Abstract
Purpose
Neuropathy is a common and potentially disabling complication of adjuvant taxane therapy. Recent studies have identified candidate single nucleotide polymorphisms associated with taxane-induced neuropathy. Therefore, we sought to determine whether neuropathy was associated with breast cancer recurrence in a clinical trial population who received adjuvant taxane therapy.
Patients and Methods
Trial E1199 included 4,554 eligible women with operable breast cancer who received up to four cycles of doxorubicin and cyclophosphamide every 3 weeks followed by paclitaxel 175 mg/m2 every 3 weeks for four cycles (P3), paclitaxel 80 mg/m2 weekly for 12 cycles (P1), docetaxel 100 mg/m2 every 3 weeks for four cycles (D3), or docetaxel 35 mg/m2 weekly for 12 cycles (D1). A Cox proportional hazards model was used to determine the relationship between neuropathy and disease-free survival (DFS), overall survival (OS), and recurrence-free survival (RFS) by treating neuropathy status as a time dependent covariate and using a landmark analysis.
Results
Of 4,554 patients who received at least one taxane dose, grade 2 to 4 neuropathy developed in 18%, 22%, 15%, and 13% of patients in the P3, P1, D3, and D1 arms, respectively. In a model that included age, race, obesity, menopausal status, tumor size, nodal status, treatment arm, neuropathy, and hyperglycemia, no significant relationship was found between neuropathy and DFS, OS, or RFS.
Conclusion
There was no association between taxane-induced neuropathy and outcome.
INTRODUCTION
The addition of adjuvant taxanes to standard anthracycline-based therapy has reduced the risk of recurrence and improved survival in patients with node-positive breast cancer.1–4 In addition, taxanes have become a standard therapy for breast cancer in the metastatic setting. Neuropathy is one of the most common nonhematologic toxicities associated with taxanes, especially weekly paclitaxel.5,6 Peripheral neuropathy has the potential to be debilitating and irreversible in some cases.7,8 The mechanism for this toxicity is not well understood.9–17In addition, no agent has been proven to definitively prevent or successfully reverse this adverse effect after it has occurred.18,19
There is substantial heterogeneity in the incidence and severity of taxane-related neurotoxicity among patients. Previous studies have demonstrated that the incidence of neuropathy was increased with older age, diabetes, alcohol use, and black race.20–23 There have been no other validated clinical characteristics that have been associated with an increased risk of neuropathy. In addition, there are no established predictive biomarkers to determine which patients are at highest risk for this toxicity. We and other authors have previously identified single nucleotide polymorphisms (SNPs) that were significantly associated with an increased risk for experiencing grade 2 to 4 peripheral neuropathy in patients with breast cancer treated with taxane-containing chemotherapy,23–25 which is a finding that requires validation in other studies.
For a biomarker to be optimally useful in the clinical setting, it must demonstrate the ability to improve the risk:benefit ratio. Thus, it is important to evaluate whether there is a relationship between patients who have the most toxicity and a superior outcome. Previously, the control arm from the N9831 trial demonstrated an association between paclitaxel-related neuropathy and improved 3-year disease-free survival (DFS).26 In this analysis, our objective was to determine whether there was a relationship between taxane-induced neuropathy and outcomes in patients who received adjuvant weekly paclitaxel and other taxane-based regimens in E1199.
PATIENTS AND METHODS
Patients
The study population included patients enrolled onto trial E1199, which included 5,052 patients with axillary node-positive or high-risk, node-negative breast cancer.6 These patients were randomly assigned to one of four treatment arms. First, all patients received four cycles of intravenous doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) every 3 weeks for four treatment cycles followed by paclitaxel 175 mg/m2 every 3 weeks for four cycles (P3), paclitaxel 80 mg/m2 weekly for 12 cycles (P1), docetaxel 100 mg/m2 every 3 weeks for four cycles (D3), or docetaxel 35 mg/m2 weekly for 12 cycles (D1). Among the 5,052 patients, 102 patients did not meet the eligibility criteria, 133 patients were enrolled through the extended-participation project, 248 patients did not receive taxane therapy, and 15 patients experienced neuropathy before the first adjuvant taxane dose; all of these patients were excluded from the analysis. Therefore, the analysis cohort included 4,554 patients who met eligibility criteria and had neuropathy information after taxane treatment (CONSORT diagram is shown in Fig 1).
Fig 1.
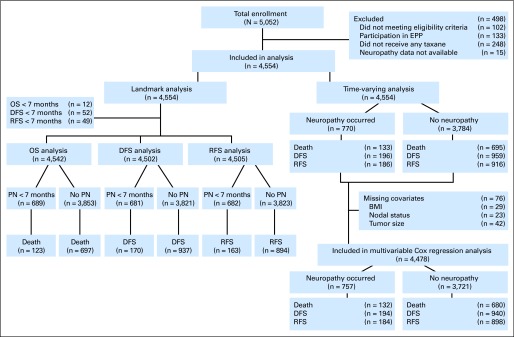
CONSORT diagram. BMI, body mass index; DFS, disease free survival; EPP, extended-participation project; OS, overall survival; PN, peripheral neuropathy; RFS, recurrence-free survival.
Phenotypes: Neuropathy and Efficacy Parameters
The phenotype evaluated was the occurrence of grade 2 to 4 neuropathy by using the National Cancer Institute Common Toxicity Criteria (version 2.0) grading scale. Investigators reported motor neuropathy, sensory neuropathy, or neuropathic pain and the last measurement that occurred 3 weeks after the conclusion of therapy. For patients who had grade 2 neuropathy, the dose was reduced by 25%. For patients who experienced grade 3 or 4 neuropathy, the dose was held until resolution to less than grade 2, at which point therapy was reduced 25%. If the grade 3 or 4 toxicity persisted longer than 3 weeks, therapy was discontinued.
The following three end points were considered: overall survival (OS), DFS, and recurrence-free survival (RFS). OS events included death as a result of any cause. DFS events included recurrence (local/regional or distant), contralateral breast cancer, or death without recurrence. RFS events were the same as DFS events but did not include contralateral breast cancer events.
Statistical Analysis
In this report, we assessed the association between the occurrence of grade 2 to 4 neuropathy and efficacy outcomes. The outcome data for the analysis were downloaded on July 2, 2010, at which time 828 of the 4,554 patients died, which included: 539 deaths as a result of breast cancer, 34 deaths as a result of protocol treatment, 105 deaths as a result of other causes, and 150 deaths with unknown causes. Among the 150 deaths with unknown causes, 118 patients had breast cancer recurrence before death. There were 1,155 DFS events and 1,102 RFS events in 4,554 patients. The median follow-up for these patients was 95.5 months (range, 3.4 to 119.1 months).
Multivariable logistic regression was used to test the association between baseline patient characteristics and neuropathy. These baseline patient characteristics included age as a categorical variable (≤ 45, 46 to 65, and > 65 years), age as a continuous variable, race (black v others), obesity (body mass index ≥ 30 kg/m2 v others), and menopausal status (premenopausal v postmenopausal). The association between the occurrence of grade 2 to 4 hyperglycemia at any time after the start of therapy and neuropathy was tested by univariate and multivariable logistic regression. Univariate logistic regression was also used to test the association between treatment and the occurrence of neuropathy.
Kaplan-Meier curves were used to estimate event-time distribution, and the log-rank test was conducted to test the significance in distribution difference between patients with or without neuropathy by using the landmark method, with 7 months from random assignment as the landmark time point. Seven months was chosen as the landmark because it corresponded to the period of adjuvant chemotherapy that spanned 24 weeks. Specifically, neuropathy was coded as present if it occurred before 7 months from random assignment and was coded as absent if it had never occurred or occurred after 7 months from random assignment. In the DFS landmark analyses, DFS was the time from the landmark time point to an event, and patients who had DFS events before the landmark time point were excluded from the analysis (Fig 1). The OS and RFS landmark analyses were conducted similarly. Cox proportional hazards methods, in which neuropathy was treated as a time-varying covariate, were used to estimate unadjusted and adjusted hazards ratios for neuropathy. In the time-varying covariate analyses, the coding of neuropathy was changed from absent to present at the time of its first occurrence. DFS was the time from random assignment to the first event. All P values were two sided, and CIs were at the 95% level. Bonferroni's correction was conducted to adjust for multiple comparisons for the survival analysis.
RESULTS
Occurrence of Peripheral Neuropathy
Among 4,554 patients, 770 patients (16.9%) experienced grade 2 to 4 neuropathy after the initiation of adjuvant taxane therapy. The highest grades of neuropathy experienced were as follows: grade 2, 71.8%; grade 3, 27.5%; and grade 4, 0.7%. The median time to neuropathy after receipt of the first dose of taxane was 3.0 months (range, 0 to 57.0 months). The start date for taxane use was missing in seven patients, and thus, these seven patients were excluded for the calculation of the median time to neuropathy. When treatment arms were compared, the percentage of grade 2 to 4 neuropathy was 17.5% (202 of 1,157 patients) in the P3 arm, 22.0% (249 of 1,130 patients) in the P1 arm, 14.7% (168 of 1,144 patients) in the D3 arm, and 13.4% (151 of 1,123 patients) in the D1 arm. The risk of grade 2 to 4 neuropathy was higher in the P1 arm than in the P3 arm (odds ratio, [OR], 1.34; 95% CI, 1.09 to 1.64). The risk of neuropathy was lower in the D1 arm (OR, 0.73; 95% CI, 0.58 to 0.92) and D3 arm (OR, 0.81; 95% CI, 0.65 to 1.02) than in the P3 arm (Table 1). The proportion of patients who required a dose reduction for any reason was not significantly different in patients who developed grade 2 to 4 neuropathy compared with those who did not develop grade 2 to 4 neuropathy in any arm. There was also no significant difference in the median relative dose intensity in patients who developed neuropathy compared with those who did not develop neuropathy in any arm.
Table 1.
Impact of Treatment Arm and Clinical Covariates on Likelihood of Peripheral Neuropathy
| Model and Covariate | All Patients |
Weekly Paclitaxel |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Model 1 | ||||||
| Age, continuous variable | 1.0 | 0.98 to 1.01 | .354 | 0.99 | 0.97 to 1.01 | .184 |
| Obese, v not | 1.15 | 0.98 to 1.35 | .095 | 1.23 | 0.91 to 1.65 | .176 |
| Black race, v others | 1.22 | 0.93 to 1.60 | .145 | 1.52 | 0.95 to 2.43 | .078 |
| Premenopausal, v postmenopausal | 0.77 | 0.61 to 0.97 | .025 | 0.70 | 0.45 to 1.06 | .092 |
| Model 2 | ||||||
| Hyperglycemia at any time, v not | 1.47 | 1.17 to 1.84 | < .001 | 1.98 | 1.25 to 3.14 | .004 |
| Model 3 | ||||||
| P1 > P3 | 1.34 | 1.09 to 1.64 | .006 | |||
| D3 > P3 | 0.81 | 0.65 to 1.02 | .070 | |||
| D1 > P3 | 0.73 | 0.58 to 0.92 | .008 | |||
NOTE: Model 1 used multivariable logistic regression to test the association between characteristics of patients at baseline and occurrence of neuropathy. Model 2 used univariate logistic regression to test the association between the occurrence of hyperglycemia and occurrence of neuropathy. Model 3 used univariate logistic regression to test the association between treatment arm and occurrence of neuropathy.
Abbreviations: D1, weekly docetaxel arm; D3, every 3-week docetaxel arm; OR, odds ratio; P1, weekly paclitaxel arm; P3, every 3-week paclitaxel arm.
Patient Characteristics Associated With Neuropathy
Multivariable logistic regression was used to evaluate the relationship between the baseline characteristics of patients and the development of neuropathy (Table 1). There was a decreased risk of neuropathy in premenopausal compared with postmenopausal patients (OR, 0.77; 95% CI, 0.61 to 0.97). Age, whether considered as a categorical or continuous variable, was not associated with neuropathy. There was a trend for a higher risk of neuropathy in blacks compared with other races (OR, 1.22; 95% CI, 0.93 to 1.60) and in obese patients compared with nonobese patients (OR, 1.14; 95% CI, 0.97 to 1.35). Information regarding a pre-existing history of diabetes was not available, but we explored the relationship between neuropathy and occurrence of treatment-related grade 2 to 4 hyperglycemia as a surrogate for glycemic instability. There was a significant association between hyperglycemia and neuropathy (unadjusted OR, 1.47; 95% CI, 1.17 to 1.84) that remained significant after adjustment for age, race, obesity, and menopausal status (adjusted OR, 1.42; 95% CI, 1.13 to 1.78). To more directly compare these associations with those seen in E5103 (in which weekly paclitaxel was the taxane backbone),23 we evaluated baseline characteristics specifically in the weekly paclitaxel arm (Table 1). Similar to in E5103, there was a strong trend for increased risk of neuropathy with black race compared with all other races (OR, 1.52; 95% CI, 0.95 to 2.43). Unlike in E5103, however, there was no association between the risk of neuropathy and age.
Analysis for Association Between Patient/Tumor Characteristics and OS
Baseline characteristics of patients were tested for associations with OS and included age, race, obesity, and menopausal status. Tumor characteristics were tested for associations and included nodal status and tumor size. In addition, treatment-related toxicities were tested and included grade 2 to 4 neuropathy and grade 2 to 4 hyperglycemia. When all arms from E1199 were evaluated, obesity, nodal status, and tumor size were all associated with survival (Appendix Table A1, online only). We also evaluated for an association within each of the four treatment arms of E1199. The only variables associated with an inferior survival across all arms included having greater than three involved nodes and having a tumor greater than 2 cm in size.
Univariate Analysis for Association Between Neuropathy and Efficacy
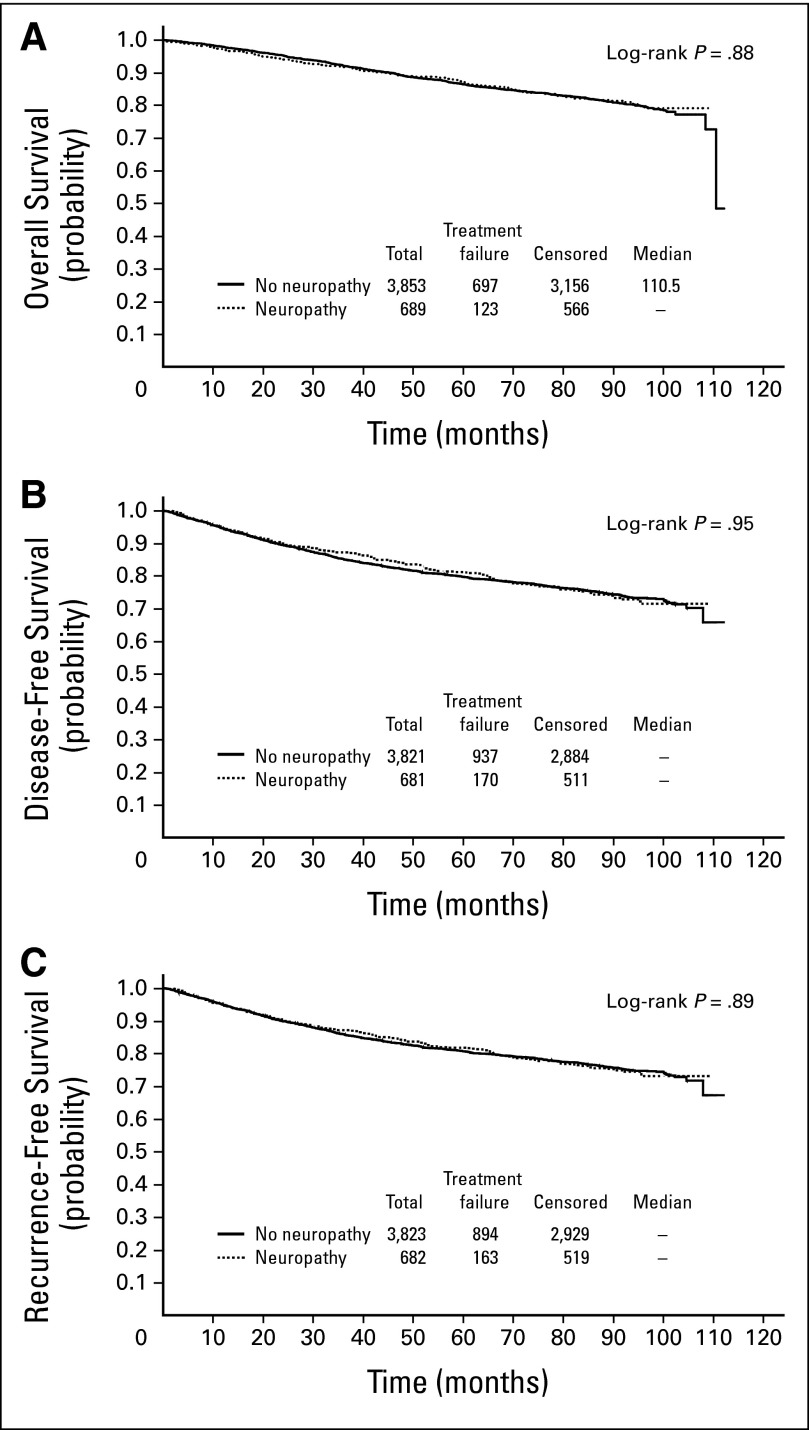
Neuropathy was tested for associations with efficacy parameters including OS, DFS, and RFS. Neuropathy was not associated with any of these end points when evaluated by Cox time-varying analysis (Table 2) and the log-rank test in landmark analysis (Fig 2). Likewise, neuropathy was tested for associations with efficacy parameters in the common clinical subgroups of tumors estrogen receptor-positive (ER+)/ human epidermal growth factor receptor 2-negative (HER2-negative), HER2-postive, or triple-negative breast cancer (as determined by institutional pathology laboratories). Neuropathy was not significantly associated with outcome in any of these specific subgroups (Table 2; Appendix Fig A1, online only).
Table 2.
Univariate Analysis for Association Between Neuropathy and Survival Outcomes Using a Cox Time-Varying Model
| Tumor Subtype | No. of Patients | Phenotype | No. of Events | HR | 95% CI | P |
|---|---|---|---|---|---|---|
| All types | 4,554 | OS | 828 | 0.89 | 0.73 to 1.07 | .21 |
| DFS | 1155 | 0.98 | 0.84 to 1.15 | .84 | ||
| RFS | 1102 | 0.99 | 0.84 to 1.16 | .88 | ||
| HER2 positive | 912 | OS | 178 | 0.72 | 0.46 to 1.14 | .17 |
| DFS | 246 | 0.77 | 0.52 to 1.12 | .17 | ||
| RFS | 232 | 0.82 | 0.56 to 1.21 | .33 | ||
| TNBC | 838 | OS | 225 | 1.21 | 0.89 to 1.66 | .23 |
| DFS | 277 | 1.17 | 0.87 to 1.56 | .29 | ||
| RFS | 261 | 1.15 | 0.85 to 1.56 | .35 | ||
| ER positive/HER2 negative | 2,645 | OS | 388 | 0.76 | 0.57 to 1.01 | .06 |
| DFS | 587 | 0.96 | 0.77 to 1.19 | .69 | ||
| RFS | 564 | 0.95 | 0.76 to 1.19 | .66 |
NOTE: Among the 4,554 patients, 459 patients had missing values for HER2 status, 48 patients had missing values for ER status, and 65 patients had missing values for progesterone receptor status. P was compared with P < .05/3 = .017 for the significance test for the three subgroup analyses.
Abbreviations: DFS, disease-free survival; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival; RFS, recurrence-free survival; TNBC, triple-negative breast cancer.
Fig 2.
(A) Kaplan Meier curve for association of neuropathy with overall survival for all patients using landmark analysis. (B) Kaplan-Meier curve for association of neuropathy with disease-free survival for all patients using landmark analysis. (C) Kaplan-Meier curve for association of neuropathy with recurrence-free survival for all patients using landmark analysis.
Multivariable Analysis for Association Between Neuropathy and Efficacy
Multivariable analysis was performed to test for associations of neuropathy with efficacy parameters with adjustment for covariates including age, race, obesity, menopausal status, tumor size, nodal status, treatment arm, and treatment-associated grade 2 to 4 hyperglycemia. Neuropathy was not associated with any clinical outcome after adjustment for covariates when evaluating by Cox time-varying analysis (Table 3). There was no association with efficacy parameters with any specific tumor subtype (ER-positive/HER2-negative, HER2-positive, and triple-negative breast cancer) when evaluating by Cox time-varying analysis (Table 3). Neuropathy did not correlate with OS after adjustment for covariates when evaluating by individual treatment arm with Cox time-varying analysis (Appendix Table A1, online only).
Table 3.
Multivariable Analysis for Association Between Neuropathy and Survival Outcomes Using a Cox Time-Varying Model
| Tumor Subtype | No. of Patients | Phenotype | No. of Events | HR | 95% CI | P |
|---|---|---|---|---|---|---|
| All types | 4,478 | OS | 812 | 0.96 | 0.79 to 1.16 | .66 |
| DFS | 1134 | 1.01 | 0.86 to 1.18 | .90 | ||
| RFS | 1082 | 1.01 | 0.86 to 1.19 | .89 | ||
| HER2 positive | 900 | OS | 177 | 0.80 | 0.51 to 1.26 | .33 |
| DFS | 244 | 0.77 | 0.52 to 1.14 | .19 | ||
| RFS | 230 | 0.83 | 0.56 to 1.23 | .35 | ||
| TNBC | 829 | OS | 219 | 1.31 | 0.95 to 1.81 | .10 |
| DFS | 270 | 1.19 | 0.89 to 1.61 | .24 | ||
| RFS | 255 | 1.17 | 0.86 to 1.59 | .32 | ||
| ER positive/HER2 negative | 2,614 | OS | 384 | 0.78 | 0.58 to 1.05 | .10 |
| DFS | 581 | 0.97 | 0.78 to 1.22 | .81 | ||
| RFS | 558 | 0.96 | 0.77 to 1.21 | .75 |
NOTE: Adjusted covariates included age (≤ 45, 46-65, and > 65 years), race (black v others), obesity (body mass index ≥ 30 kg/m2 v others), menopausal status (premenopausal v postmenopausal), hyperglycemia (yes v no), tumor size (> 2 v ≤ 2 cm), nodal status (0, 1-3 and > 3), and treatment arm (every 3-week paclitaxel, weekly paclitaxel, every 3-week docetaxel, and weekly docetaxel arms). There were 76 patients with missing values for body mass index (29 patients) and/or node status (23 patients) and/or tumor size (42 patients), and 4,478 patients were included in the multivariable Cox regression for all patients. Among the 4,478 patients, 430 patients had missing values for HER2 status, 25 patients had missing ER status, and 42 patients had missing progesterone receptor status. P was compared with P < .05/3 = .017 for the significance test for the three subgroup analyses.
Abbreviations: DFS, disease-free survival; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival; RFS, recurrence-free survival; TNBC, triple-negative breast cancer.
DISCUSSION
Taxanes are an important class of therapy for patients with breast cancer. The most common and potentially debilitating toxicity is peripheral neuropathy, which may be permanent or only partly reversible in some cases and adversely impact the quality of life.4,7,8 This toxicity is problematic in the metastatic setting in which treatment until progression or toxicity is a common approach. In addition, this toxicity can also be problematic in the adjuvant setting because it may limit the delivery of potentially curative therapy. We found that, although neuropathy was a common complication that was associated with necessary dose reductions, it was not associated with a higher risk of recurrence or inferior survival.
A limitation of this analysis was that the phenotype was determined by open toxicity assessment. The lack of a uniform and validated assessment decreased the reliability of the reporting. In addition, the lack of patient-reported outcomes made it difficult to assess the impact of the neuropathy on the quality of life of patients. The large sample size in this analysis, however, improved the likelihood that a signal would be detected despite imperfect phenotypes. Another limitation is that the duration of toxicity was not captured in E1199 with the last formal assessment at 3 weeks after the last taxane dose.
We did not capture information about comorbidities at baseline, which may have been associated with neuropathy risk, such as diabetes. To address this, we evaluated the relationship between treatment-related grade 2 to 4 hyperglycemia and neuropathy and also with clinical outcomes. Given that corticosteroid premedication was used in all arms, we hypothesized that hyperglycemia might serve as a surrogate for clinical or subclinical diabetes. Hyperglycemia during treatment was associated with an increased risk of neuropathy as hypothesized. It was also associated with inferior outcomes in the P3 arm but not in the other treatment arms or overall population. These findings, however, must be interpreted with caution because treatment-associated hyperglycemia may be multifactorial and not reflect patients with a formal diagnosis of diabetes. Race and obesity showed a strong trend toward an increased risk of neuropathy but did not reach statistical significance in the multivariable logistic regression analysis. In the weekly paclitaxel arm (which mirrored that used in E5103) there was a nonsignificant trend for increased risk with black race. The HR for race in E5103 (HR for black race v all others, 2.1) was within the CIs of this analysis for the weekly paclitaxel arm (HR, 1.52; 95% CI, 0.95 to 2.43).23 Age, which was significantly associated with the risk of neuropathy in E5103 (12.9% increased risk with each decade of life; P = .004)23 and other trials27 was not seen in this study whether we evaluated age as a continuous or a categorical variable although premenopausal status was.
The mechanism by which taxanes cause peripheral neuropathy is not conclusively elucidated. Some evidence suggested that the disruption of microtubule function may hinder axonal transport, although there are multiple other possible etiologies.9–17 Multiple agents have been tested to help prevent this toxicity or to minimize symptoms after development without success,18 which reflects the incomplete understanding of its pathophysiology and an inability to identify who is at greatest risk for developing this toxicity. To further complicate clinical decision making, there is substantial heterogeneity in the degree of neuropathy from patient to patient. Although there are a few established clinical risk factors,20–23 the majority of variation is not predictable, and there are no validated predictive biomarkers for this toxicity.
A predictive biomarker for neuropathy could prove valuable in clinical decision making; especially because patients who develop neuropathy do not derived a greater benefit from adjuvant taxane therapy. We recently reported a genome-wide association study in 2,204 patients with stage I to III breast cancer from clinical trial E5103 who received weekly paclitaxel similar to that in one of the arms in the E1199 trial.23 We identified several SNPs that were associated with the likelihood of developing paclitaxel-induced neuropathy. In addition, other groups have reported SNPs that correlate with taxane-induced neuropathy in large clinical trials.24,25 Our findings from the E1199 trial indicated no relationship between paclitaxel- or docetaxel-induced neuropathy and clinical outcomes, which therefore, provide additional rationale for the validation of these SNPs in other patient populations treated with adjuvant taxane therapy.
E1199 was a landmark study that tested two taxanes with two different schedules by using a two-by-two factorial design.6 Although an unexpected negative treatment interaction between the weekly schedule and docetaxel resulted in no significant difference when taxane types or schedules were compared, the trial was also adequately powered to compare each of the three experimental arms to the control arm of every 3-week paclitaxel treatment, which was the standard taxane regimen at the time the trial was initiated. Compared with the standard arm, the weekly paclitaxel arm was associated with improved DFS and OS, and the every 3-week docetaxel arm was associated with improved DFS but not survival at the time of the planned analysis. Because both OS and DFS were improved, and there was less overall toxicity, the weekly paclitaxel arm of this trial has become a commonly used regimen in clinical practice28 and served as the reference chemotherapy arm of E5103 that evaluated the role of adjuvant bevacizumab.23 Other studies have shown a benefit for docetaxel-containing regimens, which are also commonly used in clinical practice3,29 and are generally associated with more overall grade 3 to 4 toxicity but lower neuropathy rates than observed with weekly paclitaxel.5,6,30 The development of a biomarker predictive of neuropathy could be an important tool for choosing an adjuvant taxane regimen and facilitate improved therapeutic individualization, especially if taxane-induced neuropathy is not a pharmacodynamic marker of taxane benefit. Toward this end, we are currently studying the top candidate SNPs (from our correlative work in E5103) in E1199 with the dual goal of validating predictive biomarkers for paclitaxel and to learn whether these markers are generalizable to both commonly implemented taxanes.
Other studies have also evaluated the relationship between taxane-induced neuropathy and clinical outcomes. In patients with HER2/neu overexpressing disease treated in intergroup trial N9831 who received doxorubicin and cyclophosphamide followed by a weekly paclitaxel regimen similar to that used in E1199 and E5103, there was an association between neuropathy and improved outcomes. For that study, patients in the control arm who had grade 2 to 4 neuropathy had an improved 3-year DFS (84.2% v 77.8%; HR, 0.64; P = .01), but this was not observed in the trastuzumab-containing arms.26 In this evaluation of E1199 (which did not include trastuzumab), we found that patients with HER2/neu overexpressing disease who developed neuropathy showed a trend toward improved DFS, OS, and RFS, but this was not statistically significant. Although this trial could not exclude the possibility of a small effect for neuropathy association with outcome in this tumor subtype, any real association would likely be quite small. In addition, there is no clear biologic rationale for why the tumor subtype would influence the impact of neuropathy on outcomes.
In conclusion, this analysis of greater than 4,500 patients demonstrated that taxane-induced peripheral neuropathy does not correlate with improved outcomes in patients with operable breast cancer treated with adjuvant taxane therapy. This finding provides reassurance that biomarkers predictive for neuropathy will likely not enrich for patients who are more likely to benefit from taxane therapy and may also be useful for the identification of patients who are most likely to benefit from adjunctive therapies to mitigate neuropathy.
Supplementary Material
Appendix
Table A1.
Association of Baseline Characteristics and Development of Neuropathy With OS by Treatment Arm by Using a Cox Time-Varying Model
| Covariate | All Arms(N = 4,478; 812 events) |
P3(n = 1,141; 222 events) |
P1(n = 1,109; 188 events) |
D3(n = 1,123; 184 events) |
D1(n = 1,105; 218 events) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Neuropathy, yes v no | 0.95 | 0.79 to 1.15 | .61 | 1.00 | 0.71 to 1.42 | .99 | 0.90 | 0.62 to 1.28 | .54 | 1.05 | 0.69 to 1.58 | .82 | 0.80 | 0.53 to 1.22 | .30 |
| Age, years | |||||||||||||||
| 46-65 v ≤ 45 | 0.86 | 0.70 to 1.06 | .16 | 0.70 | 0.48 to 1.05 | .08 | 1.14 | 0.74 to 1.77 | .55 | 0.59 | 0.37 to 0.96 | .034 | 1.08 | 0.74 to 1.58 | .70 |
| > 65 v ≤ 45 | 1.01 | 0.75 to 1.36 | .95 | 0.80 | 0.45 to 1.41 | .43 | 1.43 | 0.76 to 2.70 | .27 | 0.77 | 0.40 to 1.46 | .42 | 1.00 | 0.56 to 1.80 | .99 |
| Race, black v others | 1.23 | 0.97 to 1.57 | .09 | 1.17 | 0.74 to 1.85 | .51 | 1.70 | 1.10 to 2.64 | .016 | 0.88 | 0.51 to 1.52 | .64 | 1.20 | 0.73 to 1.99 | .48 |
| Obese, BMI ≥ 30 v others | 1.20 | 1.04 to 1.38 | .01 | 1.18 | 0.90 to 1.56 | .23 | 1.20 | 0.89 to 1.62 | .24 | 1.37 | 1.01 to 1.86 | .040 | 1.13 | 0.86 to 1.49 | .39 |
| Premenopausal, v no | 0.83 | 0.68 to 1.01 | .06 | 0.79 | 0.54 to 1.16 | .23 | 0.92 | 0.62 to 1.37 | .69 | 0.61 | 0.38 to 0.96 | .034 | 0.97 | 0.68 to 1.39 | .88 |
| LN | |||||||||||||||
| 1-3 v 0 | 1.52 | 1.14 to 2.02 | .004 | 1.22 | 0.74 to 2.01 | .43 | 1.75 | 0.95 to 3.24 | .074 | 1.42 | 0.76 to 2.66 | .26 | 1.89 | 1.05 to 3.43 | .0349 |
| > 3 v 0 | 3.00 | 2.27 to 3.97 | < .001 | 2.18 | 1.35 to 3.53 | .002 | 3.22 | 1.75 to 5.92 | < .001 | 3.31 | 1.80 to 6.08 | < .001 | 3.74 | 2.09 to 6.69 | < .001 |
| Tumor size, > 2 v ≤ 2 cm | 1.91 | 1.62 to 2.25 | < .001 | 1.96 | 1.43 to 2.70 | < .001 | 2.20 | 1.55 to 3.12 | < .001 | 1.84 | 1.31 to 2.60 | < .001 | 1.74 | 1.27 to 2.37 | < .001 |
| Hyperglycemia,yes v no | 1.11 | 0.89 to 1.35 | .38 | 1.54 | 1.08 to 2.19 | .018 | 1.26 | 0.79 to 2.02 | .33 | 0.88 | 0.59 to 1.31 | .52 | 0.75 | 0.45 to 1.25 | .27 |
NOTE: Results were not adjusted for multiple comparisons.
Abbreviations: BMI, body mass index; D1, weekly docetaxel arm; D3, every 3-week docetaxel arm; HR, hazard ratio; LN, lymph node; P1, weekly paclitaxel arm; P3, every 3-week paclitaxel arm.
Fig A1.
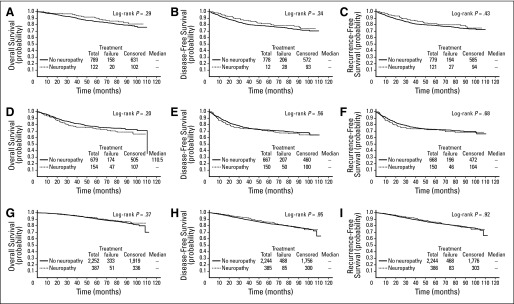
Kaplan-Meier curves for association of neuropathy with outcome using landmark analysis by tumor subtype. Kaplan-Meier curves (A to C) represent human epidermal growth factor receptor 2 (HER2) –positive subtype. Kaplan-Meier curves (G to I) represent estrogen receptor–positive and HER2-negative subtype.
Footnotes
See accompanying editorial on page 3039; listen to the podcast by Dr Rugo at www.jco.org/podcasts
Supported in part by the Department of Health and Human Services and the National Institutes of Health (Grants No. CA14958 to the Albert Einstein College of Medicine, CA23318 to the Eastern Cooperative Oncology Group [ECOG] statistical center, CA66636 to the ECOG data management center, CA21115 to the ECOG coordinating center and chairman's office, CA32012 to SWOG, CA11789 to the Cancer and Acute Leukemia Group B, CA25224 to the North Central Cancer Treatment Group, CA49883 to the Indiana University School of Medicine, and CA16116 to the Johns Hopkins Oncology Center).
Presented in part at the American Society of Clinical Oncology Breast Cancer Symposium, San Francisco, CA, September 12, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00004125.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Joseph A. Sparano, Sanofi-Aventis (C) Stock Ownership: None Honoraria: None Research Funding: Vered Stearns, Abraxis, Merck, Novartis, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Bryan P. Schneider, Vered Stearns, Edith A. Perez, George W. Sledge Jr, Joseph A. Sparano
Collection and assembly of data: Bryan P. Schneider, Fengmin Zhao, Molin Wang, Edith A. Perez, George W. Sledge Jr, Joseph A. Sparano
Data analysis and interpretation: Bryan P. Schneider, Fengmin Zhao, Molin Wang, Vered Stearns, Silvana Martino, Vicky Jones, Tom Saphner, Antonio C. Wolff, George W. Sledge Jr, William C. Wood, Nancy E. Davidson, Joseph A. Sparano
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 2.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 3.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 4.Bria E, Nistico C, Cuppone F, et al. Benefit of taxanes as adjuvant chemotherapy for early breast cancer: Pooled analysis of 15,500 patients. Cancer. 2006;106:2337–2344. doi: 10.1002/cncr.21886. [DOI] [PubMed] [Google Scholar]
- 5.Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: Final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 6.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24:1633–1642. doi: 10.1200/JCO.2005.04.0543. [DOI] [PubMed] [Google Scholar]
- 8.Swain SM, Arezzo JC. Neuropathy associated with microtubule inhibitors: Diagnosis, incidence, and management. Clin Adv Hematol Oncol. 2008;6:455–467. [PubMed] [Google Scholar]
- 9.Cavaletti G, Tredici G, Braga M, et al. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol. 1995;133:64–72. doi: 10.1006/exnr.1995.1008. [DOI] [PubMed] [Google Scholar]
- 10.Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez-Andrade JM, Peters CM, Mejia NA, et al. Sensory neurons and their supporting cells located in the trigeminal, thoracic and lumbar ganglia differentially express markers of injury following intravenous administration of paclitaxel in the rat. Neurosci Lett. 2006;405:62–67. doi: 10.1016/j.neulet.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 12.Nakata T, Yorifuji H. Morphological evidence of the inhibitory effect of taxol on the fast axonal transport. Neurosci Res. 1999;35:113–122. doi: 10.1016/s0168-0102(99)00074-7. [DOI] [PubMed] [Google Scholar]
- 13.Persohn E, Canta A, Schoepfer S, et al. Morphological and morphometric analysis of paclitaxel and docetaxel-induced peripheral neuropathy in rats. Eur J Cancer. 2005;41:1460–1466. doi: 10.1016/j.ejca.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Peters CM, Jimenez-Andrade JM, Kuskowski MA, et al. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007;1168:46–59. doi: 10.1016/j.brainres.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raine CS, Röytta M, Dolich M. Microtubule-mitochondrial associations in regenerating axons after taxol intoxication. J Neurocytol. 1987;16:461–468. doi: 10.1007/BF01668500. [DOI] [PubMed] [Google Scholar]
- 16.Theiss C, Meller K. Taxol impairs anterograde axonal transport of microinjected horseradish peroxidase in dorsal root ganglia neurons in vitro. Cell Tissue Res. 2000;299:213–224. doi: 10.1007/s004419900120. [DOI] [PubMed] [Google Scholar]
- 17.Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf S, Barton D, Kottschade L, et al. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Park SB, Krishnan AV, Lin CS, et al. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15:3081–3094. doi: 10.2174/092986708786848569. [DOI] [PubMed] [Google Scholar]
- 20.Gogas H, Shapiro F, Aghajanian C, et al. The impact of diabetes mellitus on the toxicity of therapy for advanced ovarian cancer. Gynecol Oncol. 1996;61:22–26. doi: 10.1006/gyno.1996.0090. [DOI] [PubMed] [Google Scholar]
- 21.Rowinsky EK, Chaudhry V, Cornblath DR, et al. Neurotoxicity of Taxol. J Natl Cancer Inst Monogr. 1993;15:107–115. [PubMed] [Google Scholar]
- 22.Rowinsky EK, Eisenhauer EA, Chaudhry V, et al. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20(4 suppl 3):1–15. [PubMed] [Google Scholar]
- 23.Schneider B, Li L, Miller K, et al. Genetic associations with taxane-induced neuropathy by genome wide association study (GWAS) in E5103. J Clin Oncol. 2011;29(suppl; abstr 1000):80s. [Google Scholar]
- 24.Kroetz DL, Baldwin RM, Owzar K, et al. Inherited genetic variation in EPHA5, FGD4, and NRDG1 and paclitaxel (P)-induced peripheral neuropathy (PN): Results from a genome-wide association study (GWAS) in CALGB 40101. J Clin Oncol. 2010;28(suppl; abstr 3021):238s. [Google Scholar]
- 25.Sucheston LE, Zhao H, Yao S, et al. Genetic predictors of taxane-induced neurotoxicity in a SWOG phase III intergroup adjuvant breast cancer treatment trial (S0221) Breast Cancer Res Treat. 2011;130:993–1002. doi: 10.1007/s10549-011-1671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno-Aspitia A, Dueck A, Patel T, et al. Paclitaxel-related peripheral neuropathy associated with improved outcome of patients with early stage HER2+ breast cancer who did not receive trastuzumab in the N9831 clinical trial. San Antonio Breast Cancer Symposium. 2009 [Google Scholar]
- 27.Akerley W, Herndon JE, Egorin MJ, et al. Weekly, high-dose paclitaxel in advanced lung carcinoma: A phase II study with pharmacokinetics by the Cancer and Leukemia Group B. Cancer. 2003;97:2480–2486. doi: 10.1002/cncr.11375. [DOI] [PubMed] [Google Scholar]
- 28.US Department of Health and Human Services: National Guideline Clearinghouse. http://www.guideline.gov/
- 29.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 30.Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–5551. doi: 10.1200/JCO.2005.02.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.