Abstract
Purpose
To describe the long-term (≥ 10 years) benefits of clinical human papillomavirus (HPV) DNA testing for cervical precancer and cancer risk prediction.
Methods
Cervicovaginal lavages collected from 19,512 women attending a health maintenance program were retrospectively tested for HPV using a clinical test. HPV positives were tested for HPV16 and HPV18 individually using a research test. A Papanicolaou (Pap) result classified as atypical squamous cells of undetermined significance (ASC-US) or more severe was considered abnormal. Women underwent follow-up prospectively with routine annual Pap testing up to 18 years. Cumulative incidence rates (CIRs) of ≥ grade 3 cervical intraepithelial neoplasia (CIN3+) or cancer for enrollment test results were calculated.
Results
A baseline negative HPV test provided greater reassurance against CIN3+ over the 18-year follow-up than a normal Pap (CIR, 0.90% v 1.27%). Although both baseline Pap and HPV tests predicted who would develop CIN3+ within the first 2 years of follow-up, only HPV testing predicted who would develop CIN3+ 10 to 18 years later (P = .004). HPV16- and HPV18-positive women with normal Pap were at elevated risk of CIN3+ compared with other HPV-positive women with normal Pap and were at similar risk of CIN3+ compared with women with a low-grade squamous intraepithelial Pap.
Conclusion
HPV testing to rule out cervical disease followed by Pap testing and possibly combined with the detection of HPV16 and HPV18 among HPV positives to identify those at immediate risk of CIN3+ would be an efficient algorithm for cervical cancer screening, especially in women age 30 years or older.
INTRODUCTION
On the basis of the necessary role of human papillomavirus (HPV) infection in causing virtually all cervical cancer, highly efficacious interventions based on HPV, namely prophylactic HPV vaccines for primary prevention1,2 and high-risk HPV (HR-HPV) DNA testing for screening and secondary prevention,3–7 have been developed and are being incorporated into cervical cancer prevention programs. HPV testing and cytology (Papanicolaou [Pap]) co-testing at 5-year screening intervals is now a recommended8,9 or accepted method10 for screening women age 30 years or older.
Both cross-sectional and, more importantly, prospective studies have clearly demonstrated that persistent HR-HPV infection precedes and predicts outcomes. But for how long does detection of HR-HPV predict disease? The answer to this question will provide insight into durability of population benefits for HR-HPV–based interventions. Specifically, the objective of this analysis was to assess the long-term predictive values of HR-HPV and Pap test results individually and jointly, the latter of which permits us to examine the time-specific attribution of cervical cancer risk end points for each test result.
To this end, we conducted a final analysis of the Portland Kaiser cohort of approximately 20,000 women to look at the long-term predictive values of clinical HR-HPV detection and cytology, and combinations, regarding cervical cancer risk, specifically the risk of cervical intraepithelial neoplasia grade 3 (CIN3) and invasive cervical cancer (CIN3+). We previously published a report on 10-year risk using this same algorithm11; we have extended our analysis to 18 years of follow-up. We recently reported on HPV genotype–specific risks using a research polymerase chain reaction assay with repeat testing to clarify ambiguous results.12That study excluded all prevalent disease. Here, we relied on a single test using US Food and Drug Administration–approved Hybrid Capture 2 (HC2; Qiagen, Gaithersburg, MD), a DNA assay for a pool of 13 carcinogenic HPV genotypes, as would be done in current clinical practice, and included prevalent disease to provide clinically relevant absolute risks for cervical disease. We then tested the HC2 positives for HPV16 and HPV18 using a prototype clinical assay to simulate testing in a clinical setting with the next generation of HPV DNA tests, which offer HPV16 and HPV18 detection for enhanced risk stratification.13
METHODS
Study Population
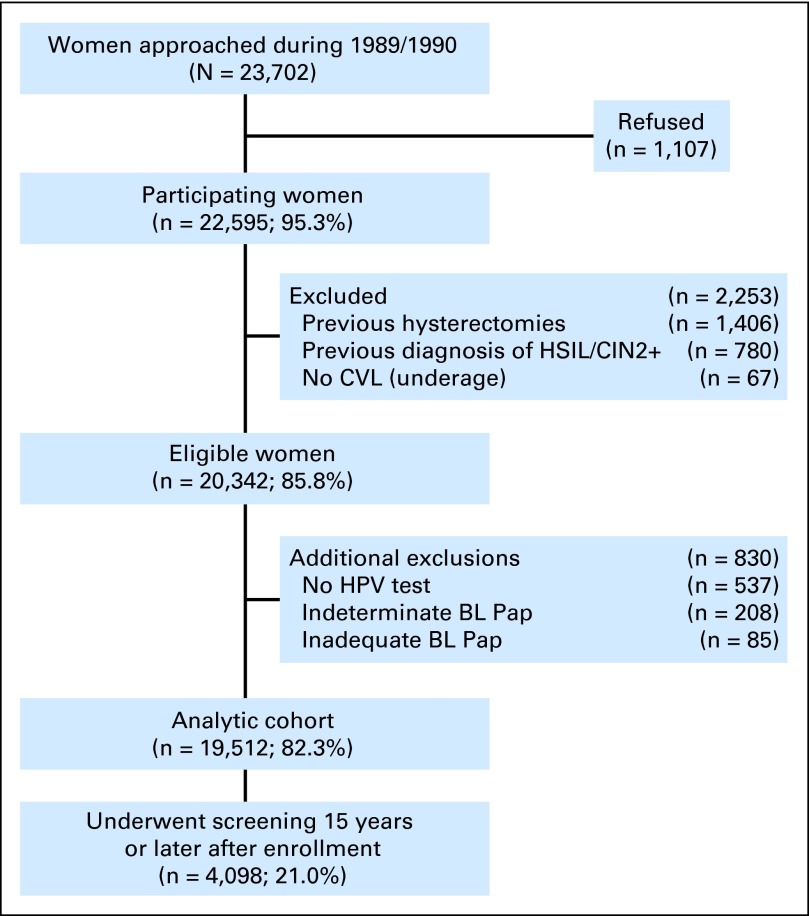
From April 1, 1989, to November 2, 1990, we approached 23,702 nonpregnant women, age 16 years or older, receiving apparently routine cytologic screening in a prepaid health plan at Kaiser Permanente in Portland, Oregon.14,15 A total of 22,595 women (95.3%) agreed to participate. A final analytic cohort of 19,512 women (86.4%) was defined after exclusions, detailed in Figure 1, with mean, median, and range of ages of 35.8, 34.0, and 16 to 94 years, respectively, at enrollment. Of those in the analytic cohort, 4,098 women (21.0%) had a least one screen 15 years or later after the cohort was initiated.
Fig 1.
CONSORT diagram for women enrolled onto the Portland Kaiser cohort in from 1989 to 1990. BL, baseline; CIN2+, cervical intraepithelial neoplasia grade 2 or more severe; CVL, cervicovaginal lavage; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; Pap, Papanicolaou.
Informed consent was obtained under the prevailing institutional review board guidelines at Kaiser Permanente and the National Institutes of Health at the time of enrollment, which permitted an opt out from minimal-intervention studies. Women at Kaiser underwent routine annual pelvic examination and cytologic screening by conventional Pap testing, which has clinical performance similar to the now more commonly used liquid-based cytology.16,17 A Pap specimen for cytology screening and cervicovaginal lavage for HPV testing were collected, as previously described16,17 (Appendix, online only).
Standard practice guidelines mandated treatment of patients with CIN2+, although some patients with CIN1 may have been treated at the discretion of the health plan physicians. HPV test results were not known by clinicians and were not used to direct patient management.
The women underwent follow-up prospectively by conventional Pap testing until November 30, 2006, for a maximum of 214 months (approximately 18 years). As previously reported, younger women (age < 30 years) had less follow-up time than older women.12
Pathology
We converted pre–Bethesda System Pap smear interpretations into Bethesda 2001 terminology for this analysis, as previous described in detail.12,18 Histologic diagnoses were converted into CIN nomenclature. Specifically, severe dysplasia and carcinoma in situ were categorized as CIN3, and moderate dysplasia was categorized as CIN2.
Histopathologic end points were defined as previously described.19,20 We used two different end points in our analysis: CIN3+ (CIN3, adenocarcinoma in situ [AIS], or cancer) and CIN2+ (CIN2 histology or high-grade squamous intraepithelial lesion [HSIL] cytology, CIN3, AIS, or cancer). We also used CIN3 or CIN2 with an immediately preceding HSIL Pap as an end point of probable precancerous lesions.
HPV DNA Testing
As previously described in detail,14,15 cervicovaginal lavage specimens were refrigerated within 1 hour of collection and transported to a laboratory for processing. Specimens were tested using HC2.14 HR-HPV–positive specimens were retested using a prototype adaptation of HC2, which included separate, individual detection of HPV16 and HPV18,21 the two most carcinogenic HPV genotypes.22,23 We used these genotyping data to simulate a clinical test for 13 HR-HPV genotypes with separate HPV16 and HPV18 detection, as previously described11 and now available for several recently approved HPV tests.24,25 HPV testing results were then categorized hierarchically according to a priori ordering of established cancer risk23,26: HPV16 positive, else HPV16 negative and HPV18 positive, else HPV16 and HPV18 negative and HR-HPV positive, else HR-HPV negative.
Statistical Analyses
We first calculated the percent test positive for each test and combinations of tests, overall and stratified by age group (< 30 years, 5-year age groups to 64 years, and ≥ 65 years). Binomial 95% CIs were calculated where noted.
Throughout the analysis, we evaluated baseline test results individually and as paired Pap and HR-HPV test results (HR-HPV positive/Pap positive [atypical squamous cells of undetermined significance (ASC-US) or more severe (ASC-US+], HR-HPV positive/Pap negative, HR-HPV negative/Pap positive, and HR-HPV negative/Pap negative) to show how these tests predicted outcomes. Because Pap and HR-HPV results (ie, HR-HPV is the primary cause of an abnormal Pap) are correlated, the paired test results were used to de-convolute which test was predicting the outcome.
We first calculated the total number of patient cases of CIN2+ and CIN3+ based on the worst diagnosis throughout follow-up after the baseline test results. Then, as previously described,11,14 we calculated the risk for CIN2+ and CIN3+ after any baseline result for each time interval by dividing the number of patients diagnosed in that interval by the number of women at risk (ie, who had undergone routine cytology screening) during that interval. Using the Kaplan-Meier method, we calculated cumulative incidence rates (CIRs) with 95% CIs for each interval up to the end of the observation time. The cumulative incidence of disease over an interval for women with a baseline test result is an estimate of the absolute risk of disease (ie, positive predictive value for a positive test result and complement of negative predictive value for negative test result), adjusted for person-time and censoring.
Of note, there were some small discrepancies in the number of patient cases of disease between the crude and cumulative incidence analyses, as a result of few women who had multiple diagnoses of CIN2+ during follow-up, with a more severe diagnosis occurring later in follow-up. For example, one woman had a CIN2 diagnosis during years 0 to 4 and a cancer diagnosis during years 10 to 18. She would have been counted as having CIN3+ in the crude analysis, whereas she would have been censored for a CIN2 diagnosis in the 0 to 4–year time bin in the cumulative incidence of CIN2+ analysis.
In some circumstances, with sufficient numbers of outcomes, we used yearly time bins after a 9-month enrollment period, except for years 16 to 18, which were collapsed into a single time bin because of the small number of events. We note that because women were only identified with end points when they had an abnormal Pap, the assumption of proportional hazards used for comparing rates of disease by baseline characteristics was not strictly correct, especially in early follow-up, when much of the disease was found. However, other approaches, such as creating a time interval for enrollment to account for differential follow-up and diagnosis by baseline test status, did not appreciably change our risk estimates (data not shown).
When we examined strata that contained smaller numbers of patient cases, such as combinations of HR-HPV and Pap results, we reverted to approximately 5-year time bins (except for the final bin, which was from years 10 to 18) to avoid imprecise estimates of risks for outcomes when a small number of women returned for a given yearly bin. Estimates based on 5-year time bins did not fully account for lower denominators that would have been obtained from more precise accounting for censoring and loss to follow-up, the latter of which was substantial by the end of 18 years. Therefore, observed rates may have been underestimated.
We used the McNemar test to compare the odds of being a patient case by the baseline testing result within a time bin as crude measure of the differences in risk within that time window. We tested for statistically significant differences in median time from enrollment to diagnosis of probable precancerous lesions using the Kruskal-Wallis test. A P value below .05 was considered significant.
RESULTS
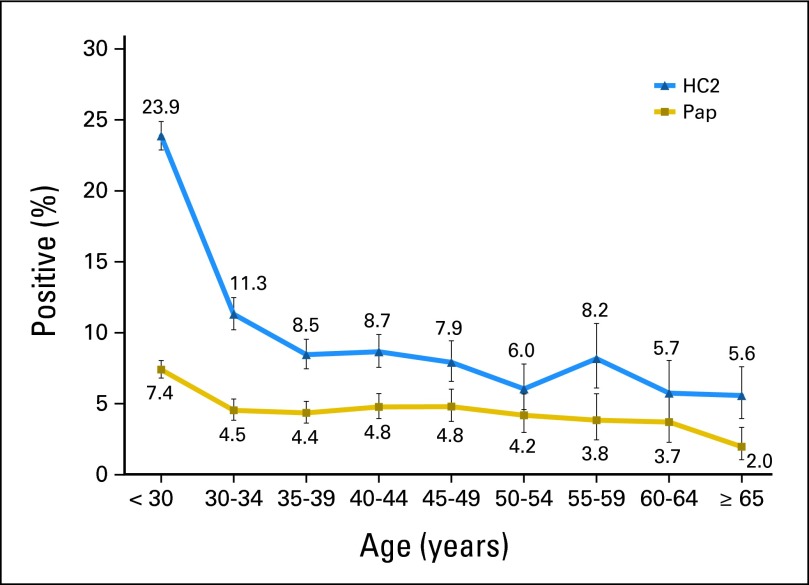
At baseline, 14.2% (95% CI, 13.7% to 14.7%) of the women tested HR-HPV positive by HC2, and 5.4% (95% CI, 5.1% to 5.8%) of women had a positive Pap; of the 12,461 women who were age 30 years or older and for whom co-testing with HR-HPV and Pap is now a screening option, 8.7% (95% CI, 8.2% to 9.2%) tested HR-HPV positive, and 4.3% (95% CI, 4.0% to 4.7%) had a positive Pap. The age group–specific percent positive (prevalence) by HR-HPV and cytology is shown in Appendix Figure A1 (online only). As expected, the positive HR-HPV tests and positive Paps were highest in women younger than age 30 years and were two-fold greater those occurring in women age 30 to 34 years. At all ages, approximately half of the women who tested HR-HPV positive had a concurrent positive Pap.
There were 396 patient cases of CIN2+ and 199 of CIN3+ diagnosed over the 18 years of the study. More patient cases of CIN2+ (215 v 136; P < .001) and CIN3+ (112 v 65; P < .001) occurred after a baseline HR-HPV–positive result versus positive Pap (Table 1). Among HR-HPV–positive women, approximately half of those with CIN2+ and CIN3+ had concurrent negative baseline Pap.
Table 1.
Worst Diagnosis Over 18-Year Follow-Up of Approximately 20,000 Women at Kaiser Permanente Northwest (Portland, OR) by Baseline Test Results
| Enrollment Test | CIN2+ |
CIN3+ |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Individual test results | ||||
| HR-HPV positive | 215 | 54 | 112 | 56 |
| HR-HPV negative | 181 | 46 | 87 | 44 |
| ASC-US+ Pap | 136 | 34 | 65 | 33 |
| Normal Pap | 260 | 66 | 134 | 67 |
| Paired test results | ||||
| HR-HPV positive/ASC-US+ | 110 | 28 | 50 | 25 |
| HR-HPV positive/normal | 105 | 27 | 62 | 31 |
| HR-HPV negative/ASC-US+ | 26 | 7 | 15 | 8 |
| HR-HPV negative/normal | 155 | 39 | 72 | 36 |
| Total | 396 | 100 | 199 | 100 |
Abbreviations: ASC-US+, atypical squamous cells of undetermined significance or more severe; CIN, cervical intraepithelial neoplasia; CIN2+, CIN grade 2 or more severe; CIN3+, CIN grade 3 or more severe; HR-HPV, high-risk human papillomavirus; Pap, Papanicolaou.
Figure 2shows the risk stratification for CIN2+ (Fig 2A) and CIN3+ (Fig 2B) achieved by the baseline HR-HPV and Pap tests separately. A positive Pap strongly predicted disease within the first 2 years, whereas an HR-HPV–positive test continued to predict those who were at risk until the end of the study. For example, HR-HPV–positive women were more likely to be diagnosed with CIN2+ (P < .001) and CIN3+ (P = .004) 10 to 18 years after enrollment compared with HR-HPV–negative women. In that same time period, women with a positive Pap were not more likely to be diagnosed with CIN2+ (P = .8) or CIN3+ (P = 1.0) than women with a negative Pap.
Fig 2.
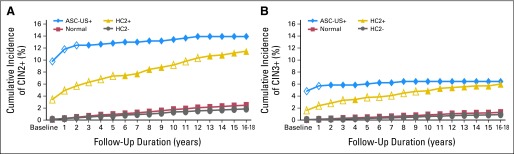
The risk of a diagnosis of (A) cervical intraepithelial neoplasia (CIN) grade 2 or more severe (CIN2+) and (B) CIN grade 3 or more severe (CIN3+) after a baseline test for human papillomavirus (HPV) and by the clinical center Papanicolaou (Pap) test. High-risk HPV (HR-HPV) testing was categorized as positive or negative; Pap smears were categorized as atypical squamous cells of undetermined significance or more severe (ASC-US+) or as normal. Open symbols indicate statistically significant patient cases of CIN2+ or CIN3+ in the test positive (HR-HPV positive or ASC-US+) versus the corresponding test negative (HR-HPV negative or normal) in that year time interval. Years 16 to 18 of follow-up were collapsed into a single time bin labeled as year 16. The 18-year cumulative incidence ratios (CIRs) of CIN2+ for women with a baseline ASC-US+ Pap, normal Pap, HPV-positive test, and HPV-negative test were 13.92% (95% CI, 11.79% to 16.39%), 2.47% (95% CI, 2.17% to 2.82%), 11.46 (95% CI, 9.88% to 13.29%), and 1.85% (95% CI, 1.58% to 2.16%), respectively. The 18-year CIRs of CIN3+ for women with a baseline ASC-US+ Pap, normal Pap, HPV-positive test, and HPV-negative test were 6.41% (95% CI, 5.00% to 8.21%), 1.27% (95% CI, 1.05% to 1.53%), 5.98% (95% CI, 4.80% to 7.43%), and 0.90% (95% CI, 0.71% to 1.14%), respectively. HC2, Hybrid Capture 2.
In a complimentary fashion, a one-time negative HR-HPV test at enrollment provided greater reassurance over the 18-year follow-up than a one-time negative Pap against CIN2+ (1.85% v 2.47%) and CIN3+ (0.90% v 1.27%). By comparison, cumulative incidence was 1.73% for CIN2+ and 0.83% for CIN3+ after one-time HPV and Pap tests that were both negative.
Table 2 lists the 18-year cumulative detection of CIN2+ and CIN3+ for years 0 to 4, 5 to 9, and 10 to 18 by combined baseline HR-HPV and Pap test results. The 18-year CIRs of CIN3+ for HR-HPV positive/Pap positive, HR-HPV positive/Pap negative, HR-HPV negative/Pap positive, and HR-HPV negative/Pap negative were 9.91%, 3.90%, 3.01%, and 0.72%, respectively. Similar patterns of risk stratification were observed for CIN2+, with a range of 18-year CIR of 21.23% for HR-HPV positive/Pap positive to 1.42% for HR-HPV negative/Pap negative.
Table 2.
Cumulative Detection of CIN2+ or CIN3+ After Baseline Test for HR-HPV and Clinical Center Pap Test
| HPV Result by Follow-Up Period (years) | Pap Result | Total No. of Patients | CIN2+ |
CIN3+ |
||||
|---|---|---|---|---|---|---|---|---|
| No. of End Points | CIR | 95% CI | No. of End Points | CIR | 95% CI | |||
| 0 to 4 | ||||||||
| HR-HPV positive | ASC-US+ | 540 | 107 | 19.81 | 16.68 to 23.45 | 48 | 8.89 | 6.67 to 11.63 |
| Normal | 2,224 | 63 | 2.83 | 2.22 to 3.61 | 37 | 1.66 | 1.21 to 2.29 | |
| HR-HPV negative | ASC-US+ | 522 | 24 | 4.60 | 3.10 to 6.79 | 14 | 2.68 | 1.59 to 4.49 |
| Normal | 16,226 | 64 | 0.39 | 0.31 to 0.50 | 19 | 0.12 | 0.07 to 0.18 | |
| 5 to 9 | ||||||||
| HR-HPV positive | ASC-US+ | 171 | 1 | 20.02 | 17.01 to 23.91 | 1 | 9.33 | 7.09 to 12.24 |
| Normal | 997 | 20 | 4.55 | 3.64 to 5.67 | 13 | 2.79 | 2.09 to 3.72 | |
| HR-HPV negative | ASC-US+ | 274 | 1 | 4.92 | 3.34 to 7.24 | 1 | 3.01 | 1.81 to 5.00 |
| Normal | 9,292 | 51 | 0.90 | 0.74 to 1.08 | 26 | 0.37 | 0.28 to 0.50 | |
| ≥ 10 | ||||||||
| HR-HPV positive | ASC-US+ | 156 | 2 | 21.23 | 17.81 to 25.19 | 1 | 9.91 | 7.48 to 13.09 |
| Normal | 788 | 20 | 6.97 | 5.67 to 8.56 | 9 | 3.90 | 2.96 to 5.13 | |
| HR-HPV negative | ASC-US+ | 216 | 1 | 5.36 | 3.63 to 7.89 | 0 | 3.01 | 1.81 to 5.00 |
| Normal | 7,567 | 40 | 1.42 | 1.20 to 1.67 | 26 | 0.72 | 0.56 to 0.91 | |
NOTE. There are some small discrepancies in number of patient cases of disease between crude (Table 1) and cumulative incidence analyses (Table 2) resulting from few women who had multiple diagnoses of CIN2+ during follow-up, with more severe diagnosis occurring later in follow-up. For example, one woman had CIN2 diagnosis during years 0 to 4 and cancer diagnosis during years 10 to 18. She would have been counted as having cancer in crude analysis, whereas she would have been censored for CIN2 diagnosis in 0 to 4–year time bin in cumulative incidence of CIN2+ analysis.
Abbreviations: ASC-US+, atypical squamous cells of undetermined significance or more severe; CIN, cervical intraepithelial neoplasia; CIN2+, CIN grade 2 or more severe; CIN3+, CIN grade 3 or more severe; CIR, cumulative incidence rate; HR-HPV, high-risk human papillomavirus; Pap, Papanicolaou.
We examined the 18-year risk of CIN3+ by enrollment screening results stratified by age groups: < 30 years (median age, 24 years), 30 to 39 years (median age, 34 years), and ≥ 40 years (median age, 47 years; Table 3). There was a greater cumulative incidence of CIN3+ among those women age younger than 30 and age 30 to 39 years who tested HPV positive/Pap normal than among those age 40 years or older. A similar age-related effect was noted in the HPV-negative/Pap-normal populations.
Table 3.
Cumulative Detection of CIN3 or CIN3+ After Baseline Test for HR-HPV and Clinical Center Pap Test Stratified by Enrollment Age Group
| HPV Result by Follow-Up Period (years) | Pap Result | Age < 30 Years |
Age 30 to 39 Years |
Age ≥ 40 Years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | No. of End Points | CIR | 95% CI | No. of Patients | No. of End Points | CIR | 95% CI | No. of Patients | No. of End Points | CIR | 95% CI | ||
| 0 to 4 | |||||||||||||
| HR-HPV positive | ASC-US+ | 382 | 27 | 7.07 | 4.89 to 10.15 | 94 | 14 | 14.89 | 9.02 to 24.05 | 64 | 7 | 10.94 | 5.26 to 21.97 |
| Normal | 1,301 | 24 | 1.84 | 1.24 to 2.74 | 496 | 8 | 1.61 | 0.81 to 3.21 | 427 | 5 | 1.17 | 0.49 to 2.80 | |
| HR-HPV negative | ASC-US+ | 140 | 6 | 4.29 | 1.93 to 9.38 | 170 | 5 | 2.94 | 1.22 to 6.98 | 212 | 3 | 1.42 | 0.45 to 4.36 |
| Normal | 5,228 | 6 | 0.11 | 0.05 to 0.26 | 5,179 | 7 | 0.14 | 0.06 to 0.28 | 5,819 | 6 | 0.10 | 0.05 to 0.23 | |
| 5 to 9 | |||||||||||||
| HR-HPV positive | ASC-US+ | 108 | 1 | 7.75 | 5.32 to 11.22 | 34 | 0 | 14.89 | 9.02 to 24.05 | 29 | 0 | 10.94 | 5.26 to 21.97 |
| Normal | 475 | 8 | 3.23 | 2.23 to 4.68 | 249 | 5 | 3.38 | 1.93 to 5.88 | 273 | 0 | 1.17 | 0.49 to 2.80 | |
| HR-HPV negative | ASC-US+ | 41 | 0 | 4.29 | 1.93 to 9.38 | 86 | 0 | 2.94 | 1.22 to 6.98 | 147 | 1 | 2.07 | 0.77 to 5.52 |
| Normal | 2,214 | 15 | 0.70 | 0.45 to 1.09 | 3,030 | 5 | 0.28 | 0.16 to 0.51 | 4,048 | 6 | 0.24 | 0.14 to 0.43 | |
| ≥ 10 | |||||||||||||
| HR-HPV positive | ASC-US+ | 97 | 1 | 8.70 | 5.86 to 12.83 | 33 | 0 | 14.89 | 9.02 to 24.05 | 26 | 0 | 10.94 | 5.26 to 21.97 |
| Normal | 375 | 7 | 5.04 | 3.54 to 7.15 | 202 | 1 | 3.85 | 2.23 to 6.62 | 211 | 1 | 1.64 | 0.71 to 3.77 | |
| HR-HPV negative | ASC-US+ | 34 | 0 | 4.29 | 1.93 to 9.38 | 65 | 0 | 2.94 | 1.22 to 6.98 | 117 | 0 | 2.07 | 0.77 to 5.52 |
| Normal | 1,775 | 13 | 1.43 | 1.01 to 2.03 | 2,648 | 8 | 0.59 | 0.37 to 0.92 | 3,144 | 5 | 0.40 | 0.25 to 0.66 | |
Abbreviations: ASC-US+, atypical squamous cells of undetermined significance or more severe; CIN3, cervical intraepithelial neoplasia grade 3; CIN3+, CIN3 or more severe; CIR, cumulative incidence rate; HR-HPV, high-risk human papillomavirus; Pap, Papanicolaou.
After negative HPV and Pap tests in women age 30 years or older (n = 12,461), the 3-year risks, relevant to past cotesting screening guidelines, of CIN2+ and CIN3+ were 0.23% and 0.08%, respectively. If the screening intervals were extended to 5 years according to current cotesting screening guidelines,26a the risks were 0.36% and 0.16%, respectively. Of the 37 patient cases of CIN3+ diagnosed in this subgroup, only five (14%) were diagnosed within 3 years and 13 (35%) within 5 years.
Finally, we wanted to look at the 18-year risks of CIN2+ and CIN3+ for different combinations of Pap interpretations and HPV risk groups (Table 4 ). In general, cervical cancer risk increased with more severe Pap interpretations and higher-risk HPV genotypes. Notably, women who tested HPV16 positive had a similar or higher 18-year CIR than women with any Pap interpretation other than HSIL.
Table 4.
18-Year Cumulative Detection of CIN2+ or CIN3+ by Baseline Hierarchic HPV Status and Pap Test Results
| Baseline Result | No. of Patients | CIN2+ |
CIN3+ |
||
|---|---|---|---|---|---|
| CIR | 95% CI | CIR | 95% CI | ||
| HPV16 positive | |||||
| Normal | 307 | 20.53 | 14.79 to 28.08 | 13.70 | 9.06 to 20.42 |
| ASC-US | 51 | 15.69 | 7.98 to 29.52 | 7.84 | 2.92 to 20.17 |
| LSIL | 75 | 37.33 | 27.30 to 49.60 | 20.00 | 12.44 to 31.27 |
| HSIL | 19 | 89.47 | 69.44 to 98.61 | 73.68 | 51.21 to 91.65 |
| Any | 452 | 25.59 | 20.82 to 31.22 | 16.76 | 12.78 to 21.82 |
| HPV18 positive | |||||
| Normal | 110 | 10.74 | 4.87 to 22.76 | 6.44 | 2.15 to 18.42 |
| ASC-US | 20 | 5.00 | 0.60 to 35.55 | 0.00 | |
| LSIL | 20 | 32.00 | 9.51 to 77.43 | 24.00 | 4.46 to 80.84 |
| HSIL | 5 | 80.00 | 24.93 to 99.99 | 20.00 | 0.99 to 99.33 |
| Any | 155 | 14.81 | 8.74 to 24.46 | 7.78 | 3.38 to 17.36 |
| HR-HPV positive (HPV16 negative and HPV18 negative) | |||||
| Normal | 1,807 | 4.68 | 3.52 to 6.21 | 2.30 | 1.54 to 3.42 |
| ASC-US | 153 | 13.84 | 8.51 to 22.08 | 5.09 | 2.07 to 12.25 |
| LSIL | 183 | 12.02 | 8.05 to 17.75 | 2.73 | 1.14 to 6.49 |
| HSIL | 14 | 57.14 | 31.23 to 85.30 | 21.43 | 6.38 to 58.63 |
| Any | 2,157 | 6.30 | 5.12 to 7.74 | 2.67 | 1.92 to 3.71 |
| HR-HPV negative | |||||
| Normal | 16,226 | 1.42 | 1.20 to 1.67 | 0.72 | 0.56 to 0.91 |
| ASC-US | 406 | 1.94 | 0.83 to 4.50 | 1.15 | 0.42 to 3.12 |
| LSIL | 103 | 11.65 | 6.73 to 19.77 | 4.85 | 2.02 to 11.43 |
| HSIL | 13 | 61.54 | 34.00 to 88.89 | 46.15 | 21.47 to 79.52 |
| Any | 16,748 | 1.54 | 1.32 to 1.80 | 0.79 | 0.63 to 0.98 |
| Any | |||||
| Normal | 18,450 | 2.02 | 1.78 to 2.30 | 1.06 | 0.89 to 1.27 |
| ASC-US | 630 | 6.03 | 4.22 to 8.58 | 2.59 | 1.49 to 4.49 |
| LSIL | 381 | 17.57 | 14.04 to 21.87 | 7.40 | 5.08 to 10.71 |
| HSIL | 51 | 72.55 | 59.52 to 84.24 | 47.06 | 34.13 to 62.05 |
| Any | 19,512 | 2.64 | 2.38 to 2.93 | 1.36 | 1.17 to 1.58 |
Abbreviations: ASC-US+, atypical squamous cells of undetermined significance or more severe; CIN, cervical intraepithelial neoplasia; CIN2+, CIN grade 2 or more severe; CIN3+, CIN grade 3 or more severe; CIR, cumulative incidence rate; HPV, human papillomavirus; HR-HPV, high-risk HPV; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; Pap, Papanicolaou.
DISCUSSION
It is well established that clinical HPV DNA testing is more sensitive for the detection of current and future cervical precancer (CIN2, CIN3, and AIS) and cervical cancer3–7,27–29 than cervical cytology, which can then reduce the incidence of cervical cancer4,6,7 and cervical cancer–related mortality29 within a decade. Using a current US Food and Drug Administration–approved HPV test, including prevalent and incidence disease, and without retesting of specimens to clarify any equivocal results, as was done previously,12 we simulated in a clinically relevant manner the long-term risk stratification achievable by HPV testing at single time point. Remarkably, we showed that clinical HPV DNA detection at one time point predicts cervical cancer risk for more than 15 years, identifying not only women who have prevalent CIN3+ but also those whose HPV infections will persist and develop into disease in the future. In contrast, as previously discussed,14 cytologic abnormalities are a specific diagnostic marker for immediate, microscopic, and visible disease, but it does not forecast clinically important disease beyond 1 or 2 years, hence the need to repeat it at much shorter intervals.
A previous 12-year follow-up analysis of a subcohort of Pap-normal Danish women30 found similar risk stratification by HPV testing, also using HC2, but no comparison was made with Pap testing. The risks for CIN3+ among HPV-positive and -negative women (approximately 6% and 3%, respectively) in that study were somewhat greater than those observed in this study (4.3% and 0.7% at year 12, respectively; data not shown). Likewise, the risk for CIN3+ among the HPV16-positive women in that study was twice what we observed in this study among Pap-negative women. Whether these differences are the result of differences in the population risk, diagnostic differences between pathologists, or both is unclear. However, the general patterns between the two studies are similar, and more importantly, we have shown that these patterns continue for another 5 to 6 years longer than observed in the Danish study.
We acknowledge several limitations of our study. Most importantly, during follow-up, women were referred to colposcopy based on an abnormal Pap interpretation only. Therefore, women with persistently positive HPV test results were not immediately referred to colposcopy, as is now recommended when HPV testing is used in conjunction with cervical cytology for women age 30 years or older.31,32 As a result, there was a delay in some women with clinically relevant disease undergoing colposcopy compared with current practice, probably leading to: one, less risk stratification by HPV early in the study, and greater stratification a few years later, when the prevalent disease was subsequently diagnosed; and two, exaggeration of the differences in timing of diagnosis between ASC-US+ and HPV positivity. Second, we converted Pap terminology from a previous classification to the 2001 Bethesda System,18 and our ASC-US does not perfectly represent current-day ASC-US. Third, aggressive treatment of mild histologic changes associated with HPV infection early on in the study could have censored some HPV infections that might have otherwise later developed into CIN3+.
Finally, as a consequence of using a lavage rather than directly exfoliating cells from the os of the cervix, HPV testing was slightly less sensitive for HPV and related lesions. For example, only 38 (74.5%) of 51 baseline HSIL cytology results tested HC2 positive, rather than the expected 90% to 95%.33We believe that the loss of analytic sensitivity for HPV was nondifferential. That is, the negative impact of using this specimen collection on detection of HPV DNA was comparable for both women who had or developed CIN3+ and those who did not. Nevertheless, using current standards for specimen collection, we would predict higher sensitivity for CIN3+ and therefore even greater reassurance against CIN3+ among HPV-negative women than observed in this study.
As previously reported,11 separate HPV16 and HPV18 detection adds further risk stratification beyond what can be achieved by cytology alone among HPV positives; we also found that CIN3 after HPV16 detection developed sooner after baseline than after other HPV genotype detection, consistent with reports that HPV16-related CIN334 and cervical cancer23,35 develop at younger ages than other HPV genotypes. Among HPV-positive women with normal cytology, HPV16 and HPV18 detection can identify a group of women who have substantial risk of CIN3+. Using a more conservative approach to estimating the risks, we observed a lower risk after HPV18 than we previously observed.11 Our previous estimates used 1 year as the unit of time, leading to a larger absolute risk than justified by the number of events because of compounded unstable estimates. Yet the risk of CIN3+ after one-time detection of HPV18 was substantially higher than for the other HR-HPV genotypes in aggregate. HPV18 is strongly associated with adenocarcinoma and AIS,22,23,36 which is on the rise in Western countries37,38 and is preferentially missed by cytologic methods.39 It is therefore rational to monitor for HPV18 as well as HPV16 separately in screening.
Despite the limitations of the study, the data presented provide additional support for the use of HPV testing in routine screening in women age 30 or older. Importantly, an HPV test provides greater reassurance against CIN3 and cervical cancer than Pap testing and thus might be used as the screen to rule out disease in healthy women, whereas Pap is useful as a secondary diagnostic test to identify HPV-positive women at immediate risk of CIN3+. There is also evidence from other studies6,7,40 suggesting that HPV testing might help to identify women at risk for AIS and invasive adenocarcinoma, which are poorly detected by cytology-based screening alone.38,41
Appendix
Materials and Methods
For each patient, clinicians collected a Papanicolaou (Pap) specimen using an Ayre spatula and cytobrush and made a standard, ethanol-fixed Pap smear. Then, the cervix was rinsed with 10 mL of sterile saline using a 3.25-inch flexible intracatheter extender attached to a disposable syringe (Sherman ME, Lorincz AT, Scott DR, et al: J Natl Cancer Inst 95:46-52, 2003; Schiffman MH, Bauer HM, Hoover RN, et al: J Natl Cancer Inst 85:958-964, 1993). The pooled fluid was collected from the posterior vaginal fornix and processed for human papillomavirus (HPV) testing. This method of specimen collection was used at a time when the HPV assays available (eg, southern blot) required more cellular material and does not represent the current methods of specimen collection.
Results
We also observed that the median time from enrollment to a probable precancerous diagnosis (grade 3 cervical intraepithelial neoplasia [CIN3] or CIN2 and high-grade squamous intraepithelial lesion Pap) after being HPV16 positive (n = 75; 264 days) was shorter than that after being HPV18 positive (n = 12; 675 days; P = .2) or positive for other carcinogenic HPV genotypes (n = 52; 551 days; P = .08 [Kruskal Wallis]); among those with normal cytology, the median time to diagnosis for HPV16 positives (n = 33; 1,404 days) was similar to that for HPV18 positives (n = 7; 1,008 days; P = .9) and other carcinogenic HPV positives (n = 30; 1,206 days; P = .9).
Discussion
Treatment primarily by less-invasive procedures like cryotherapy of all HPV-positive women, as is being evaluated and considered for lower-resource countries (Denny L, Kuhn L, Hu CC, et al: J Natl Cancer Inst 102:1557-1567, 2010) that do not have sufficient infrastructure for diagnostic verification, would result in significant overtreatment; however, such a screen-and-treat approach would also likely have long-lasting, cancer-reducing effects.
As valid clinical tests with separate HPV16 and HPV18 detection become available, with an analytic sensitivity similar to the detection of HPV16 and HPV18 used in this study, detection of HPV16 and HPV18 as triage for HPV-positive, cytology-negative patients may be preferable to 1-year follow-up of everyone in this category.
After an HPV-negative/Pap-normal result, cumulative—presumably incident—CIN3+ decreased with increasing age. Women in the youngest age group (< 30 years) were still many years older than the optimal age of HPV vaccination, as recommend by the Advisory Committee on Immunization Practices (Markowitz LE, Dunne EF, Saraiya M, et al: MMWR Recomm Rep 56:1-24, 2007) and American Cancer Society (Saslow D, Castle PE, Cox JT, et al: CA Cancer J Clin 57:7-28, 2007). Therefore, HPV vaccination will prevent little disease in the general population of women who need screening (ie, women age ≥ 30 years) and should not be generally recommended for them.
From a practical viewpoint, HPV testing efficiently separates a small group of HPV-positive women who need clinical follow-up and repeated Pap testing with or without HPV genotyping from the much larger group of women who test negative for the necessary cause of cervical cancer and therefore do not need any screening for several years. The decision to use HPV testing adjunctively with cytology as a co-test or alone as the primary screen is a matter of tradeoffs in sensitivity and cost. Co-testing increases the sensitivity for detection of prevalent CIN3+ by a few percent (2% to 5%; Katki HA, Kinney WK, Fetterman B, et al: Lancet Oncol 12:663-672, 2011; Castle PE, Stoler MH, Wright TC Jr, et al: Lancet Oncol 12:880-890, 2011; Arbyn M, Ronco G, Meijer CJ, et al: Lancet Oncol 10:935-936, 2009; Rijkaart DC, Berkhof J, Rozendaal L, et al: Lancet Oncol 13:78-88, 2012) compared with HPV primary testing alone, at the cost of conducting concurrent Pap testing on the entire population versus in the 5% to 10% of the population who test HPV positive.
The durability of the risk stratification by a single HPV test has an important clinical implication. Three-year intervals after a negative HPV test, as was previously recommended in women age 30 years or older (Wright TC Jr, Schiffman M, Solomon D, et al: Obstet Gynecol 103:304-309, 2004; Wright TC Jr, Massad LS, Dunton CJ, et al: J Low Genit Tract Dis 11:201-222, 2007), may still be too short given the low risk and the high likelihood that any HPV found after an earlier negative screening is new and many years from being clinically important (Rodriguez AC, Schiffman M, Herrero R, et al: J Natl Cancer Inst 102:315-324, 2010; Katki HA, Kinney WK, Fetterman B, et al: Lancet Oncol 12:663 to 672, 2011). There is now evidence from this study and others (Dillner J, Rebolj M, Birembaut P, et al: BMJ 337:a1754, 2008) that screening at an interval of 5 to 7 years after a negative HPV test is safe. Current recommendations (Saslow D, Solomon D, Lawson HW, et al: CA Cancer J Clin 62:147-172, 2012; Saslow D, Solomon D, Lawson HW, et al: J Low Genit Tract Dis [epub ahead of print on March 13, 2012]; Moyer VA: Ann Intern Med [epub ahead of print on March 14, 2012]) of co-testing every 5 years reflect the added safety afforded by HPV testing. Unfortunately, it is increasingly evident that in the United States, clinicians are using HPV testing to screen women age 30 years or older more frequently than every 3 years (Saraiya M, Berkowitz Z, Yabroff KR, et al: Arch Intern Med 170:977-985, 2010). In addition to inflating health care costs, overscreening can lead to overtreatment and possibly negative reproductive health outcomes (Arbyn M, Kyrgiou M, Simoens C, et al: BMJ 337:a1284, 2008). Achieving extended screening intervals will likely require substantial changes in clinician and patient attitudes (Coughlin SS, King J: BMC Public Health 10:146, 2010).
Fig A1.
The percent positive (prevalence) by Hybrid Capture 2 (HC2) and Papanicolaou (Pap; atypical squamous cells of undetermined significance or more severe) with 95% CIs (indicated by vertical bars) by age group.
Footnotes
See accompanying editorial on page 3037
Supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Philip E. Castle, Merck (C), Qiagen (U), Roche (U); Attila T. Lorincz, Qiagen (C) Stock Ownership: Attila T. Lorincz, Qiagen Honoraria: None Research Funding: Attila T. Lorincz, Qiagen Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Andrew G. Glass, Sholom Wacholder
Administrative support: Philip E. Castle
Collection and assembly of data: Andrew G. Glass, Brenda B. Rush, David R. Scott, Attila T. Lorincz
Data analysis and interpretation: Philip E. Castle, Nicolas Wentzensen, Julia C. Gage, Julie Buckland, Greg Rydzak, Sholom Wacholder
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Kjaer SK, Sigurdsson K, Iversen OE, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila Pa) . 2009;2:868–878. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 2.Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet . 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 3.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med . 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 4.Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet . 2007;370:1764–1772. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 5.Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med . 2007;357:1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 6.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: A randomised controlled trial. Lancet Oncol . 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 7.Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: Randomised study within organised screening programme. BMJ. 2010;340:c1804. doi: 10.1136/bmj.c1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin . 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. doi: 10.1097/LGT.0b013e31824ca9d5. [epub ahead of print on March 13, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. doi: 10.7326/0003-4819-156-12-201206190-00424. [epub ahead of print on March 14, 2012] [DOI] [PubMed] [Google Scholar]
- 11.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst . 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman M, Glass AG, Wentzensen N, et al. A long-term prospective study of type-specific human papillomavirus infection and risk of cervical neoplasia among 20,000 women in the Portland Kaiser Cohort Study. Cancer Epidemiol Biomarkers Prev . 2011;20:1398–1409. doi: 10.1158/1055-9965.EPI-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castle PE, Stoler MH, Wright TC, Jr, et al. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: A subanalysis of the ATHENA study. Lancet Oncol . 2011;12:880–890. doi: 10.1016/S1470-2045(11)70188-7. [DOI] [PubMed] [Google Scholar]
- 14.Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: A 10-year cohort analysis. J Natl Cancer Inst . 2003;95:46–52. doi: 10.1093/jnci/95.1.46. [DOI] [PubMed] [Google Scholar]
- 15.Schiffman MH, Bauer HM, Hoover RN, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst . 1993;85:958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 16.Arbyn M, Bergeron C, Klinkhamer P, et al. Liquid compared with conventional cervical cytology: A systematic review and meta-analysis. Obstet Gynecol . 2008;111:167–177. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 17.Siebers AG, Klinkhamer PJ, Grefte JM, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: A randomized controlled trial. JAMA . 2009;302:1757–1764. doi: 10.1001/jama.2009.1569. [DOI] [PubMed] [Google Scholar]
- 18.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA . 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 19.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis . 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 20.Liaw KL, Glass AG, Manos MM, et al. Detection of human papillomavirus DNA in cytologically normal women and subsequent cervical squamous intraepithelial lesions. J Natl Cancer Inst . 1999;91:954–960. doi: 10.1093/jnci/91.11.954. [DOI] [PubMed] [Google Scholar]
- 21.Castle PE, Lorincz AT, Scott DR, et al. Comparison between prototype hybrid capture 3 and hybrid capture 2 human papillomavirus DNA assays for detection of high-grade cervical intraepithelial neoplasia and cancer. J Clin Microbiol . 2003;41:4022–4030. doi: 10.1128/JCM.41.9.4022-4030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer . 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 23.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol . 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 24.Stoler MH, Wright TC, Jr, Sharma A, et al. High-risk human papillomavirus testing in women with ASC-US cytology: Results from the ATHENA HPV study. Am J Clin Pathol . 2011;135:468–475. doi: 10.1309/AJCPZ5JY6FCVNMOT. [DOI] [PubMed] [Google Scholar]
- 25.Einstein MH, Martens MG, Garcia FA, et al. Clinical validation of the Cervista HPV HR and 16/18 genotyping tests for use in women with ASC-US cytology. Gynecol Oncol . 2010;118:116–122. doi: 10.1016/j.ygyno.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens: Part B—Biological agents. Lancet Oncol . 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 26a.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 28.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst . 2008;100:492–501. doi: 10.1093/jnci/djn065. [DOI] [PubMed] [Google Scholar]
- 29.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med . 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 30.Kjaer SK, Frederiksen K, Munk C, et al. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: Role of persistence. J Natl Cancer Inst . 2010;102:1478–1488. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright TC, Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol . 2004;103:304–309. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 32.Wright TC, Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis . 2007;11:201–222. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 33.Castle PE, Fetterman BJ, Thomas Cox J, et al. The age-specific relationships of abnormal cytology and HPV DNA results with the risk of cervical precancer and cancer. Obstet Gynecol . 2010;116:76–84. doi: 10.1097/AOG.0b013e3181e3e719. [DOI] [PubMed] [Google Scholar]
- 34.Castle PE, Schiffman M, Wheeler CM, et al. Human papillomavirus genotypes in cervical intraepithelial neoplasia grade 3. Cancer Epidemiol Biomarkers Prev . 2010;19:1675–1681. doi: 10.1158/1055-9965.EPI-10-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler CM, Hunt WC, Joste NE, et al. Human papillomavirus genotype distributions: Implications for vaccination and cancer screening in the United States. J Natl Cancer Inst . 2009;101:475–487. doi: 10.1093/jnci/djn510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castellsagué X, Díaz M, de Sanjosé S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: Implications for screening and prevention. J Natl Cancer Inst . 2006;98:303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 37.Reimers LL, Anderson WF, Rosenberg PS, et al. Etiologic heterogeneity for cervical carcinoma by histopathologic type, using comparative age-period-cohort models. Cancer Epidemiol Biomarkers Prev . 2009;18:792–800. doi: 10.1158/1055-9965.EPI-08-0965. [DOI] [PubMed] [Google Scholar]
- 38.Bray F, Carstensen B, Møller H, et al. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev . 2005;14:2191–2199. doi: 10.1158/1055-9965.EPI-05-0231. [DOI] [PubMed] [Google Scholar]
- 39.Sasieni P, Castanon A, Cuzick J. Screening and adenocarcinoma of the cervix. Int J Cancer . 2009;125:525–529. doi: 10.1002/ijc.24410. [DOI] [PubMed] [Google Scholar]
- 40.Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: A population-based study in routine clinical practice. Lancet Oncol . 2011;12:663–672. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang SS, Sherman ME, Hildesheim A, et al. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer. 2004;100:1035–1044. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]