Abstract
Purpose
After a report from the Women's Health Initiative (WHI) in 2002, a precipitous decline in menopausal hormonal therapy (MHT) use in the United States was linked to a decline in breast cancer incidence rates. Given that MHT use is also associated with increased ovarian cancer risk, we tested whether ovarian cancer incidence rates changed after 2002.
Methods
Using the North American Association of Central Cancer Registries database (1995 to 2008; N = 171,142 incident ovarian cancers), we applied standard analytic approaches and age-period-cohort (APC) models to estimate ovarian cancer incidence rate changes before (1995 to 2002) and after (2003 to 2008) the WHI report.
Results
Among women age ≥ 50 years, age-standardized ovarian cancer incidence declined by 0.8% per year (95% CI, −1.8% to −0.5% per year) before the WHI announcement; after the WHI report, the rate declined by 2.4% per year (95% CI, −2.5% to −2.2% per year). APC models confirmed an accelerated decline in ovarian cancer incidence after the WHI report, adjusted for age and birth cohort effects. This sudden change was notable among women most likely to have used MHT (ie, women age 50 to 69 years, white women, and residents of regions with highest MHT prescription frequency). The largest changes were found for the endometrioid histologic subtype.
Conclusion
After a marked reduction in MHT use around 2002, ovarian cancer incidence rates demonstrated an accelerated decline, with the largest changes for endometrioid carcinomas. This strong temporal association, although not proving a causal role of hormones in ovarian carcinogenesis, suggests that future analytic research supporting cancer control efforts should clarify the role of hormonal exposures on the development and behavior of subtypes of ovarian cancer.
INTRODUCTION
Ovarian cancer is the most lethal gynecologic malignancy in the United States, accounting for a projected 22,280 cases and 15,500 related deaths in 2012.1 Most women with ovarian cancer present with advanced-stage disease, which is often fatal despite aggressive treatment. Accordingly, developing improved methods of early detection or prevention of these tumors based on a deeper understanding of their pathogenesis is a research priority.
Historical examples have shown that links between sudden temporal changes in the prevalence of specific hormonal exposures and rapid changes in cancer incidence may provide insights into carcinogenesis. In the 1970s, the correlation between increased use of exogenous unopposed estrogens and increasing endometrial cancer rates provided evidence for an etiologic relationship.2 In 2002, results from the Women's Health Initiative (WHI) linked combined estrogen plus progestin menopausal hormone therapy (MHT) to adverse health effects,3 which was followed by a sharp decrease in MHT use, irrespective of formulation or geographic region in the United States,4–6 along with a decrease in breast cancer rates,7 supporting a link between these agents and breast cancer. However, a comparable analysis of ovarian cancer incidence rates has not been performed.
A meta-analysis of population-based case-control and prospective studies found that use of estrogen-only MHT increased ovarian cancer risk by 22% (risk ratio, 1.22; 95% CI, 1.18 to 1.27) and that combined estrogen plus progestin therapy increased risk by 10% (risk ratio, 1.10; 95% CI, 1.04 to 1.16).8 Three additional cohort studies have been published since the meta-analysis9–11; the largest demonstrated a statistically significant link between combined MHT use and ovarian cancer risk, whereas the smaller studies reported increased but nonsignificant risks. Before 2002, ovarian cancer incidence rates were declining slowly, but it is unknown whether this trend changed after the WHI announcement. Given the epidemiologic evidence that unopposed estrogens increase ovarian cancer risk and more recent evidence suggesting a similar, albeit weaker, association for combined MHT, we hypothesized that the WHI announcement in 2002 would be followed by a subsequent accelerated decline in ovarian cancer incidence rates.
To test our hypothesis, we analyzed temporal trends in ovarian cancer incidence before and after the WHI announcement in mid-2002. Given that rates of ovarian cancer incidence have been declining for decades,12 we applied joinpoint regression models13 and also used age-period-cohort (APC) models to analyze temporal changes, adjusted for the interrelated effects of age and birth cohort.14,15 Specifically, we tested whether rates of decline suddenly accelerated after 2002. In addition, we performed stratified analyses by age, race, histologic subtype, and US regions categorized by the frequency of MHT prescriptions. To achieve the statistical power required for this analysis, we used data from the large-scale North American Association of Central Cancer Registries (NAACCR), which covers greater than 80% of the US population.16
METHODS
Data Source
We used the Cancer Incidence in North America (CINA) Analytic File for Expanded Races provided by NAACCR (www.naaccr.org) for this analysis. Cancer incidence data that are provided by the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program or the Centers for Disease Control and Prevention's National Program of Cancer Registries and that meet high-quality standards are included in the CINA analytic data set.17 Ovarian cancer incidence data were available for 42 consenting population-based cancer registries of the 56 included in CINA (1995 to 2008; Appendix Table A1, online only).
Study Population
The analytic file included 240,912 malignant ovarian tumors, which we further restricted to 211,534 carcinomas, based on International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes, as follows: serous (ICD-O-3 codes 8441, 8442, 8460, 8461, 8462, and 9014), endometrioid (8380, 8381, 8560, and 8570), clear cell (8310 and 8313), mucinous (8470, 8471, 8472, 8480, 8481, 8482, 8490, and 9015), and other/unspecified. For analyses restricted to women age 50 years and older, a reasonable population-based surrogate for postmenopausal status18 and a relevant age range for MHT use, the analytic file included 192,075 malignant ovarian tumors, which we further restricted based on ICD-O-3 codes to 171,142 carcinomas (Appendix Table A2, online only).
Population counts used for estimation of age-standardized incidence were derived from the 2000 US Census in 1-year age intervals. The 2005 incidence data and underlying population data for Louisiana, Alabama, Mississippi, and Texas were adjusted to account for displacement related to Hurricanes Katrina and Rita.
Subgroup Variables
We computed ovarian cancer incidence by age (< v ≥ 50 years [50 to 69 v 70 to 84 years]), race (white, black, or other/unknown), and histologic subtypes (serous, mucinous, endometrioid, or clear cell; other/unspecified carcinomas were not separately analyzed). We also computed rates in regions previously identified as having comparatively low MHT use (Middle Atlantic: New Jersey, New York, and Pennsylvania) and comparatively high MHT use (West South Central: Arkansas, Louisiana, and Texas) during the study period.6
Statistical Analysis
Age-standardized ovarian carcinoma incidence rates per 100,000 women-years with 95% CIs were generated using SEER*Stat Software (version 7.0.9; http://www.seer.cancer.gov/seerstat)19 and plotted on a linear scale. Trends in cancer incidence were analyzed by fitting a linear regression to the natural log of the incidence rate, with the slope representing the annual percent change for a specified time period,13 and we compared the annual percent change between the before (1995 to 2002) and after WHI (2003 to 2008) time periods. To further test for significance of temporal trends in ovarian cancer rates and to identify the point at which the trends changed, we performed a joinpoint analysis (Joinpoint Regression Program version 3.5.1 software; http://www.srab.cancer.gov/joinpoint),13 which fits a series of joined straight lines to the log of the annual age-standardized rates.
Although SEER*Stat and joinpoint regression can identify significant temporal trends in age-standardized incidence and estimate the years of transition, they do not distinguish between influences that occurred in specific time periods for all age groups (ie, period effects) versus effects associated with year of birth (ie, generational or birth cohort effects). Accordingly, to develop more refined estimates of period changes adjusted for both age and birth cohort effects, we analyzed period effects using APC models.14,15
For the APC analysis, we used 32 2-year age groups (21 through 22, 23 through 24, and so on to 83 through 84 years) and seven 2-year time periods (1995 through 1996, 1997 through 1998, 1999 through 2000, 2001 through 2002, 2003 through 2004, 2005 through 2006, and 2007 through 2008), spanning 38 partially overlapping 4-year birth cohorts referred to by midyear of birth (1912, 1914, and so on to 1986). APC models were fitted to the entire data set and in substrata according to age (50 to 69 v 70 to 84 years old), race (white, black, or other/unknown), histologic subtypes (serous, mucinous, endometrioid, or clear cell), or low- and high-MHT region.
To explore changes in the APC period deviations circa 2002, we used the Tarone-Chu method.20,21 Specifically, we contrasted the three 2-year time periods (1995 through 1996, 1997 through 1998, and 1999 through 2000) before the 2-year interval 2001 through 2002 with the three 2-year time periods (2003 through 2004, 2005 through 2006, and 2007 through 2008) after 2001 through 2002. In addition, using a method similar to one recently developed by one of the authors (P.S.R.) for cohort relative risks,22 we estimated the corresponding period relative risks compared with the referent 2002 time period.
As sensitivity analyses, we restricted analysis to 30 registries that achieved gold/silver NAACCR certification status for 10 or more years between 1995 and 200823 and restricted analysis to 31 registries included in the NAACCR 2011 annual report on the status of cancer.16 To account for the expected reporting delays and data corrections that most frequently occur in the most recent 1 to 3 years of incidence data, we additionally examined the delay-adjusted rates in 13 SEER registries from 1992 to 2009.24
RESULTS
Trends in Ovarian Cancer Incidence
Figure 1 compares the age-standardized incidence rates between women younger than age 50 years and women age 50 years and older. Joinpoint regression models identified a single joinpoint at the year 2001 (range, 1999 to 2002) among women age 50 years and older and no joinpoint among women younger than age 50 years. That is, ovarian cancer incidence rates decreased significantly from 1995 to 2001 in the older age group by −0.8% per year, and by 1.6% per year more rapidly thereafter (ie, by −2.4% per year from 2001 to 2008). However, ovarian cancer incidence decreased steadily for the entire time period among the younger age group, (ie, −2.2% per year; 95% CI, −2.5% to −1.8% per year). Accordingly, we further analyzed data for women age 50 years and older.
Fig 1.
Age-standardized ovarian cancer incidence rates in the United States (North American Association of Central Cancer Registries Incidence, 1995 to 2008). Age standardized to the 2000 US population.
Among the older women (Table 1), SEER*Stat analyses confirmed that ovarian cancer incidence rates decreased by 21.1% from 1995 to 2008, with an annual percent change of −1.87% per year (95% CI, −2.09% to −1.64% per year). Before the WHI announcement (1995 to 2002), rates declined by 1.17%, whereas afterward (2003 to 2008), rates decreased by 2.19% per year. Sensitivity analyses restricted to various subsets of registries and accounting for reporting delays (see Methods) showed similar trends (data available on request), and therefore, we present results for the entire data set.
Table 1.
Ovarian Carcinomas Among US Women Age ≥ 50 Years (NAACCR Incidence, 1995 to 2008)
| Year | Age-Standardized Rate per 100,000* | No. of Ovarian Carcinomas† | Population (No.) | Ovarian Cancer Incidence Rates (%) |
||
|---|---|---|---|---|---|---|
| Percent Change‡ | Annual Percent Change | 95% CI | ||||
| Total | 34.32 | 171,142 | 495,041,648 | |||
| 1995 | 38.22 | 9,336 | 23,591,415 | |||
| 1996 | 38.06 | 9,675 | 24,545,508 | |||
| 1997 | 37.11 | 11,061 | 29,051,668 | |||
| 1998 | 37.00 | 11,504 | 30,495,803 | |||
| 1999 | 36.65 | 12,047 | 32,422,731 | |||
| 2000 | 36.36 | 12,198 | 33,146,759 | |||
| 2001 | 35.98 | 12,948 | 35,671,176 | |||
| 2002 | 34.94 | 13,202 | 37,529,315 | |||
| 2003 | 33.85 | 13,011 | 38,375,429 | |||
| 2004 | 32.95 | 13,392 | 40,676,844 | |||
| 2005 | 32.36 | 12,639 | 39,152,508 | |||
| 2006 | 31.80 | 13,531 | 42,642,491 | |||
| 2007 | 31.03 | 13,350 | 43,341,843 | |||
| 2008 | 30.15 | 13,248 | 44,398,158 | |||
| 1995-2002 | −8.57 | −1.17§ | −1.44 to −0.89 | |||
| 2003-2008 | −10.92 | −2.19§∥ | −2.45 to −1.93 | |||
| 1995-2008 | −21.10 | −1.87§ | −2.09 to −1.64 | |||
NOTE. Data from the SEER*Stat Database (“NAACCR Incidence, CINA Analytic File, 1995-2008, for Expanded Races, Standard File”).
Abbreviation: NAACCR, North American Association of Central Cancer Registries.
Rates are per 100,000 population and age standardized to the 2000 US population (single ages to 84 years; Census P25-1130).
Ovarian cancer (International Classification of Diseases code 56.9); included only malignant carcinomas and excluded noncarcinomas (n = 20,821).
Percent changes were calculated using 1 year for each end point; annual percent changes were calculated using the weighted least squares method.
The annual percent change is significantly different from zero (P < .05).
The annual percent changes is significantly different from the annual percent changes for 1995 to 2002 (P < .05).
APC Model
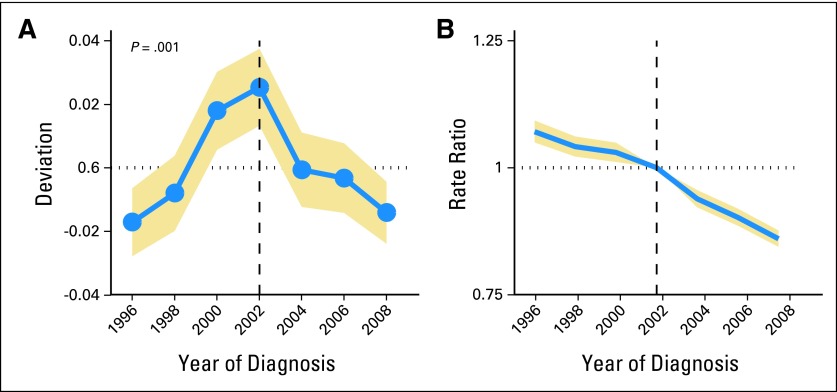
The observed rates from SEER*Stat and fitted rates from the APC model were similar, confirming a greater deceleration in the incidence of ovarian cancer after 2002 compared with earlier years, adjusted for both age and cohort effects. The period deviations declined with a slope difference of −1.2% after 2002 (P for change adjusted for age and cohort effects = .001; Fig 2A). Additionally, the period relative risk for ovarian cancer overall was 7% higher (period relative risk, 1.07; 95% CI, 1.05 to 1.09) in 1995 compared with the 2002 referent time period and 14% lower (period relative risk, 0.86; 95% CI, 0.84 to 0.88) in 2008 compared with 2002 (Fig 2B).
Fig 2.
Age-period-cohort effects among US women age ≥ 50 years (North American Association of Central Cancer Registries Incidence, 1995 to 2008). Point estimates are shown in blue, with 95% CIs shaded in gold. (A) The significance of period deviations was assessed by contrasting the three time periods before the Women's Health Initiative (WHI; 1995 to 1996, 1997 to 1998, and 1999 to 2000) with the three time periods after WHI (2003 to 2004, 2005 to 2006, and 2007 to 2008). P value is for change in the slopes of the period deviations, adjusted for age and cohort effects. (B) Period relative risks were calculated as rate ratios adjusted for age and birth cohort effects, comparing the ovarian cancer incidence rates for a given time period with the rate of a referent period (the 2002 time period in this analysis). The period relative risks declined from more than 1.0 before the 2002 referent period, after which the period relative risks were significantly less than 1.0.
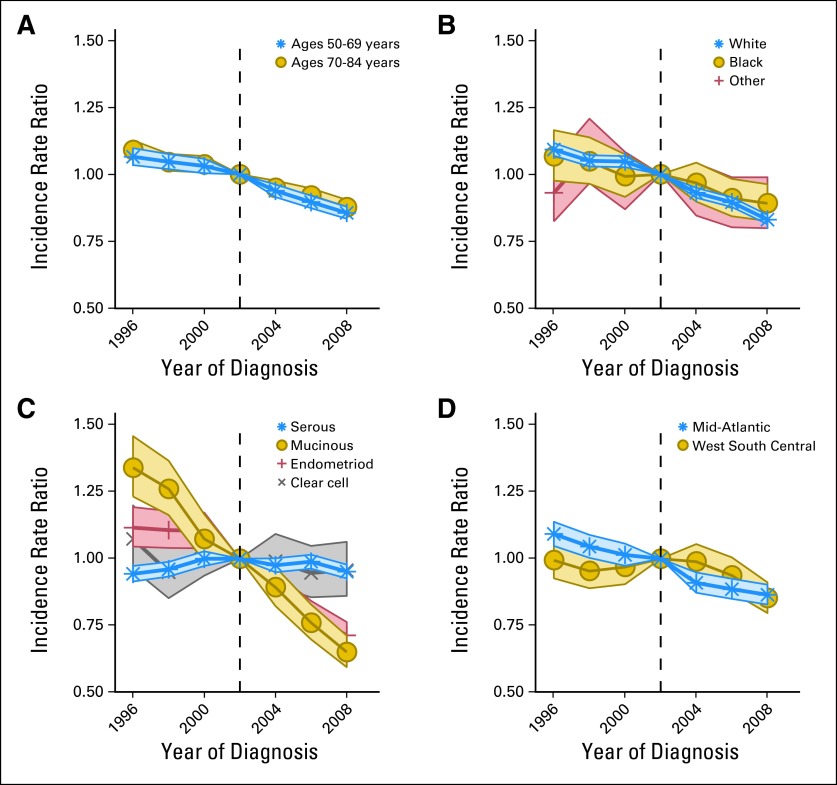
Ovarian Cancer Stratified by Key Factors
We estimated the period relative risk by age at ovarian cancer diagnosis (Fig 3A), race (Fig 3B), histologic subtype (Fig 3C), and US geographic regions associated with low (Middle Atlantic) and high (West South Central) MHT use (Fig 3D). Period relative risks generally declined over the entire study period. The most notable sudden declines circa 2002 occurred among women most likely to have used MHT (women age 50 to 69 years, white women, and residents of regions with the highest MHT prescription frequency; P for change adjusted for age and cohort effects < .02). The largest change points circa 2002 were found for endometrioid histologic subtype. Clear cell subtype showed no decline or change point. Mucinous tumors decreased steadily without a change point circa 2002. In contrast, serous carcinomas showed a unique pattern, in which the incidence increased then decreased slightly but significantly around 2002. The change in the period relative risks was also statistically significant for both low- and high-grade serous carcinomas (data not shown). However, for low-grade serous carcinomas, the relative risks decreased slowly before 2002, after which the rate of decline accelerated; whereas for high-grade serous carcinomas, the relative risks increased before 2002, after which the rate of increase slowed significantly.
Fig 3.
Age-period-cohort period relative risks among US women age ≥ 50 years (North American Association of Central Cancer Registries Incidence, 1995 to 2008). The period relative risks by (A) age at ovarian cancer diagnosis, (B) race, (C) histologic subtype, and (D) US geographic regions associated with high (West South Central) and low (Mid-Atlantic) menopausal hormone therapy frequency. For most strata, the period relative risks declined from more than 1.0 before the 2002 referent period, after which the period relative risks were less than 1.0 (see text for further details).
Finally, we analyzed ovarian cancer trends by stage at diagnosis (using the SEER summary and historic stage variables). We found decreasing trends for local, regional, and distant stages (data not shown), albeit results were only statistically significant for regional cancers.
DISCUSSION
Our analysis shows that the decline in ovarian cancer rates, which has been occurring for many years, accelerated around 2002 among women age 50 years and older, coinciding with health warnings emanating from the WHI study related to use of MHT.25 Furthermore, using APC modeling to refine results of joinpoint regression, we demonstrate that the more rapid decline among women age 50 years and older is independent of both age and cohort influences. The decline in ovarian cancer rates after the WHI announcement parallels previously reported observations linking a reduction in MHT prescriptions in 2002 to decreasing breast cancer rates.7 The sudden changes in rates for these two hormonally sensitive cancers during the same time period strengthens the evidence linking MHT use to the declining incidence of both tumors and is consistent with the view that hormones have a promoter effect for both breast and ovarian carcinogenesis.26
The APC period relative risks showed the most significant declines among groups of women most likely to have used MHT (ie, women age 50 to 69 years, white women, and residents in regions with the highest frequency of MHT prescriptions). We also observed statistically significant period changes circa 2002 for both endometrioid and serous histologic subtypes, with the largest changes in incidence rates for endometrioid ovarian tumors. For serous tumors overall, the period relative risks showed a unique pattern, with a slight increase before the decline after 2002. However, the relative risks varied by histologic subtype, which might be related to etiologic heterogeneity, with hormonal exposures impacting some types of ovarian cancers more than others.
We considered alternatives to reduced MHT use as an explanation for the ovarian cancer rate declines after 2002, but view these as less likely. For an exposure to account for the abrupt accelerated decline in ovarian cancer incidence rates after 2002, the prevalence of the exposure would have to shift rapidly around that time period. To the best of our knowledge, we do not know of any other calendar-period changes (eg, screening) with a temporal relationship that is similar to MHT use. Furthermore, we used APC models to adjust for potentially confounding age and birth cohort influences, such as those related to oral contraceptive use.27 The decline is unlikely an artifact of oophorectomy prevalence in the United States because performance of oophorectomy in the United States decreased from 2002 to 2006, placing more women rather than fewer women at risk.28 Indeed, although oophorectomy is effective in reducing ovarian cancer risk and its benefit is well documented among high-risk women such as BRCA1/2 mutation carriers, there has been a general lack of awareness of the potential prophylactic effect of oophorectomy for ovarian cancer risk reduction in past years.29 Increased recognition of metastases to the ovary30,31 and better recognition of fallopian tube primary tumors32 also could affect rates modestly, but the effects would likely have been small during the years of analysis and would not have shown the temporal, demographic, and regional specificity that we identified. This concern is most relevant for mucinous carcinomas, which have decreased at least in part secondary to better recognition of GI metastases to the ovary. Lastly, use of selective estrogen receptor modulator agents for chemoprevention, such as tamoxifen or raloxifene, is unlikely to account for the observed data because these agents have not been shown to lower ovarian cancer risk33,34 and use of aromatase inhibitors is a more recent practice that would not impact the ovarian cancer incidence during the time period we evaluated.35
The main strengths of this analysis include the availability of a large number of data points from a representative, regionally diverse population in the United States and use of APC modeling to specifically estimate period effects adjusted for both age and cohort effects. A major limitation of ours and any other descriptive analysis is that it can only be used to generate a hypothesis about carcinogenesis and cannot determine the causes of incidence trends observed. Other limitations include our inability to link an individual's MHT use to cancer development and the potential for pathologic misclassification. Another limitation is that we used modeled results (joinpoint and APC analyses), but our findings for the observed and modeled data were consistent and provided complementary evidence.
Our data suggest a possible link between the decline in MHT use after the WHI announcement in 2002 and the accelerated decline in ovarian cancer incidence that is parallel to the declining breast cancer incidence rates.36 It is unclear whether ovarian cancer rates will continue to decrease beyond our study period, and our descriptive study cannot establish a direct causal relationship between exogenous hormone exposure and ovarian cancer. Nonetheless, our results provide compelling temporal evidence that hormonal exposures contribute to ovarian carcinogenesis with the clearest suggestion for endometrioid carcinomas. In fact, changes in rates for serous carcinomas (the numerically predominant cause of ovarian cancer deaths) were significant but of considerably smaller magnitude than for endometrioid tumors. Although there are some large studies linking MHT use to an increased risk for serous carcinomas, this association does not seem to be established at this time. Therefore, our findings for serous carcinomas should not be overinterpreted.
In summary, analytic studies show that oral contraceptive use at early ages is highly protective for ovarian cancer and that MHT use increases ovarian cancer risk, and limited data suggest that aromatase inhibitors may have value in treating ovarian cancers, all of which support the concept that hormonal mechanisms are important to the etiology of some ovarian cancers. Our descriptive findings based on 171,000 incident cases are not intended to inform an individual woman's decision about MHT use. Rather, they are presented to clarify the national patterns and also highlight the need to increase our knowledge of hormonal mechanisms in ovarian cancer development, which ultimately may allow women to make evidence-based personal decisions as well as guide policies that have implications for cancer control.
Appendix
Table A1.
North American Association of Central Cancer Registries Included in Analysis
| Registry |
|---|
| Alabama |
| Alaska |
| Arizona |
| Arkansas |
| California (including Greater Bay and Los Angeles) |
| Colorado |
| Connecticut |
| Delaware |
| Florida |
| Georgia (including Atlanta) |
| Hawaii |
| Iowa |
| Kentucky |
| Louisiana |
| Maine |
| Massachusetts |
| Michigan |
| Mississippi |
| Montana |
| Nebraska |
| Nevada |
| New Hampshire |
| New Jersey |
| New York |
| North Carolina |
| North Dakota |
| Ohio |
| Oregon |
| Pennsylvania |
| Rhode Island |
| South Carolina |
| South Dakota |
| Tennessee |
| Texas |
| Utah |
| Virginia |
| Washington |
| West Virginia |
| Wyoming |
Table A2.
Demographics and Clinical Characteristics Among US Women Age ≥ 50 Years With Ovarian Carcinoma (NAACCR Incidence, 1995 to 2008)
| Demographic or Clinical Characteristic | Women With Malignant Cancer* (N = 171,142) |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| 50-59 | 45,701 | 27 |
| 60-69 | 48,250 | 28 |
| 70-79 | 47,312 | 28 |
| ≥ 80 | 29,879 | 17 |
| Race | ||
| White | 153,178 | 90 |
| Black | 11,390 | 7 |
| Other | 6,574 | 4 |
| Histologic subtype | ||
| Serous | 75,495 | 44 |
| Endometrioid | 16,518 | 10 |
| Clear cell | 6,960 | 4 |
| Mucinous | 10,503 | 6 |
| Other carcinoma | 61,666 | 36 |
| MHT use by US region | ||
| High use (West South Central) | 15,319 | 9 |
| Low use (Mid-Atlantic) | 35,250 | 21 |
Abbreviations: MHT, menopausal hormone therapy; NAACCR, North American Association of Central Cancer Registries.
Excluded women with borderline malignant cancer.
Footnotes
Supported entirely by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Hannah P. Yang, William F. Anderson, Mark E. Sherman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society. Atlanta, GA: American Cancer Society; 2012. Cancer Facts & Figures. [Google Scholar]
- 2.Weiss NS, Szekely DR, Austin DF. Increasing incidence of endometrial cancer in the United States. N Engl J Med. 1976;294:1259–1262. doi: 10.1056/NEJM197606032942303. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042–1050. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 5.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: Annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Kim N, Gross C, Curtis J, et al. The impact of clinical trials on the use of hormone replacement therapy: A population-based study. J Gen Intern Med. 2005;20:1026–1031. doi: 10.1111/j.1525-1497.2005.0221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 8.Pearce CL, Chung K, Pike MC, et al. Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin. Cancer. 2009;115:531–539. doi: 10.1002/cncr.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mørch LS, Løkkegaard E, Andreasen AH, et al. Hormone therapy and ovarian cancer. JAMA. 2009;302:298–305. doi: 10.1001/jama.2009.1052. [DOI] [PubMed] [Google Scholar]
- 10.Hildebrand JS, Gapstur SM, Feigelson HS, et al. Postmenopausal hormone use and incident ovarian cancer: Associations differ by regimen. Int J Cancer. 2010;127:2928–2935. doi: 10.1002/ijc.25515. [DOI] [PubMed] [Google Scholar]
- 11.Tsilidis KK, Allen NE, Key TJ, et al. Menopausal hormone therapy and risk of ovarian cancer in the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2011;22:1075–1084. doi: 10.1007/s10552-011-9782-z. [DOI] [PubMed] [Google Scholar]
- 12.Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiol Biomarkers Prev. 2009;18:132–139. doi: 10.1158/1055-9965.EPI-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39:311–324. [PubMed] [Google Scholar]
- 15.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: Ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;20:1263–1268. doi: 10.1158/1055-9965.EPI-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker TC, Howe HL. Measuring the quality of population-based cancer registries: The NAACCR perspective. J Reg Manage. 2001;28:41–44. [Google Scholar]
- 18.Morabia A, Flandre P. Misclassification bias related to definition of menopausal status in case-control studies of breast cancer. Int J Epidemiol. 1992;21:222–228. doi: 10.1093/ije/21.2.222. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. National Cancer Institute SEER*Stat software (ed version 7.0.9) http://seer.cancer.gov/seerstat/software/
- 20.Tarone RE, Chu KC. Evaluation of birth cohort patterns in population disease rates. Am J Epidemiol. 1996;143:85–91. doi: 10.1093/oxfordjournals.aje.a008661. [DOI] [PubMed] [Google Scholar]
- 21.Anderson WF, Camargo MC, Fraumeni JF, Jr, et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jemal A, Ma J, Rosenberg PS, et al. Increasing lung cancer death rates among young women in southern and midwestern states. J Clin Oncol. 2012;30:2739–2744. doi: 10.1200/JCO.2012.42.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.North American Association of Central Cancer Registries. Who is certified. http://www.naaccr.org/Certification/WhoisCertified.aspx.
- 24.Clegg LX, Feuer EJ, Midthune DN, et al. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 25.Haas JS, Kaplan CP, Gerstenberger EP, et al. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 26.Colditz GA. Decline in breast cancer incidence due to removal of promoter: Combination estrogen plus progestin. Breast Cancer Res. 2007;9:108. doi: 10.1186/bcr1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gnagy S, Ming EE, Devesa SS, et al. Declining ovarian cancer rates in U.S. women in relation to parity and oral contraceptive use. Epidemiology. 2000;11:102–105. doi: 10.1097/00001648-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Asante A, Whiteman MK, Kulkarni A, et al. Elective oophorectomy in the United States: Trends and in-hospital complications, 1998-2006. Obstet Gynecol. 2010;116:1088–1095. doi: 10.1097/AOG.0b013e3181f5ec9d. [DOI] [PubMed] [Google Scholar]
- 29.Greene MH, Piedmonte M, Alberts D, et al. A prospective study of risk-reducing salpingo-oophorectomy and longitudinal CA-125 screening among women at increased genetic risk of ovarian cancer: Design and baseline characteristics—A Gynecologic Oncology Group study. Cancer Epidemiol Biomarkers Prev. 2008;17:594–604. doi: 10.1158/1055-9965.EPI-07-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young RH. From Krukenberg to today: The ever present problems posed by metastatic tumors in the ovary—Part II. Adv Anat Pathol. 2007;14:149–177. doi: 10.1097/PAP.0b013e3180504abf. [DOI] [PubMed] [Google Scholar]
- 31.Young RH. From Krukenberg to today: The ever present problems posed by metastatic tumors in the ovary—Part I. Historical perspective, general principles, mucinous tumors including the Krukenberg tumor. Adv Anat Pathol. 2006;13:205–227. doi: 10.1097/01.pap.0000213038.85704.e4. [DOI] [PubMed] [Google Scholar]
- 32.Carlson J, Roh MH, Chang MC, et al. Recent advances in the understanding of the pathogenesis of serous carcinoma: The concept of low- and high-grade disease and the role of the fallopian tube. Diagn Histopathol (Oxf) 2008;14:352–365. doi: 10.1016/j.mpdhp.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook LS, Weiss NS, Schwartz SM, et al. Population-based study of tamoxifen therapy and subsequent ovarian, endometrial, and breast cancers. J Natl Cancer Inst. 1995;87:1359–1364. doi: 10.1093/jnci/87.18.1359. [DOI] [PubMed] [Google Scholar]
- 34.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer. 2012;12:835–848. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- 36.Cronin KA, Ravdin PM, Edwards BK. Sustained lower rates of breast cancer in the United States. Breast Cancer Res Treat. 2009;117:223–224. doi: 10.1007/s10549-008-0226-8. [DOI] [PubMed] [Google Scholar]