2004;61:573–581. doi: 10.1111/j.1365-2265.2004.02132.x. [DOI] [PubMed] [Google Scholar]
- 9.Eichelbaum M, Burk O. CYP3A genetics in drug metabolism. Nat Med. 2001;7:285–287. doi: 10.1038/85417. [DOI] [PubMed] [Google Scholar]
- 10.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 11.Borst L, Wallerek S, Dalhoff K, et al. The impact of CYP3A5*3 on risk and prognosis in childhood acute lymphoblastic leukemia. Eur J Haematol. 2011;86:477–483. doi: 10.1111/j.1600-0609.2011.01608.x. [DOI] [PubMed] [Google Scholar]
- 12.Davies SM, Bhatia S, Ross JA, et al. Glutathione S-transferase genotypes, genetic susceptibility, and outcome of therapy in childhood acute lymphoblastic leukemia. Blood. 2002;100:67–71. doi: 10.1182/blood.v100.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Thapar A, O'Donovan M, Owen MJ. The genetics of attention deficit hyperactivity disorder. Hum Mol Genet. 2005;14:R275–R282. doi: 10.1093/hmg/ddi263. [DOI] [PubMed] [Google Scholar]
- 14.Krämer UM, Cunillera T, Càmara E, et al. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. J Neurosci. 2007;27:14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsky RH, Sparling MB, Ryan LM, et al. Association of COMT Val158Met genotype with executive functioning following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17:465–471. doi: 10.1176/jnp.17.4.465. [DOI] [PubMed] [Google Scholar]
- 16.Balasa M, Gelpi E, Antonell A, et al. Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology. 2011;76:1720–1725. doi: 10.1212/WNL.0b013e31821a44dd. [DOI] [PubMed] [Google Scholar]
- 17.Shadli RM, Pieter MS, Yaacob MJ, et al. APOE genotype and neuropsychological outcome in mild-to-moderate traumatic brain injury: A pilot study. Brain Inj. 2011;25:596–603. doi: 10.3109/02699052.2011.572947. [DOI] [PubMed] [Google Scholar]
- 18.Jordan BD. Genetic influences on outcome following traumatic brain injury. Neurochem Res. 2007;32:905–915. doi: 10.1007/s11064-006-9251-3. [DOI] [PubMed] [Google Scholar]
- 19.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 20.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wechsler D. New York, NY: Harcourt Brace Jovanovich; 1989. Wechsler Preschool and Primary Scales of Intelligence: Revised. [Google Scholar]
- 22.Wechsler D. ed 3. San Antonio, TX: Psychological Corporation; 1991. Wechsler Intelligence Scale for Children. [Google Scholar]
- 23.Wechsler D. ed 3. San Antonio, TX: Psychological Corporation; 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- 24.Conners CK. Toronto, Ontario: Canada, Multi-Health Systems; 1995. Conners' Continuous Performance Test. [Google Scholar]
- 25.Conners CK. New York, NY: Multi-Health Systems; 1997. Conners' Rating Scales: Revised User's Manual. [Google Scholar]
- 26.Strauss E, Sherman EMS, Spreen O. ed 3. Oxford, United Kingdom: Oxford University Press; 2006. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary; pp. 562–575. [Google Scholar]
- 27.Ogg RJ, Zou P, Allen DN, et al. Neural correlates of a clinical continuous performance test. Magn Reson Imaging. 2008;26:504–512. doi: 10.1016/j.mri.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Conklin HM, Krull KR, Reddick WE, et al. Cognitive outcomes following contemporary treatment without cranial irradiaiton for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104:1386–1395. doi: 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 30.Watkins D, Ru M, Hwang HY, et al. Hyperhomocysteinemia due to methionine synthase deficiency, cblG: Structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L. Am J Hum Genet. 2002;71:143–153. doi: 10.1086/341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almawi WY, Khan A, Al-Othman SS, et al. Case-control study of methylenetetrahydrofolate reductase mutations and hyperhomocysteinemia and risk of stroke. J Stroke Cerebrovasc Dis. 2009;18:407–408. doi: 10.1016/j.jstrokecerebrovasdis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Parnetti L, Caso V, Santucci A, et al. Mild hyperhomocysteinemia is a risk-factor in all etiological subtypes of stroke. Neurol Sci. 2004;25:13–17. doi: 10.1007/s10072-004-0219-5. [DOI] [PubMed] [Google Scholar]
- 33.Strange RC, Spiteri MA, Ramachandran S, et al. Glutathione-S-transferase family of enzymes. Mutat Res. 2001;482:21–26. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 34.Cases O, Lebrand C, Giros B, et al. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. J Neurosci. 1998;18:6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumagae Y, Matsui Y, Iwata N. Deamination of norepinephrine, dopamine, and serotonin by type A monoamine oxidase in discrete regions of the rat brain and inhibition by RS-8359. Jpn J Pharmacol. 1991;55:121–128. doi: 10.1254/jjp.55.121. [DOI] [PubMed] [Google Scholar]
- 36.Gershon MD, Sherman DL, Pintar JE. Type-specific localization of monoamine oxidase in the enteric nervous system: Relationship to 5-hydroxytryptamine, neuropeptides, and sympathetic nerves. J Comp Neurol. 1990;301:191–213. doi: 10.1002/cne.903010205. [DOI] [PubMed] [Google Scholar]
- 37.Ferreri F, Lapp LK, Peretti CS. Current research on cognitive aspects of anxiety disorders. Curr Opin Psychiatry. 2011;24:49–54. doi: 10.1097/YCO.0b013e32833f5585. [DOI] [PubMed] [Google Scholar]
- 38.Lane SJ, Reynolds S, Thacker L. Sensory over-responsivity and ADHD: Differentiating using electrodermal responses, cortisol, and anxiety. Front Integr Neurosci. 2010;4:8. doi: 10.3389/fnint.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvim RO, Freitas SR, Ferreira NE, et al. APOE polymorphism is associated with lipid profile, but not with arterial stiffness in the general population. Lipids Health Dis. 2010;9:128. doi: 10.1186/1476-511X-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park TS, Panek RL, Rekhter MD, et al. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis. 2006;189:264–272. doi: 10.1016/j.atherosclerosis.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 41.Jin YP, Østbye T, Feightner JW, et al. Joint effect of stroke and APOE 4 on dementia risk: The Canadian Study of Health and Aging. Neurology. 2008;70:9–16. doi: 10.1212/01.wnl.0000284609.77385.03. [DOI] [PubMed] [Google Scholar]
- 42.Ryan L, Walther K, Bendlin BB, et al. Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage. 2011;54:1565–1577. doi: 10.1016/j.neuroimage.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spector SA, Singh KK, Gupta S, et al. APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS. 2010;24:1471–1479. doi: 10.1097/QAD.0b013e328339e25c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gozal D, Capdevila OS, Kheirandish-Gozal L, et al. APOE epsilon 4 allele, cognitive dysfunction, and obstructive sleep apnea in children. Neurology. 2007;69:243–249. doi: 10.1212/01.wnl.0000265818.88703.83. [DOI] [PubMed] [Google Scholar]
- 45.Edwards MC, Gardner ES, Chelonis JJ, et al. Estimates of the validity and utility of the Conners' Continuous Performance Test in the assessment of inattentive and/or hyperactive-impulsive behaviors in children. J Abnorm Child Psychol. 2007;35:393–404. doi: 10.1007/s10802-007-9098-3. [DOI] [PubMed] [Google Scholar]
- 46.Mahone EM, Martin R, Kates WR, et al. Neuroimaging correlates of parent ratings of working memory in typically developing children. J Int Neuropsychol Soc. 2009;15:31–41. doi: 10.1017/S1355617708090164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore IM, Hockenberry MJ, Anhalt C, et al. Mathematics intervention for prevention of neurocognitive deficits in childhood leukemia. Pediatr Blood Cancer. 2011;59:278–284. doi: 10.1002/pbc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]