Abstract
OBJECTIVES
To describe vaporized hydrogen peroxide (VHP) as an adjuvant in the control of multidrug-resistant (MDR) Acinetobacter baumannii infection in a long-term acute care hospital (LTACH) and to describe the risk factors for acquisition of MDR A. baumannii infection in the LTACH population.
DESIGN
Outbreak investigation, case-control study, and before-after intervention trial.
SETTING
A 54-bed LTACH affiliated with a tertiary care center in northeastern Ohio.
METHODS
Investigation of outbreak with clinical and environmental cultures, antimicrobial susceptibility testing, polymerase chain reaction assay of repetitive chromosomal elements to type strains, and case-control study; and intervention consisting of comprehensive infection control measures and VHP environmental decontamination.
RESULTS
Thirteen patients infected or colonized with MDR A. baumannii were identified from January 2008 through June 2008. By susceptibility testing, 10 (77%) of the 13 isolates were carbapenem-resistant. MDR A. baumannii was found in wound samples, blood, sputum, and urine. Wounds were identified as a risk factor for MDR A. baumannii colonization. Ventilator–associated pneumonia was the most common clinical syndrome caused by the pathogen, and the associated mortality was 14% (2 of the 13 case patients died). MDR A. baumannii was found in 8 of 93 environmental samples, including patient rooms and a wound care cart; environmental and clinical cultures were genetically related. Environmental cultures were negative immediately after VHP decontamination and both 24 hours and 1 week after VHP decontamination. Nosocomial acquisition of the pathogen in the LTACH ceased after VHP intervention. When patients colonized with MDR A. baumannii reoccupied rooms, environmental contamination recurred.
CONCLUSION
Environmental decontamination using VHP combined with comprehensive infection control measures interrupted nosocomial transmission of MDR A. baumannii in an LTACH. The application of this novel approach to halt the transmission of MDR A. baumannii warrants further investigation.
Multidrug-resistant (MDR) Acinetobacter baumannii is an increasingly common pathogen in healthcare settings.1 This pathogen is a significant threat to hospitalized patients, especially those supported with mechanical ventilation, receiving longstanding antibiotic therapy, or having chronic wounds.2 MDR A. baumannii is also difficult to eradicate from the environment, surviving for up to several weeks on hospital surfaces.3,4 Environmental persistence of MDR A. baumannii is thought to facilitate horizontal transmission by means of the hands of healthcare workers after they touch colonized patients and contaminated medical equipment and/or contaminated environment.5 Consequently, MDRA. baumannii causes outbreaks that lead to lengthy patient hospitalizations and increased mortality, making it a consummate healthcare pathogen.6 The widespread emergence of resistance to carbapenems among MDR A. baumannii isolates makes its successful control a public health priority.7
The use of vaporized hydrogen peroxide (VHP) to disinfect a sealed patient care area represents a new approach to tackling environmental contamination within healthcare settings.8–10 The infusion of dry vapor is delivered by means of a portable unit, and disinfection is achieved by maintaining a targeted hydrogen peroxide concentration within the area. At the end of a preprogrammed cycle, the hydrogen peroxide reduces to innocuous water and oxygen. VHP has been previously used to interrupt outbreaks caused by methicillin- resistant Staphylococcus aureus,11 although contamination of surfaces recurred when patients were reintroduced to the environment.12
We describe an outbreak of MDR A. baumannii infection in a long-term acute care hospital (LTACH) in which VHP, in combination with comprehensive infection control measures, was used to break the cycle of nosocomial transmission. We highlight the risk factors for acquisition of MDR A. baumannii and the importance of environmental cleaning in outbreak control and prevention.
METHODS
Setting
The University Hospitals Extended Care Campus is a healthcare facility in suburban Cleveland, Ohio. The patient care unit consists of a 54-bed LTACH (divided into 2 wards with mostly single-occupancy rooms). This facility also houses a skilled nursing unit, an assisted living unit, and a dementia care unit. The outbreak occurred in the LTACH wards only.
Epidemiologic Investigation
In late January 2008, a cluster of 3 cases of MDR A. baumannii infection was noted by infection control personnel. The cases were located within a single ward, and the clinical isolates appeared phenotypically similar by antibiotic susceptibilities. On February 11, 2008, the administration was alerted to a possible outbreak, and an outbreak investigation was initiated. Infection control staff conducted weekly meetings with leadership among medical, nursing, environmental services, respiratory, and physical therapy personnel. The initial focus was tightening of basic infection control strategies, such as hand hygiene, environmental cleaning, and adherence to use of personal protective equipment. Although pulsatile wound lavage activity was infrequent and was not included in the care plan of the 3 initial case patients, it was suspended in February because it may aerosolize MDR A. baumannii.13 In February, only 1 new case was identified; however, 2 cases occurred in early March, prompting a point prevalence survey of all patients on the affected ward who were not known to be colonized or infected with MDR A. baumannii on March 25; the point prevalence survey identified 3 additional patients. Isolation of case patients was prolonged and included droplet precautions in addition to contact precautions, when indicated. The affected ward was closed to admissions on April 7, and an environmental survey was performed. The use of VHP for decontamination of rooms began April 30, 2008, after staff had been trained in the VHP technology.
Multidrug resistance was defined as resistance to more than 3 classes of antimicrobial agents. A case patient was defined as a person who was found to be infected or colonized with MDR A. baumannii more than 48 hours after admission to the University Hospitals Extended Care Campus, from January 1 through June 30, 2008. Patients known to be colonized or infected with MDR A. baumannii within the preceding year were excluded.
Microbiologic Methods
In addition to bacterial cultures of clinical samples (ie, blood, urine, sputum, and wound samples) obtained as part of routine medical care, point prevalence surveillance cultures were performed on samples obtained from wounds, respiratory tracts, and perirectal areas of patients to investigate undetected colonization with MDR A. baumannii. An environmental point prevalence study was performed on the affected wards to detect inanimate reservoirs of MDR A. baumannii. Specimens from the patient rooms were obtained by sampling the call bell, bedside table, and bed rails with cotton-tipped swabs moistened with sterile saline. Similarly, samples were collected from common areas of the wards, such as charts, computer keyboards, and medication carts. Swab samples were then inoculated onto MacConkey agar and incubated overnight at 37°C. Non–lactose-fermenting colonies from the MacConkey plates were subcultured and identified.
Bacterial species and antibiotic susceptibilities were determined with the MicroScan system (Siemens Healthcare Diagnostics) using conventional breakpoint gram-negative trays. Antibiotic susceptibilities were interpreted in accordance with criteria of the Clinical and Laboratory Standards Institute.14 Isolates with reduced susceptibility (intermediate or resistant) to at least 3 antibiotic classes (eg, extended-spectrum cephalosporins, aminoglycosides, and quinolones) were defined as MDR.
To determine the genetic relatedness among MDR A. baumannii isolates, bacterial DNA extracted from each isolate (UltraClean Microbial DNA Isolation Kit; Mo Bio Laboratories) was subjected to genotyping using the automated DiversiLab system (bioMérieux), which is based on polymerase chain reaction assay of repetitive chromosomal elements (rep- PCR). Rep-PCR was performed using the DiversiLab Acinetobacter kit. A 2100 Bioanalyzer (Agilent Technologies) was used to separate and to detect DNA fragments. Results were analyzed with the web-based DiversiLab software, version 3.3 (bioMérieux), using the modified Kullback-Leibler statistical method to generate a dendrogram, as previously demonstrated for A. baumannii.15,16 Isolates that clustered more than 95% were considered to be related.
VHP Decontamination
After terminal cleaning to remove organic and porous materials, VaproSure (Steris) technology was used for room decontamination. VHP was generated to reach a peak concentration of 240 ppm for 90 minutes, and the mean total cycle duration was 8 hours. Chemical and biological indicators were used for quality assurance. Hydrogen peroxide vapor was not detected by handheld sensors outside of the contained areas. Each patient room in the LTACH underwent processing once with this method.
Case-Control Study
A case-control study was conducted to assess risk factors for acquisition of the outbreak strain of MDR A. baumannii. Control subjects were randomly selected from LTACH patients hospitalized from January 1 through June 30, 2008. The control subjects hospitalized during the point prevalence survey had negative culture results for MDR A. baumannii. Neither case patients nor control subjects had a history of A. baumannii infection or colonization within the preceding year. The ratio of control subjects to case patients was 2 : 1. All control subjects had a least 1 culture of a clinical sample of urine, sputum, wound, or blood during the study period. Control subjects were frequency matched to case patients by ward assignment.
Data were collected about the study patients by reviewing medical records after institutional review board approval. The following variables were examined for each study patient: age; sex; length of acute care stay before LTACH admission; presence of a tracheostomy, feeding tube, urinary catheter, and/ or central venous line on admission; end-stage renal disease; diabetes; admission pre-albumin level; intravenous antibiotics on admission; transmission-based precautions on admission; presence of wounds; participation in group therapy during LTACH stay; and respiratory care during LTACH stay, including tracheostomy care, mechanical ventilation, and nebulized medications.17,18
Statistical Analysis
The primary outcome was MDR A. baumannii acquisition. Descriptive statistics were performed using 2-tailed Student t tests for continuous variables and χ2 tests for dichotomous variables. Univariate analyses were conducted to evaluate associations. All statistical analyses were performed using SPSS software, version 17.0 (SPSS).
RESULTS
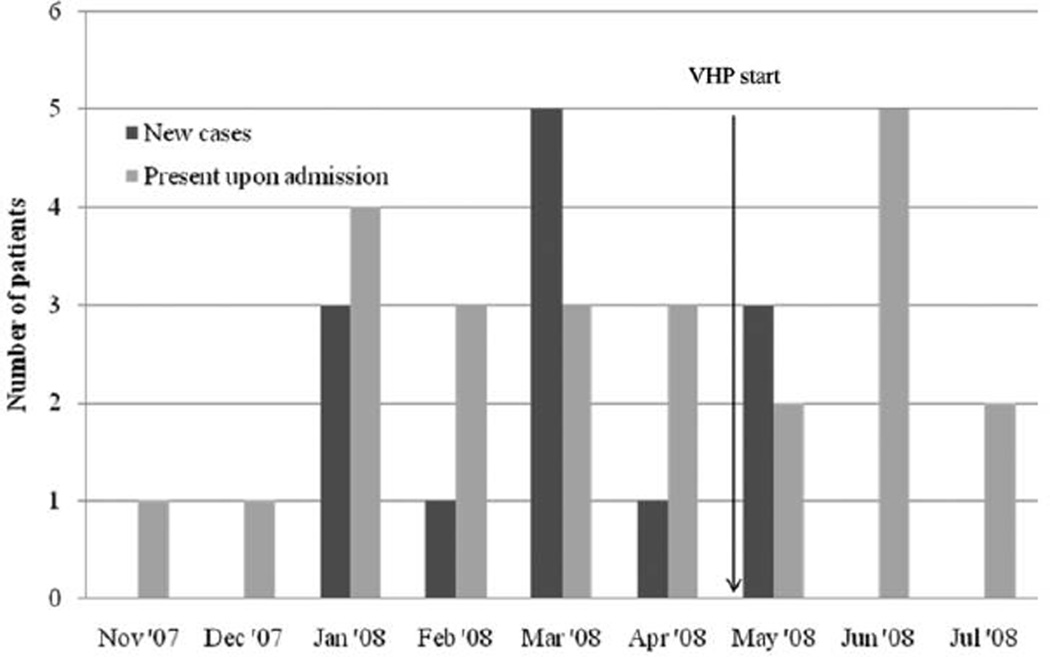
A total of 13 case patients infected or colonized with MDR A. baumannii were identified from January through June 2008 (Figure 1). This number includes the 3 original patients identified in January 2008 and 10 other patients who were identified during the investigation of the outbreak, even after reinforcement of basic infection control protocols. Five case patients developed ventilator–associated pneumonia, and 1 case patient developed a bloodstream infection. Death resulted from MDR A. baumannii infection in 2 patients with pneumonia (14% of the total 13 case patients). Respiratory (n = 5), wound (n = 1), or urinary tract (n = 1) colonization was present in the 7 remaining case patients. The pathogens were predominantly resistant to carbapenems (10 [77%]), ceftazidime (12 [92%]), tobramycin (10 [77%]), and ampicillin-sulbactam (11 [85%]), and all were resistant to ciprofloxacin and cefepime.
Figure 1.
Epidemiologic curve of new cases and patients hospitalized with known multidrug-resistant Acinetobacter baumannii colonization or infection on admission to the long-term acute care hospital wards of University Hospitals Extended Care Campus in northeastern Ohio, from November 2007 through July 2008.
In response to the increase in cases in March 2008, the affected LTACH ward was closed and environmental samples were collected and cultured for Acinetobacter species. Among 93 environmental samples collected, 11 A. baumannii isolates were obtained, 8 of which were MDR (all carbapenem resistant). Seven of the 8 isolates were recovered from patient rooms (bed rail, bedside table, or call bell), and the remaining isolate was obtained from a wound care cart, suggesting that high-touch objects in the patients’ immediate environment were contaminated with the pathogen. Molecular typing by rep-PCR demonstrated more than 95% similarity among environmental and patient isolates (with 1 exception), indicating that they were genetically related.
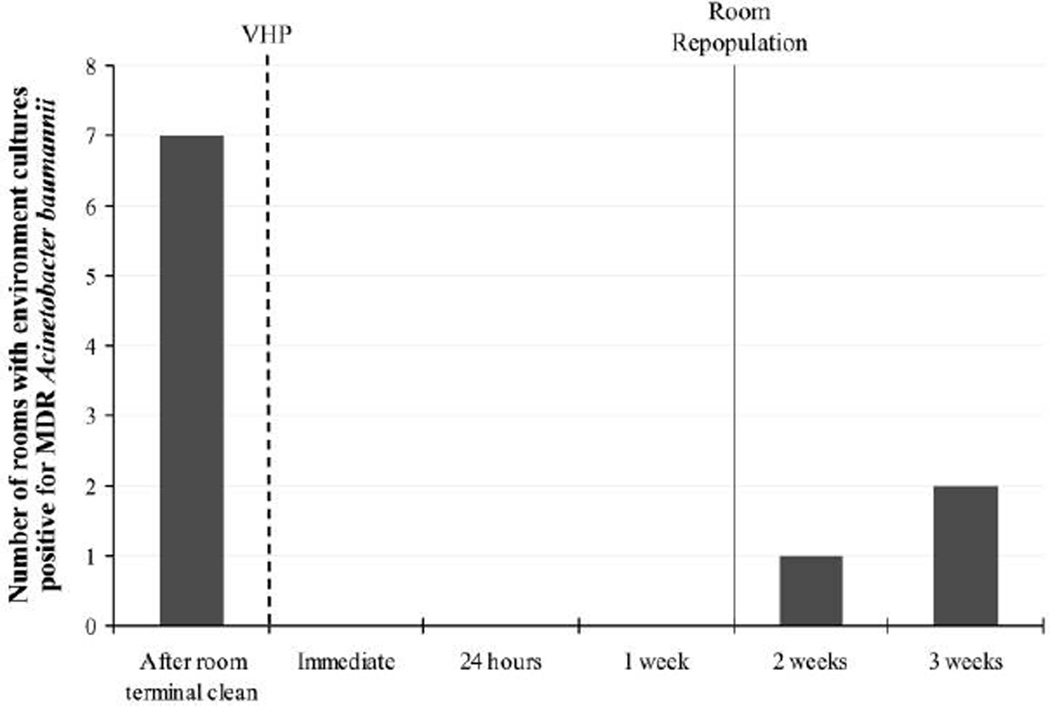
To eliminate the pathogen from the patient environment, the affected patient rooms and the wound care cart were disinfected using VHP for 8 hours. Environmental sampling was performed after room terminal clean as a baseline and repeated immediately after treatment with VHP and at 4 time points thereafter: at 24 hours, and at 1, 2, and 3 weeks (Figure 2). The environmental specimens yielded MDR A. baumannii 1 week after patient repopulation (2 weeks after decontamination). Of the 3 rooms that became recontaminated, only 1 housed a patient with known colonization. After the completion of VHP room decontamination in the LTACH wards, no further cases of nosocomial acquisition of MDR A. baumannii colonization or infection were detected clinically through the end of 2009. When the ward was reopened, active surveillance was performed routinely on admission to identify incoming colonized patients (samples from wounds, respiratory tracts, and perirectal areas were obtained and cultured).
Figure 2.
Results of environmental microbiologic sampling before and after vaporized hydrogen peroxide (VHP) sterilization of patient rooms. Time of sterilization is indicated by the dotted line and of room repopulation by the solid line, with sampling intervals after VHP use indicated on the x-axis.
Risk factors for nosocomial acquisition of MDR A. baumannii were identified by a case-control study. The 13 case patients were frequency matched to control subjects (n = 27) by ward location. The case patients and control subjects were similar with respect to age, sex, pre-albumin level, and presence of a feeding tube, central line, and urinary catheter (Table 1). Case patients were more likely to have wounds (odds ratio [OR], 12.92; P = .01), to have tracheostomy tubes (OR, 9.60; P = .03), and to have received intravenous antibiotics on admission to the LTACH (OR, 6.86; P = .04). Transmission-based precautions (gowns, gloves, and masks when appropriate) designed to prevent the spread of drug-resistant organisms from patient to patient, were more common on admission with regard to case patients (OR, 5.6; P = .03). Case patients did not differ significantly from control subjects with respect to attendance of group therapy during LTACH stay or the diagnoses of diabetes or end-stage renal disease. The length of hospitalization preceding the LTACH stay was significantly longer for the case patients (34 vs 22 days; P = .02)
TABLE 1.
Comparison of Risk Factors for Infection or Colonization with Multidrug-Resistant Acinetobacter baumannii in a Long-Term Acute Care Hospital (LTACH)
| Risk factor | Case patients (n = 13) |
Control subjects (n = 27) |
OR (95% CI) | P |
|---|---|---|---|---|
| Age, mean, years | 70.4 | 69.7 | … | .89 |
| Length of stay in LTACH, mean, days | 34.2 | 21.6 | … | .02 |
| Pre-albumin level, mean, mg/L | 20.2 | 19.9 | … | .92 |
| Female sex | 4 (31) | 11 (41) | 1.54 (0.38–6.31) | .73 |
| Tracheostomy | 12 (92) | 15 (56) | 9.60 (1.09–84.64) | .03 |
| Feeding tube | 11 (85) | 17 (63) | 3.24 (0.59–17.66) | .27 |
| Central line(s) | 10 (77) | 15 (56) | 2.67 (0.59–11.92) | .30 |
| Urinary catheter | 12 (92) | 18 (67) | 5.33 (0.59–48.30) | .23 |
| Diabetes | 7 (54) | 10 (37) | 4.82 (1.43–16.23) | .50 |
| End-stage renal disease | 1 (8) | 4 (15) | 0.48 (0.048–4.78) | >.99 |
| Respiratory treatments | 13 (100) | 20 (74) | … | |
| Group therapy | 3 (23) | 13 (48) | 0.32 (0.073–1.44) | .18 |
| Transmission-based precautions | 8 (62) | 6 (22) | 5.6 (1.33–23.62) | .03 |
| Intravenous antibiotics | 6 (46) | 3 (11) | 6.86 (1.35–34.71) | .04 |
| Wounds | 12 (92) | 13 (48) | 12.92 (1.47–113.78) | .01 |
NOTE. CI, confidence interval; OR, odds ratio.
DISCUSSION
This outbreak of MDR A. baumannii colonization and infection in an LTACH was controlled by patient room decontamination with VHP, in addition to comprehensive infection control measures. The outbreak affected 13 patients and resulted in 2 deaths. Although this outbreak involved a small number of patients, it is notable because it affected patients in a nontraditional setting (LTACH), involved organisms resistant to multiple antibiotics—including carbapenems, and resulted in substantial mortality. To our knowledge, this is the first study to demonstrate elimination of MDR A. baumannii from the environment of an LTACH using VHP, potentially contributing to the interruption of nosocomial transmission of the pathogen.
Importantly, this outbreak afforded the opportunity to specifically identify risk factors that contributed to the pathogen’s acquisition in an LTACH. Given the expanding role of LTACHs in caring for the chronically critically ill,19 identification of risk factors is of interest, especially because of the typically poor outcomes,20 complex comorbidities,21 and reported high prevalence of MDR A. baumannii colonization22–24 of this population. Results of this case-control study suggest an association between MDR A. baumannii colonization and the presence of wounds, tracheostomy, and intravenous antibiotic treatment, as well as prolonged hospitalization preceding transfer to LTACH and transmission-based precautions on admission to LTACH (Table 1). Although MDR A. baumannii survives well in wounds,2,9 wounds have seldom been reported as a risk factor for colonization with MDR A. baumannii, except for trauma and burn patients. It is of interest that in the University Hospitals Extended Care Campus LTACH, wounds are managed by a specialized wound care team with dedicated personnel and equipment. The wound care cart was the only common-area environmental object from which the pathogen was cultured. Analysis of MDR A. baumannii isolates recovered from affected patients and from the environment (including the wound care cart) indicated that they were genetically related. This strongly suggests the presence of an environmental reservoir that enhanced horizontal transmission of the pathogen. These findings highlight the importance of educating and monitoring of all ancillary staff on appropriate infection control measures, such as hand hygiene, use of personal protective equipment, and proper environmental decontamination. Promotion of standard infection control measures has successfully controlled the spread of MDR A. baumannii in intensive care units and hospitals.25,26
Bedrails, bedside tables, and other high-touch objects in hospital rooms are classified as “noncritical,” and as such they warrant only routine low-level disinfection.27 The standard decontamination of such items is the manual application of a liquid disinfectant, such as a quaternary ammonium compound, and this practice was routinely followed at the University Hospitals Extended Care Campus LTACH. However, conventional manual cleaning with disinfectants has been reported to leave 10% of sites contaminated,28 and adherence to recommended disinfectant application procedures is challenging. Evidence of contaminated environmental surfaces contributing to transmission of Acinetobacter species is emerging.5 Thus, in addition to strict adherence to hand hygiene and transmission-based precautions, development of improved decontamination strategies, including new technologies such as VHP, is desirable. VHP is favorable in part because of its portability, low vapor temperature, and lack of harmful residue.8 VHP decontamination does not afford any residual biocidal effect. Therefore, it is not surprising that we documented recontamination of hospital rooms with MDR A. baumannii after they were reoccupied.
We did not perform routine surveillance cultures on admission to the LTACH during this outbreak investigation but rather a point prevalence study. Therefore, we may have underestimated the number of patients who harbored MDR A. baumannii before LTACH admission and who did not have documented colonization. Many of these patients were already in transmission-based precautions on admission to the LTACH, indicating that they may have been colonized or infected with MDR organisms (including MDR A. baumannii) at the referring institutions. Although the presence of transmission-based precautions on admission intuitively may be thought to be protective against acquisition of MDR A. baumannii, the case-control study did not support this hypothesis. This suggests alternative hypotheses for MDR A. baumannii acquisition, such as isolation as a marker of increased severity of illness and thus increased risk, poor adherence to isolation precautions, or other factors, such as previously undetected MDR A. baumannii colonization. Also, closure of the affected LTACH ward reduced opportunities for transmission because the remaining patient population dwindled, which may have confounded the effect of VHP. Efforts are needed to identify patients who acquire and who are at risk of acquiring MDR A. baumannii before their transfer to LTACHs, to limit the impact of such organisms in this setting. In addition, antibiotic use patterns among LTACH patients with MDR A. baumannii need to be further delineated to discern the role of antibiotic exposure in the acquisition of MDR A. baumannii. Most importantly, future evaluation of strategies incorporating environmental decontamination with VHP necessitates prospective and controlled studies, ideally a cluster-randomized trial.
In summary, this report describes an outbreak of MDR and carbapenem-resistant A. baumannii colonization and infection occurring in an LTACH. Wounds were found to be an important risk factor for acquisition of the pathogen, and a wound care cart served as a common environmental source. Comprehensive infection control efforts, combined with environmental decontamination with VHP, helped to control this outbreak. Given the increasing importance of LTACHs in our healthcare system, preventing the transmission of nosocomial pathogens in this setting is urgent. Because patients are often transferred between different facilities and between levels of care, interrupting the cycle of transmission within LTACHs may have an impact on healthcare-associated infections caused by MDR A. baumannii in other settings as well.
ACKNOWLEDGMENTS
We thank the administration and staff of University Hospitals Extended Care Campus for their continued commitment to excellent care and infection prevention.
Financial support. Veterans Administration Research and Development Service (Merit Review Award to R.A.B.); National Institute of Allergy and Infectious Diseases (AI072219, AI063517, and AI081036 to R.A.B.); Steris (to F.P. and R.A.B.); Case Western Reserve University (Wyeth Fellowship in Antimicrobial Resistance to F.P.).
Footnotes
Potential conflicts of interest. A.R. reports that she is on the speakers’ bureau of Steris. All other authors report no conflicts of interest relevant to this article.
Presented in part: 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Meeting of the Infectious Diseases Society of America; Washington, DC; October 2008.
REFERENCES
- 1.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial- resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 2.Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358(12):1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 3.Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol. 1998;36(7):1938–1941. doi: 10.1128/jcm.36.7.1938-1941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wendt C, Dietze B, Dietz E, Ruden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35(6):1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol. 2003;24(4):284–295. doi: 10.1086/502205. [DOI] [PubMed] [Google Scholar]
- 6.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51(10):3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otter JA, Cummins M, Ahmad F, van Tonder C, Drabu YJ. Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapour decontamination. J Hosp Infect. 2007;67(2):182–188. doi: 10.1016/j.jhin.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Kahnert A, Seiler P, Stein M, Aze B, McDonnell G, Kaufmann SH. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis. Lett Appl Microbiol. 2005;40(6):448–452. doi: 10.1111/j.1472-765X.2005.01683.x. [DOI] [PubMed] [Google Scholar]
- 10.Otter JA, Puchowicz M, Ryan D, et al. Feasibility of routinely using hydrogen peroxide vapor to decontaminate rooms in a busy United States hospital. Infect Control Hosp Epidemiol. 2009;30(6):574–577. doi: 10.1086/597544. [DOI] [PubMed] [Google Scholar]
- 11.Blythe D, Keenlyside D, Dawson SJ, Galloway A. Environmental contamination due to methicillin-resistant Staphylococcus aureus (MRSA) J Hosp Infect. 1998;38(1):67–69. doi: 10.1016/s0195-6701(98)90176-1. [DOI] [PubMed] [Google Scholar]
- 12.Hardy KJ, Gossain S, Henderson N, et al. Rapid recontamination with MRSA of the environment of an intensive care unit after decontamination with hydrogen peroxide vapour. J Hosp Infect. 2007;66(4):360–368. doi: 10.1016/j.jhin.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Maragakis LL, Cosgrove SE, Song X, et al. An outbreak of multidrugresistant Acinetobacter baumannii associated with pulsatile lavage wound treatment. JAMA. 2004;292(24):3006–3011. doi: 10.1001/jama.292.24.3006. [DOI] [PubMed] [Google Scholar]
- 14.Clinical Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing: 18th Informational Supplement. Wayne, PA: CLSI; 2008. M100-S18. [Google Scholar]
- 15.Saeed S, Fakih MG, Riederer K, Shah AR, Khatib R. Interinstitutional and intrainstitutional transmission of a strain of Acinetobacter baumannii detected by molecular analysis: comparison of pulsed-field gel electrophoresis and repetitive sequence-based polymerase chain reaction. Infect Control Hosp Epidemiol. 2006;27(9):981–983. doi: 10.1086/507286. [DOI] [PubMed] [Google Scholar]
- 16.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem- resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65(2):233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 17.Hamill RJ, Houston ED, Georghiou PR, et al. An outbreak of Burkholderia (formerly Pseudomonas) cepacia respiratory tract colonization and infection associated with nebulized albuterol therapy. Ann Intern Med. 1995;122(10):762–766. doi: 10.7326/0003-4819-122-10-199505150-00005. [DOI] [PubMed] [Google Scholar]
- 18.Wexler MR, Rhame FS, Blumenthal MN, Cameron SB, Juni BA, Fish LA. Transmission of gram-negative bacilli to asthmatic children via home nebulizers. Ann Allergy. 1991;66(3):267–271. [PubMed] [Google Scholar]
- 19.Medicare Payment Advisory Commission. Medpac home page. [Accessed March 9, 2010]; http://www.medpac.gov. Published 2003.
- 20.Carson SS, Bach PB, Brzozowski L, Leff A. Outcomes after long-term acute care: an analysis of 133 mechanically ventilated patients. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1568–1573. doi: 10.1164/ajrccm.159.5.9809002. [DOI] [PubMed] [Google Scholar]
- 21.Munoz-Price LS. Long-term acute care hospitals. Clin Infect Dis. 2009;49(3):438–443. doi: 10.1086/600391. [DOI] [PubMed] [Google Scholar]
- 22.Furuno JP, Hebden JN, Standiford HC, et al. Prevalence of methicillinresistant Staphylococcus aureus Acinetobacter baumannii in a long-term acute care facility. Am J Infect Control. 2008;36(7):468–471. doi: 10.1016/j.ajic.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephens C, Francis SJ, Abell V, DiPersio JR, Wells P. Emergence of resistant Acinetobacter baumannii in critically ill patients within an acute care teaching hospital and a long-term acute care hospital. Am J Infect Control. 2007;35(4):212–215. doi: 10.1016/j.ajic.2006.04.208. [DOI] [PubMed] [Google Scholar]
- 24.Lolans K, Rice TW, Munoz-Price LS, Quinn JP. Multicity outbreak of carbapenem-resistant Acinetobacter baumannii isolates producing the carbapenemase OXA-40. Antimicrob Agents Chemother. 2006;50(9):2941–2945. doi: 10.1128/AAC.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Baño J, Garcia L, Ramirez E, et al. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive“bundle” approach. Am J Infect Control. 2009;37(9):715–722. doi: 10.1016/j.ajic.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apisarnthanarak A, Pinitchai U, Thongphubeth K, Yuekyen C, Warren DK, Fraser VJ. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a Thai tertiary care center: a 3-year study. Clin Infect Dis. 2008;47(6):760–767. doi: 10.1086/591134. [DOI] [PubMed] [Google Scholar]
- 27.Rutala WA. APIC guideline for selection and use of disinfectants. 1994, 1995, and 1996 APIC Guidelines Committee. Association for Professionals in Infection Control and Epidemiology, Inc. Am J Infect Control. 1996;24(4):313–342. doi: 10.1016/s0196-6553(96)90066-8. [DOI] [PubMed] [Google Scholar]
- 28.Wisplinghoff H, Schmitt R, Wohrmann A, Stefanik D, Seifert H. Resistance to disinfectants in epidemiologically defined clinical isolates of Acinetobacter baumannii. J Hosp Infect. 2007;66(2):174–181. doi: 10.1016/j.jhin.2007.02.016. [DOI] [PubMed] [Google Scholar]