Abstract
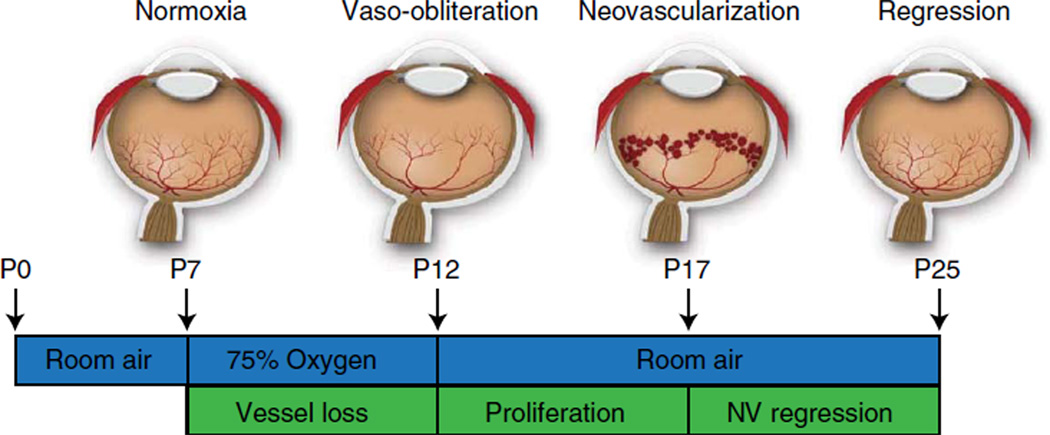
The mouse model of oxygen-induced retinopathy (OIR) has been widely used in studies related to retinopathy of prematurity, proliferative diabetic retinopathy and in studies evaluating the efficacy of antiangiogenic compounds. In this model, 7-d-old (P7) mouse pups with nursing mothers are subjected to hyperoxia (75% oxygen) for 5 d, which inhibits retinal vessel growth and causes significant vessel loss. on P12, mice are returned to room air and the hypoxic avascular retina triggers both normal vessel regrowth and retinal neovascularization (NV), which is maximal at P17. neovascularization spontaneously regresses between P17 and P25. although the OIR model has been the cornerstone of studies investigating proliferative retinopathies, there is currently no harmonized protocol to assess aspects of angiogenesis and treatment outcome. In this protocol we describe standards for mouse size, sample size, retinal preparation, quantification of vascular loss, vascular regrowth, NV and neovascular regression.
INTRODUCTION
Retinopathy of prematurity
Retinopathy of prematurity (ROP) and other ocular diseases with pathological neovascularization (NV) present in two phases. In the first phase there is a loss of pre-established vessels and cessation of normal vessel development. Phase I is precipitated by the addition of factors in the extrauterine environment, notably oxygen, which is above intrauterine levels even in room air. The relative hyperoxia is exacerbated by oxygen supplementation used to overcome pulmonary insufficiency and brain ischemia in these infants. This shift from the relatively hypoxic in utero environment to relative hyperoxia leads to the suppression of oxygen-regulated growth factors such as vascular endothelial growth factor (VEGF) and erythropoietin1,2. Phase I is also precipitated after premature birth by the loss of factors normally provided by the mother in utero, such as insulin-like growth factor (IGF-1) and ω-polyunsaturated fatty acids3,4.
As the retina matures after birth, it becomes more metabolically active and the avascular retina becomes hypoxic, leading to phase II of ROP. The hypoxia of phase II induces a rapid increase in hypoxia inducible factor (HIF)-regulated growth factors (VEGF and Epo) that were suppressed during phase I. Factors missing from the mother may rise slightly if the fetal liver and other organs that produce them have matured sufficiently, but may still be below normal intrauterine levels.
Retinal vascularization is completed around 36 weeks of gestation and is incomplete in premature infants5. The use of animal models in studying oxygen-induced retinopathy (OIR) has provided pivotal insights into the pathogenesis and underlying mechanism of this disease process. Retinal vascularization occurs ex utero in several animals. Thus, many animals such as mice, rats, rabbits, kittens and beagle pups have incompletely vascularized retinas at birth. This incomplete vascular development resembles the immature retinal vascular development occurring in premature infants6. The association between oxygen exposure and vaso-obliteration has also been observed in mice, rats, rabbits, kittens and puppy animal models7–11.
Models to study retinal vascular development
One of the first models used to study retinal vascular development was the kitten6. The kitten and beagle puppy retinal vascular development have similarities to the human12, but a major drawback to these models is cost and availability of reagents. An additional model utilizes rabbits to study OIR. In this model, the retinal vessels are limited to a small region of the retina and do not mimic the retinal vascular development found in the human infant6. The rat model of OIR is more commonly used, because it is economical with less variability. This model relies on alternating oxygen levels between 50% and 10% for 12 h cycles from postnatal day 0 through 14 (ref. 6). The rat pups are then maintained at room air (normoxia) for 4–7 d allowing the NV phase to develop6.
Our laboratory has developed a mouse model of ROP, which allows for the study of molecular pathways involved in the disease7. Proliferative retinopathy in the mouse model develops reliably (and quantifiably) over 17 d. This model enables users to take advantage of the genetic manipulation possible in mice, allowing them to tease apart pathways involved in ROP and will be described in depth below7. Other animal models may not be as consistent, economical or have the advantages of genetic manipulation that the mouse OIR model has.
To study retinopathy, a well-characterized and standardized model is necessary (Fig. 1). The mouse model of OIR has proven useful in delineating the molecular changes in both phases of neovascular eye diseases1,4, as well as defining the mechanisms by which developmental and abnormal angiogenesis occurs13–15. In addition, it allows the user to take advantage of the well established genetic manipulation platforms for mice7. In this model, proliferative retinopathy develops reliably (and quantifiably) over 17 d, unlike rodent models of diabetes, which do not develop proliferative disease. Neonatal mice are exposed to 75% oxygen from postnatal day (P)7 until P12 (ref. 7) (Fig. 1). Hyperoxia causes vessel regression in the central retina and the cessation of normal radial vessel growth, mimicking the first phase of ROP8. The extent of vaso-obliteration can be determined by measuring the avascular area in retinal whole-mount at P12 (Fig. 2)1,4. This readout is of particular interest in studies that probe vasoprotective strategies or investigate genes that are hypothesized to be involved in vascular obliteration. Beyond ischemic retinopathies, the degeneration of microvasculature within nervous tissue16,17 is a cardinal feature of ischemic brain injuries such as strokes. Halting this vascular decay reduces brain mass loss and preserves function18,19. As in retinopathies, a neovascular phase occurs subsequent to the initial vessel loss18. Therefore, it is conceivable to employ the ROP model as a complementary tool in investigating the pathogenesis of ischemic brain injury.
Figure 1.
Cartoon schematic of the mouse OIR model. Neonatal mice and their nursing mother are kept in room air from birth through P7 and normal vascular development ensues. At P7, mice are exposed to 75% oxygen, which inhibits retinal vessel growth and causes significant vessel loss. Mice are returned to room air at P12; the avascular retina becomes hypoxic, triggering both normal vessel regrowth and a pathological neovascular response. Neovascularization (NV) reaches its maximum at P17. Shortly thereafter, the NV spontaneously regresses, and the retina reaches resolution by P25.
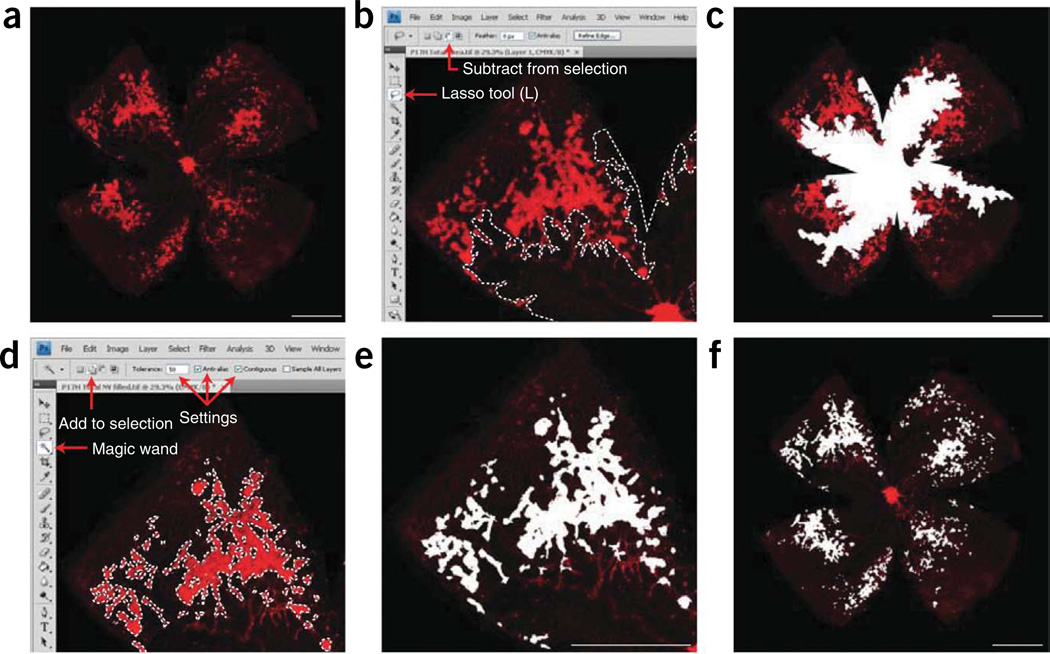
Figure 2.
Vasculature and quantification of P12H vaso-obliteration. (a) Image of ×5 P12H retinal whole-mount stained for endothelial cells with isolectin B4-594 and obtained using the Zeiss AxioCam MRm, Zeiss AxioObserver.Z1 microscope and AxioVision 4.6.3.0 software. (b) Screenshot of one retinal quadrant with the total vascular area traced. The tools used to obtain total retinal area in CS4 photoshop, the Polygonal Lasso Tool and the ‘Add to selection’ function, are highlighted by the red arrows. (c) ×5 P12H retinal whole-mount with the avascular area (vaso-obliterated area) highlighted. (d) Screenshot of one retinal quadrant with quantification of avascular zone. The functions and settings utilized, the ‘Lasso Tool’ and ‘Subtract from selection,’ are highlighted by red arrows. (e) ×5 P12H retinal whole-mount with the total avascular area highlighted in white. (f) Histogram function with ‘Refresh’ key, and pixel record highlighted by red arrow. Once the desired area is outlined, click the ‘Refresh’ key and record the number of pixels in the area. Scale bars are 1,000 µm. These studies adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Children’s Hospital Boston Animal Care and Use Committee.
Upon return to room air, the avascular areas of retina become hypoxic20,21. This hypoxia induces the expression of angiogenic factors, resulting in the regrowth of normal retinal vessels, and depending on the extent of initial vessel loss during phase 1, retinal NV. The neovascular phase in the OIR model is similar to the second phase of ROP in humans and, in addition, mimics many aspects of other proliferative retinopathies and other neovascular eye diseases.
In phase II, both vascular regrowth and NV develop between P12 and P174.Vascular regrowth can be quantified in whole mounted retinas by comparing the avascular area to total retinal area (Fig. 3). NV is maximal at P17. Several approaches to measure the extent of NV have been developed: in retinal cross-sections, counting preretinal nuclei2,7,22; in retinal whole-mounts, scoring retinal NV in a grading system23,24; and in retinal whole-mounts, manually measuring the area of neovascular tufts1,4,25. This protocol will address the latter quantification method of outlining NV structures in Adobe Photoshop (or a similar program) and comparing total NV area with the total retinal area (Fig. 3)1,4. This method reflects more accurately the extent of disease for a given eye. Retinal NV is of interest in studies investigating anti angiogenic compounds or genes thought to be involved in pathological neovascular growth.
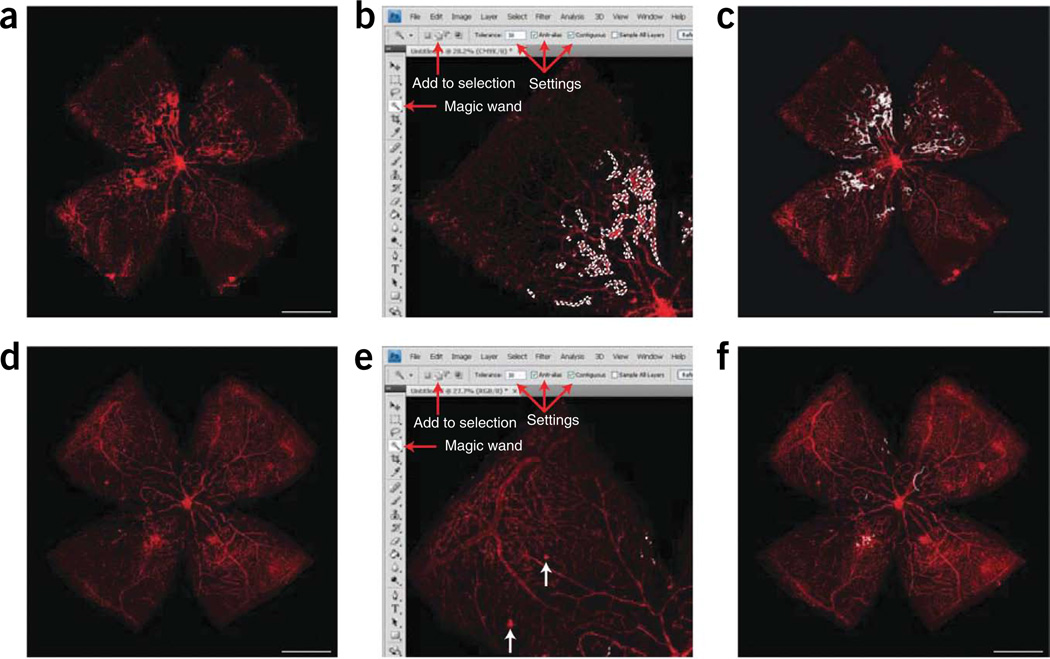
Figure 3.
Quantification of vaso-obliteration and neovascularization (NV) at P17H. (a) Image of ×5 P17H retinal whole-mount stained for endothelial cells with isolectin B4-594 and obtained using a Zeiss AxioCam MRm, Zeiss AxioObserver.Z1 microscope and AxioVision 4.6.3.0 software. (b) Screenshot of a retinal quadrant with the avascular area highlighted. The tools necessary to make this measurement are highlighted by red arrows: the Lasso Tool and ‘subtract from selection’ keys. As at P12, this measurement should be made after finding the pixels in total retina. (c) P17H retinal whole-mount with entire avascular (vaso-obliterated) area highlighted in white. (d) Screenshot of a retinal quadrant with the neovascular tufts highlighted. The necessary tools and settings are noted with red arrows; use the ‘magic wand’ and ‘add to selection’ tools to highlight NV. The tolerance should be set to 50, and the ‘anti-alias’ and ‘contiguous’ boxes should be checked. (e) One quadrant of a retina with NV highlighted in white. (f) ×5 P17H retinal whole-mount with all neovascular tufts highlighted in white. Scale bars represent 1,000 µm. These studies adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Children’s Hospital Boston Animal Care and Use Committee.
Resolution of NV from P17 through P25 can also be assessed using the OIR model26. Quantification of NV in whole-mounted retinas during this stage can be more subjective due to the resolving nature of the neovessels. We identify resolving neovessels by the following four criteria: shape, vessel diameter, fluorescent intensity and protrusion above the superficial layer (Fig. 4). At present, there is an insufficient understanding of how pathological NV regresses. This readout is of interest for establishing strategies to suppress already-formed pathological vessels.
Figure 4.
Quantification of neovascularization (NV) during regression: P18H–P25H. (a) Image of ×5 retinal whole-mount at P21H stained for endothelial cells with isolectin B4-594 and obtained using Zeiss AxioCam MRm, Zeiss AxioObserver.Z1 microscope, and AxioVision 4.6.3.0 software. (b) Screenshot of a P21H retinal quadrant with the NV highlighted. The necessary tools and settings are noted with red arrows. Use the ‘magic wand’ and ‘add to selection’ tools to highlight NV. The tolerance should be set to 30 or lower and the ‘anti-alias’ and ‘contiguous’ boxes should be checked. (c) ×5 P21H retinal whole-mount with all NV highlighted in white. (d) ×5 retinal whole-mount at P25H stained with isolectin B4-594. (e) Screenshot of a P25H retinal quadrant with the NV highlighted. White arrows point to background artifacts or precipitates that do not connect to vessels. Red arrows indicate tools and settings as listed above in (b). (f) ×5 P25H retinal whole-mount with all NV highlighted in white. Scale bars are 1,000 µm. These studies adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Children’s Hospital Boston Animal Care and Use Committee.
As with all model systems involving the use of animals, the welfare of subjects is a concern in the mouse OIR system. It is important that animals be killed at the time of ocular enucleation. Appropriate care should be used to maintain animal welfare.
Experimental design
The OIR procedure7
The mouse model of OIR exposes P7 mouse litters to 75% oxygen for 5 d (until P12). This results in the regression or vaso-obliteration of the central retinal vasculature. The mice are then returned to room air until P17, during which time neovessels form at the junction between the vascularized and non-vascularized retina. As described above, this model is useful in delineating mechanism involved in initial vessel loss (P7–P12), vascular regrowth (P12–P17), neovessel formation (P14–P17) and neovascular regression (P17–P25). Our laboratory has found that the greatest limitation in assessing these aspects of retinopathy is the technical difficulty of retinal dissections and quantification of NV and vaso-obliteration. For the experienced researcher, we have found that ~50 eyes may be dissected or whole mounted and 10 eyes may be quantified per day. This protocol has been widely adopted and described in detail in earlier publications1–4,7,20,25–28.
Controls
When defining a phenotype, it is essential to have high n values, with animals from at least three litters to overcome biological variability. This variability occurs even with the use of the same strain; therefore, intralitter controls are the gold standard but this may not be feasible in some studies, wherein several genes are knocked out. If not, the exact background strain of the genetically modified mice must be used as a control. Ideally, control and experimental mice should be subjected to model conditions at the same time; however, we have found that staggering experimental litters in oxygen does not significantly alter the phenotype. The retinal angiogenic response of mice is very much strain dependent with 129S strain being much more angiogenic than C57BL6/J (ref. 29).
Moreover, mothers occasionally expire because of oxygen toxicity, have a large litter (10 or more pups) or stop feeding their pups resulting in growth restricted litters that exhibit an altered vascular phenotype30. Given this, we have found that mice under 6 g at P17 should not be included in the analysis.
Materials
REAGENTS
2,2,2-Tribromo ethanol (Sigma-Aldrich, cat. no. T48402)
 2,2,2-Tribromo ethanol can cause skin and eye irritations and is toxic if inhaled or swallowed. Solutions containing this compound should be made in a chemical hood while wearing the appropriate protective equipment (laboratory safety coat and gloves).
2,2,2-Tribromo ethanol can cause skin and eye irritations and is toxic if inhaled or swallowed. Solutions containing this compound should be made in a chemical hood while wearing the appropriate protective equipment (laboratory safety coat and gloves).Isopentanol (isoamyl alcohol) (Sigma-Aldrich, cat. no. W205710)
 Isopentanol is harmful if inhaled and flammable. Solutions containing this compound should be made in a chemical hood while wearing the appropriate protective equipment (laboratory safety coat and gloves).
Isopentanol is harmful if inhaled and flammable. Solutions containing this compound should be made in a chemical hood while wearing the appropriate protective equipment (laboratory safety coat and gloves).16% Paraformaldehyde (PFA) (Electron Microscopy Sciences, cat. no. 15710)
 Paraformaldehyde is toxic if inhaled or swallowed. Solutions containing this compound should be made in a chemical hood while wearing the appropriate protective equipment (laboratory safety coat and gloves).
Paraformaldehyde is toxic if inhaled or swallowed. Solutions containing this compound should be made in a chemical hood while wearing the appropriate protective equipment (laboratory safety coat and gloves).1× PBS pH 7.4 (Gibco, cat. no. 10010)
10× PBS (Gibco, cat. no. 0885)
dH2O
1 M CaCl2 (Sigma-Aldrich, cat. no. C1016)
Isolectin B4–594 (Alexa Fluor 594 – I21413, Molecular Probes)
Steriflip tubes
Sodium azide (NaN3) (Sigma-Aldrich, cat. no. S2002)
 Sodium azide is toxic if inhaled or swallowed and can irritate the skin. Solutions containing this compound should be made in a chemical hood while wearing the appropriate protective equipment (laboratory safety coat and gloves).
Sodium azide is toxic if inhaled or swallowed and can irritate the skin. Solutions containing this compound should be made in a chemical hood while wearing the appropriate protective equipment (laboratory safety coat and gloves).Triton X-100 (Roche Applied Science, cat. no. 11 332 481 001)
SlowFade anti-fade reagent (Invitrogen, cat. no. S2828)
Neonatal mice and nursing mother
 All animal experiments must comply with national laws and institutional regulations.
All animal experiments must comply with national laws and institutional regulations.
EQUIPMENT
BioSpherix Proox Model 110 Hyperoxia Chamber (BioSpherix, Ltd., cat. no. A-30274)
Oxygen tanks (100%)
Automatic Changeover Manifold system (Airgas East, cat. no. Y11 CP104D540)
1ml disposable syringe (Becton, Dickson and Company, cat. no. 309602)
Curved forceps (Roboz, cat. no. RS-5111)
Smooth, fine tipped, No. 5 dissecting forceps (Wilson Ophthalmic, cat. no. 603-0001025-00)
Tissue culture plate, 24-well, flat bottom with low evaporation lid (Falcon, cat. no. 35 2847)
Superfrost/Plus microscope slides (Fisher, cat. no. 12-550-15)
VWR micro cover glass, 18 mm × 18 mm No. 1½ (VWR, cat. no. 48366 205)
Surgical blade (Cincinnati Surgical, cat. no. BS2982 ISO 7740)
Disposable transfer pipettes (Fine tip) (VWR, cat. no. 16001-194)
Transfer pipettes (Wide tip) (Fisherbrand, cat. no. 13-711-9A)
Dissecting microscope
Aluminum foil
Zeiss AxioCam MRm and Zeiss AxioObserver.Z1 microscope (Zeiss)
AxioVision 4.6.3.0. (Zeiss)
Adobe CS3 or later versions (Photoshop)
REAGENT SETUP
Avertin
Under a chemical hood, dissolve 0.5 g of 2,2,2-tribromo ethanol in 40 ml dH2O by heating on a hot plate for 15 min with occasional agitation. Once the solid has dissolved, add 310 µl of isopentanol and mix thoroughly. Allow the solution to cool in a chemical hood for 20 min. Aliquot avertin solution into 50 ml conical tubes and store at 4 °C for up to 4 weeks.
4% PFA
Prepare 500 ml 1.33× PBS from 10× stock by mixing 67 ml of 10× PBS with 433 ml of dH2O. In a chemical hood, prepare 4% PFA (vol/vol) by combining 10 ml of 16% PFA (one whole vial) with 30 ml of 1.33× PBS. Store the final 4% PFA (vol/vol) solution in a conical tube at 4 °C for up to 7 d.
1 mM CaCl2
 CaCl2 precipitates out of solution easily
CaCl2 precipitates out of solution easily
To dilute, use a clean 150 ml flask, a stir bar and a hot plate with stir function. Add 99.90 ml of 1× PBS to 150 ml Erlenmeyer flask and place on stir plate set to 3 (no heat necessary). Add 100 µl of 1 M CaCl2 slowly in a drop-wise manner to the stirring PBS and let the solution stir for 1 h. Aliquot solution into 50 ml vials. Use a vacuum hose and steriflip tubes to filter the solution into 50 ml conical tubes. Store the final 1mM CaCl2 solution at 4 °C.
Lectin solution
Reconstitute conjugated lectin from 500 µg powder form by adding 500 µl of 1 mM CaCl2 in 1× PBS (made above). Reconstituted lectin is then combined with 49 ml of 1 mM CaCl2 and 500 µl of 200 mM NaN3. This corresponds to a final concentration of 10 µg ml−1 lectin, 1 mM CaCl2, 2 mM NaN3 and 1× PBS. Lectin solution may be stored at 4 °C for 2 weeks.
0.5% Triton X-100
To make 30 ml of 0.5% Triton X-100 (vol/vol), dilute 150 µl of pure Triton X-100 in 29.85 ml of 1× PBS. Vortex the resulting product to mix thoroughly. Store the solution at 4 °C.
PROCEDURE
Oxygen induced retinopathy  12–25 d
12–25 d
1| Set up hyperoxia chamber according to manufacturer’s instructions. This chamber should contain oxygen sensors that monitor and maintain the oxygen level at 75%. A manifold setup with the capability to switch between several oxygen tanks is useful.
2| Record the neonate’s date of birth (DOB).
 DOB is the postnatal day (P)0.
DOB is the postnatal day (P)0.
3| On P7, expose neonates and mother to 75% oxygen in a BioSpherix Proox Model 110 until P12. Key points in the disease process are as follows: (a) at P12, maximum vaso-obliteration occurs. (b) At P17, maximum neovascular response occurs; the level of normal vessel regrowth and the severity of NV can be quantified (see below). (c) Between P17 and P25, regression of NV occurs; the level and rate of NV regression can be quantified (see below).
 Mice should be weighed at P17 before proceeding. Mice that are underdeveloped (low weights) have abnormal phenotypes and should be discarded from data sets.
Mice should be weighed at P17 before proceeding. Mice that are underdeveloped (low weights) have abnormal phenotypes and should be discarded from data sets.
![]()
Enucleation and fixation of the ocular globe  1 h and 15 min
1 h and 15 min
4| Fill a 1 ml syringe with avertin and remove any air bubbles. Inject ~200–300 µl i.p. Injecting slowly and keeping the needle inside the mouse reduces reflux.
 All animal experiments must comply with national laws and institutional regulations.
All animal experiments must comply with national laws and institutional regulations.
5| Once the mouse is killed (usually after 1–2 min), position it under a dissection microscope using one hand to hold open the ocular orbit so that the eye is visible.
6| With the curved forceps (curve facing upwards), apply pressure to the temporal side of the orbit until the ocular globe protrudes. Press down, but do not clamp, and straddle the ocular globe until the optic nerve is accessible. Clamp the curved forceps onto the optic nerve and make a quick motion to the right to enucleate the eye.
7| Immediately transfer the eye to an appropriately labeled well in a tissue culture plate, filled with 4% PFA. Allow the eye to fix for 1 h at room temperature (22.5 °C). After 1 h, wash the eyes two to three times in 1× PBS. Transfer eyes to a well containing 1× PBS for storage.
 Eyes can be stored in 1× PBS at 4 °C for upto 5 d after fixation.
Eyes can be stored in 1× PBS at 4 °C for upto 5 d after fixation.
Retina isolation and vascular staining  2–5 min per retinal isolation vascular staining: 1 d or overnight
2–5 min per retinal isolation vascular staining: 1 d or overnight
8| To isolate the retina, place the fixed eye in a pool of 1× PBS under a dissecting microscope. Hold the base of the optic nerve with one set of dissecting forceps and pinch the cornea with the other set of forceps. Once the cornea is secured, release hold on the optic nerve and obtain a surgical blade with your free hand. Using a surgical blade, make a radial incision through the cornea. Starting at the incision, carefully peel away the sclera toward the optic nerve with forceps. Remove the cornea, the sclera, the optic nerve and RPE, leaving just the retina and lens. Also see supplementary Video 1.
 Do not move forceps too deep (toward the retina or lens) or the retina will tear. This step takes experience and much practice.
Do not move forceps too deep (toward the retina or lens) or the retina will tear. This step takes experience and much practice.
9| Remove the lens from the retina and discard.
10| Using forceps, clean all debris, loose vessels and hyaloid vessels out of the retinal cup.
![]()
11| Begin the staining procedure (below).
 Retinas may be stored for upto 2 d in 1× PBS at 4 °C.
Retinas may be stored for upto 2 d in 1× PBS at 4 °C.
12| (Optional) If the intermediate or deep vascular plexes need to be visualized for the experiment, permeabalize retinas overnight by incubating in 500 µl of 0.5% Triton X-100 at 4 °C. Rinse retinas three times in 1× PBS at room temperature before proceeding.
13| To stain retinal vasculature, remove PBS from the cell culture plate well using a disposable fine tip pipette.
 Use caution not to puncture any retina while removing PBS.
Use caution not to puncture any retina while removing PBS.
14| Immediately add 500 µl lectin solution and cover the plate with aluminum foil to protect from light. Stain retinas in the lectin solution by shaking/rocking at room temperature overnight.
15| Wash the retinas three to four times for 15 + min in 1× PBS.
Retina whole-mount  2 min per retina
2 min per retina
16| Under a dissecting microscope, place the stained retina in a pool of 1× PBS on a microscope slide so that the entire retinal cup is visible. Also see supplementary Video 2.
 Clean any debris or remaining hyaloid vessels out of the retinal cup with smooth, fine tipped, no. 5 dissecting forceps. Artifacts may affect the quality of disease quantification in later steps.
Clean any debris or remaining hyaloid vessels out of the retinal cup with smooth, fine tipped, no. 5 dissecting forceps. Artifacts may affect the quality of disease quantification in later steps.
17| With your forceps in the one hand, secure the retina so that it does not move. Using a surgical blade, make an incision at 3 o’clock. Move from the center of the retina, 1 mm away from the optic nerve, towards the peripheral retina. Apply pressure to make the first incision.
18| Rotate the retina 180°. Repeat incision, from periphery to the center at 3 o’clock.
19| Rotate the retina 90°. Repeat incision.
20| Rotate the retina 180°. Repeat incision.
21| At this point, the retina should have four incisions, dividing it into four equal-sized quadrants. Remove the 1× PBS with a fine tip pipette. Use forceps to ensure that all four quadrants are lying flat on the slide, with the photoreceptor side facing down. Remove any excess debris. Place a drop of SlowFade anti-fade reagent (mounting media) on a coverslip and drop it over the retina.
22| Store the retinas at 4 °C, protected from light.
23| Image the sample.
![]()
Imaging  depends on the microscope
depends on the microscope
24| Images can be obtained using the Zeiss AxioCam MRm, Zeiss AxioObserver.Z1 microscope and AxioVision 4.6.3.0 software. Obtain whole-mount retinal images at ×5 magnification using the mosaiX function (4 rows × 5 columns with 15% overlap). Merge retinal images using the stitching module in AxioVision, crop using the same function and convert to a single image with the convert tile image module. Alternatively, four images can be taken at ×5 on a confocal or fluorescence microscope. These images can be imported and merged together in Adobe Photoshop (or similar software) to produce an image of the entire retina for further analysis.
Quantification of vaso-obliteration and NV  20–50 min per retina depending on the level of NV
20–50 min per retina depending on the level of NV
25| Import retinal image into Adobe Photoshop CS3 or later versions. All detail given from this point forward is specific for Adobe Photoshop CS3 and CS4, though the procedure can be carried out in similar programs with the same accuracy.
26| Using the Polygonal Lasso tool with the ‘add to selection’ function (on the top tool bar) concurrently selected, trace the vascular area of the entire retina (Fig. 2). This tool creates straight lines; clicking the left mouse button allows you to pivot and change direction, to closely follow the vascular boundary. If a mistake is made while tracing the vascular area, the procedure should be repeated from the beginning. Once the vascular area is highlighted, the number of pixels needs to be obtained. In the histogram function, hit the refresh icon (circular arrows in the upper right hand corner) and record the number of pixels in the total retina. The number of pixels is listed at the bottom right corner of the histogram function.
![]()
27| Having selected total retinal area, use the Lasso tool and the ‘subtract from selection’ icon to selectively remove the vascularized retina, leaving behind only the avascular area. The Lasso tool (as opposed to the Polygonal Lasso tool) makes it possible to create freeform borders. We suggest zooming in on one quadrant, in order to maintain precision while tracing along the avascular front. If errors are made during this step, the step-back function may be used to undo the last action. Once the avascular region is selected, click the refresh icon again to obtain the number of pixels in the avascular area (Figs. 2 and 3).
![]()
28| To analyze the selected avascular area, or to keep an image for laboratory records, select the paint bucket tool and click the outlined avascular area. This will fill in the area with a selected color. Click the ‘Save As’ button and rename the image with filled avascular area, so that the original image is not corrupted.
29| If analyzing NV, reopen the original image. Select the magic wand tool from the side tool panel on the left side of the screen. On the top tool panel, set the tolerance to a level that will pick up NV while excluding normal vessels (we suggest beginning at 50). Click the add to selection key and make sure the boxes next to ‘contiguous’ and ‘Anti-alias’ are checked. Select regions of NV by clicking on them using the magic wand tool. The areas of NV will fluoresce more intensely than surrounding normal vessels. The Magic Wand tool functions by selecting areas that are similarly colored and the tolerance determines how similar in color the pixels have to be to be selected. If the NV in the sample is of similar intensity to normal vessels, the tolerance may be adjusted downward to increase the sensitivity to subtle intensity differences (Fig. 3).
![]()
30| Once neovessels are selected, zoom in on the area of interest by holding the ‘Alt’ key on the keyboard and scrolling up. Make sure that the entire area of interest is selected.
 Often, highly fluorescent areas of NV are not selected with the initial quantification. If nonselected shapes appear within selected areas, click the center of these areas with the magic wand tool to add them to the overall selection. If too much area is selected, pressing ‘Control’, ‘Alt’ and ‘Z’ all at the same time will undo the previous step. Alternatively, selecting the edit, ‘undo’ dropdown will accomplish the same backstep.
Often, highly fluorescent areas of NV are not selected with the initial quantification. If nonselected shapes appear within selected areas, click the center of these areas with the magic wand tool to add them to the overall selection. If too much area is selected, pressing ‘Control’, ‘Alt’ and ‘Z’ all at the same time will undo the previous step. Alternatively, selecting the edit, ‘undo’ dropdown will accomplish the same backstep.
31| Once all NV is selected and checked, click the refresh icon and record the total number of pixels in the NV area.
32| To quantify NV at later time points, such as P21 and P25, use the magic wand tool with a lower tolerance. We recommend beginning with a tolerance of 30 and moving down to increase the specificity (Fig. 4).
![]()
Interpretation of data from quantifications  variable
variable
33| To determine the amount of vaso-obliteration, divide the number of pixels in the avascular area (determined above) by the number of pixels in the total retinal area.
34| Determine the amount of NV by dividing the number of pixels of NV by the number of pixels in the total retinal area.
35| To evaluate the relative rate of neovascular regression from P17 to P25, take the percentage of NV that remains unresolved at P21 and P25 and normalize it to the amount of NV at P17. Achieve this by adjusting P17 NV to 100%, indicating maximal NV. Divide NV remaining at P21 and P25 by the NV at P17, revealing the relative degree of NV that remained unresolved at each of those time points. A sufficient n value is required to carry out the regression experiments. Here multiple litters for each experimental group are used to reduce the variability. The slope of this line is the overall rate of neovascular resolution.
36| Assess vessel regrowth in a similar manner. Adjust the amount of vaso-obliteration to 100% at P12, indicating maximal vaso-obliteration. Divide vaso-obliteration remaining at P15 and P17 by the vaso-obliteration at P12, revealing the relative degree of vessel regrowth at each respective time-point.
![]()
Steps 1–3, Oxygen-induced retinopathy: 12–25 d
Steps 4–7, Enucleation and fixation of the ocular globe: 1 h and 15 min
Steps 8–10, Retinal isolation: 2–5 min per retinal isolation (1 d or overnight)
Steps 11–15, Vascular staining: 12–24 h (usually overnight)
Steps 16–23, Retinal whole-mount: 2 min per retina
Step 24, Imaging: depends on the microscope
Steps 25–28, Quantification of vaso-obliteration: 5–10 min
Steps 29–32, Quantification of NV: 10–40 min
Steps 33–36, Interpretation of data: variable
![]()
Step 3: A potential problem is the nursing mother expiring because of oxygen toxicity, having a large litter (10 or more pups) or stopping feeding her pups resulting in growth restricted litters that exhibit an altered vascular phenotype30. Given this, we have found that mice under 6 g at P17 should not be included in the analysis. If the nursing mother expires while in oxygen (generally occurs at P12), a surrogate mother may be used in its place. To reduce the runty phenotype, mothers should nurse no more than eight pups per litter. We have not found that pup depletion affects the reproducibility of the OIR model.
We have found that CO2 levels may get elevated within the hyperoxia chamber. In other models, high CO2 values (>3%) have been shown to have an adverse effect on vascular development, leading to vaso-attenuation. Although we have not seen any significant change in the vascular phenotype in the OIR model when CO2 is elevated, we recommend attempting to maintain CO2 values below 3%. 50 g of soda lime may be placed at the bottom of the chamber to serve as a quencher.
Step 10: In the case of young eyes (postnatal day 12 and younger), we recommend fixing with PFA for 10 min on ice, isolating the retina and fixing in PFA for an additional 30 min before storing in PBS. This may reduce the adhesiveness of the RPE.
Step 23: Lectin tends to diffuse from vessels if the image is not acquired within 48 h, creating a fuzzy image and unreliable quantification in the following steps.
Step 26: If the retina is punctured during the dissection/flat mounting or staining steps, this puncture will affect the total retinal area. The punctured area should be excluded during this step using the lasso tool and ‘subtract from’ function (see Fig. 2 for details on these settings). Once all punctured regions are removed, click the refresh arrow icon in the histogram and record the total number of pixels in the retina. If three or more punctures are present in any given retina, that sample should be excluded from the data set to avoid inaccurate and inconsistent quantifications.
Step 27: For precision, the selected avascular area can be modified by clicking the appropriate ‘add to selection’ or ‘subtract from selection’ icons. For instance, if the boundary is not close enough to the vascular front, select the ‘add to selection’ function and trace the vascular boundary more closely. Conversely, if the boundary drawn is overlapping with the vascular region, select the ‘subtract from selection’ function and trace a new boundary along the vascular front.
Step 29: If unwanted (non-neovascular) areas are selected, adjust the tolerance to 30, 20 or 10. This increases the sensitivity of the selection, making it more precise (see Fig. 4). If excess (non-neovascular) NV is selected, use the dropdown ‘Edit’ menu, and the Step back function, to undo the previous selection(s). This may be done alternatively by holding the ‘Control’, ‘Alt’ and ‘Z’ keys simultaneously.
Step 32: Use care not to confuse background or precipitates for regressing tufts. Zoom in on the area of interest and make sure NV is connected to normal vessels. See example (white arrows, Fig. 4e).
ANTICIPATED RESULTS
The OIR model is a widely used and established way of studying vascular loss, vessel regrowth, neovascular tuft formation and vascular regression, as seen in ROP and diabetic retinopathy. Here, we outline the preparation of retinal whole-mounts and delineate the method by which we quantify vaso-obliteration, vessel regrowth, NV and neovascular regression. Vaso-obliteration occurs in response to high (75%) oxygen exposure (Fig. 2a). Maximum vaso-obliteration is quantified at P12 by comparing the number of pixels in the total retina (Fig. 2b,c) with the number of pixels in the avascular area (Fig. 3d,e). Vessel regrowth is then assessed by quantifying the avascular area of the retina at P17 (Fig. 3b,c), normalizing control and test groups to their respective maximum VO values from P12, and calculating the percentage of regrowth (Step 36). Neo-vascularization forms from P13 through P17 in response to hypoxic conditions in the retina (Fig. 3a). Neo-vascularization is quantified at P17, when pathological response is at a maximum. To obtain the quantitative level of NV, NV tufts are highlighted, giving the number of pixels in the NV area (Fig. 3d,e). This value is divided by the number of pixels in the total retinal area (Fig. 3f) to give the percentage of NV. Tuft regression occurs between P18 and P25 and may be quantified at various time points during this process. In order to determine the rate of NV regression, the percentage of NV that remains unresolved at P21 and P25 is normalized to maximum NV as calculated at P17 (see Step 35; Fig. 4).
Supplementary Material
ACKNOWLEDGMENTS
This research was generously supported by the V. Kann Rasmussen Foundation, the US National Institutes of Health (EY008670, EY017017, EY14811 (L.E.H.S.); 5 T32 EY07145, 1 F32 EY017789-01 (K.M.C.)), William Randolph Hearst Fund (K.M.C.) and Children’s Hospital Boston Mental Retardation and Developmental Disabilities Research Center, P01 HD18655 (L.E.H.S.). Support from the Research to Prevent Blindness Lew Wasserman Merit Award (L.E.H.S). Juvenile Diabetes Research Foundation International (3-2006-278, 10-2008-603 (J.C.). The sponsors had no role in the design or conduct of the study, in the collection, analysis and interpretation of data and in the preparation, review or approval of the manuscript.
Footnotes
Note: Supplementary information is available via the HTML version of this article.
AUTHOR CONTRIBUTIONS K.M.C. wrote the manuscript with N.M.K., R.J.D. and L.E.H.S. with input from C.M.A., J.C., K.G., P.S., A.S. and K.L.W. All authors have worked on and modernized this protocol.
References
- 1.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith LE, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 1999;5:1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 3.Smith LE, et al. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276:1706–1709. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- 4.Connor KM, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer EA, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991;98:1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 6.Madan A, Penn JS. Animal models of oxygen-induced retinopathy. Front Biosci. 2003;8:d1030–d1043. doi: 10.2741/1056. [DOI] [PubMed] [Google Scholar]
- 7.Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 8.Ashton N. Animal experiments in retrolental fibroplasia. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1954;58:51–53. discussion, 53–54. [PubMed] [Google Scholar]
- 9.Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. Br. J. Ophthalmol. 1953;37:513–520. doi: 10.1136/bjo.37.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penn JS, Tolman BL, Henry MM. Oxygen-induced retinopathy in the rat: relationship of retinal nonperfusion to subsequent neovascularization. Invest. Ophthalmol. Vis. Sci. 1994;35:3429–3435. [PubMed] [Google Scholar]
- 11.Flower RW. Perinatal ocular physiology and ROP in the experimental animal model. Doc. Ophthalmol. 1990;74:153–162. doi: 10.1007/BF02482604. [DOI] [PubMed] [Google Scholar]
- 12.Flower RW, McLeod DS, Lutty GA, Goldberg B, Wajer SD. Postnatal retinal vascular development of the puppy. Invest. Ophthalmol. Vis. Sci. 1985;26:957–968. [PubMed] [Google Scholar]
- 13.Mammoto A, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellstrom M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 15.Kubota Y, Hirashima M, Kishi K, Stewart CL, Suda T. Leukemia inhibitory factor regulates microvessel density by modulating oxygen-dependent VEGF expression in mice. J. Clin. Invest. 2008;118:2393–2403. doi: 10.1172/JCI34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Checchin D, et al. Hypercapnia prevents neovascularization via nitrative stress. Free Radic. Biol. Med. 2006;40:543–553. doi: 10.1016/j.freeradbiomed.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Brault S, et al. Selective neuromicrovascular endothelial cell death by 8-Iso-prostaglandin F2alpha: possible role in ischemic brain injury. Stroke. 2003;34:776–782. doi: 10.1161/01.STR.0000055763.76479.E6. [DOI] [PubMed] [Google Scholar]
- 18.Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1105–R1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- 19.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 20.Gardiner TA, et al. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am. J. Pathol. 2005;166:637–644. doi: 10.1016/s0002-9440(10)62284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, et al. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest. Ophthalmol. Vis. Sci. 2009;50:1329–1335. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aiello LP, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins RD, et al. Diltiazem reduces retinal neovascularization in a mouse model of oxygen induced retinopathy. Curr. Eye Res. 1999;18:20–27. doi: 10.1076/ceyr.18.1.20.5390. [DOI] [PubMed] [Google Scholar]
- 24.Lange C, et al. Intravitreal injection of the heparin analog 5-amino-2-naphthalenesulfonate reduces retinal neovascularization in mice. Exp. Eye Res. 2007;85:323–327. doi: 10.1016/j.exer.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Ritter MR, et al. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J. Clin. Invest. 2006;116:3266–3276. doi: 10.1172/JCI29683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies MH, Stempel AJ, Powers MR. MCP-1 deficiency delays regression of pathologic retinal neovascularization in a model of ischemic retinopathy. Invest. Ophthalmol. Vis. Sci. 2008;49:4195–4202. doi: 10.1167/iovs.07-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorrell MI, Aguilar E, Scheppke L, Barnett FH, Friedlander M. Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc. Natl. Acad. Sci. USA. 2007;104:967–972. doi: 10.1073/pnas.0607542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lofqvist C, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc. Natl. Acad. Sci. USA. 2007;104:10589–10594. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan CK, et al. Differential expression of pro- and antiangiogenic factors in mouse strain-dependent hypoxia-induced retinal neovascularization. Lab. Invest. 2005;85:721–733. doi: 10.1038/labinvest.3700277. [DOI] [PubMed] [Google Scholar]
- 30.Vanhaesebrouck S, et al. Oxygen-induced retinopathy in mice: amplification by neonatal IGF-I deficit and attenuation by IGF-I administration. Pediatr. Res. 2009;65:307–310. doi: 10.1203/PDR.0b013e3181973dc8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.