Abstract
Purpose
Advanced follicular lymphomas (FL) are considered incurable with conventional chemotherapy and there is no consensus on the best treatment approach. Southwest Oncology Group (SWOG) and Cancer and Leukemia Group B compared the safety and efficacy of two immunochemotherapy regimens for FL in a phase III randomized intergroup protocol (SWOG S0016) that enrolled 554 patients with previously untreated, advanced-stage FL between March 1, 2001, and September 15, 2008.
Patients and Methods
Patients were eligible for the study if they had advanced-stage (bulky stage II, III, or IV) evaluable FL of any grade (1, 2, or 3) and had not received previous therapy. In one arm of the study, patients received six cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy at 3-week intervals with six doses of rituximab (CHOP-R). In another arm of the study, patients received six cycles of CHOP followed by consolidation with tositumomab/iodine I-131 tositumomab radioimmunotherapy (RIT).
Results
After a median follow-up period of 4.9 years, the 2-year estimate of progression-free survival (PFS) was 76% on the CHOP-R arm and 80% on the CHOP-RIT arm (P = .11). The 2-year estimate of overall survival (OS) was 97% on the CHOP-R arm and 93% on the CHOP-RIT arm (P = .08).
Conclusion
There was no evidence of a significant improvement in PFS comparing CHOP-RIT with CHOP-R. However, PFS and OS were outstanding on both arms of the study. Future studies are needed to determine the potential benefits of combining CHOP-R induction chemotherapy with RIT consolidation and/or extended rituximab maintenance therapy.
INTRODUCTION
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma and is characterized by an indolent clinical course presenting with generalized lymphadenopathy and marrow involvement.1 Eighty-five percent of patients have disseminated disease at the time of diagnosis, and such patients are considered to be incurable with conventional therapies.1 Nevertheless, survival of FL patients has markedly improved in the last 15 years as a result of innovations in patient management, including implementation of immunochemotherapy regimens incorporating both chemotherapy and monoclonal antibodies.2–4 To date, however, there is no consensus on the best immunochemotherapeutic regimen for front-line management of advanced FL. A recent patterns of care study assessed treatment of 2,728 patients at 265 centers between 2004 and 2007.5 The initial therapy was chemotherapy plus rituximab in 52%, observation in 18%, rituximab monotherapy in 14%, and other treatments in 15%. Among patients treated with rituximab chemotherapy, cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) plus rituximab (CHOP-R) was administered to 55%; cyclophosphamide, vincristine, prednisone, and rituximab (CVP-R) was administered to 23%; and fludarabine plus rituximab regimens were administered to 15%.5 A recent large international clinical trial revealed similar distributions, with 74% of 1,202 patients receiving CHOP-R and 23% CVP-R.6
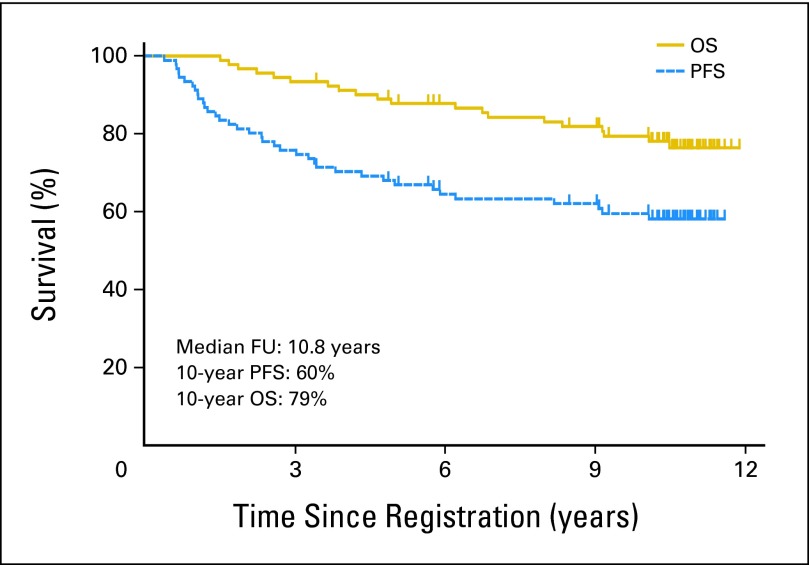
Southwest Oncology Group (SWOG) has conducted sequential studies of various regimens for FL, with protocol SWOG S9911 being the most promising. This phase II study administered six cycles of CHOP followed by tositumomab and 131I-tositumomab consolidative radioimmunotherapy (RIT).7–8 The updated progression-free survival (PFS) and overall survival (OS) curves after a median follow-up period of 10.8 years show that 60% of patients are projected to be progression-free and 79% are projected to be alive after 10 years (Fig 1), which compares favorably with other studies in advanced-stage FL. At least six other phase II-III studies also support the promise of front-line RIT for management of lymphomas.9–15 These promising results encouraged SWOG to perform a phase III trial comparing the experimental arm from S9911 (CHOP plus 131I-tositumomab) with CHOP chemotherapy alone (the SWOG standard in 1999 when the study was designed) and with CHOP plus six doses of rituximab (a promising new immunochemotherapy regimen in 1999).16 Cancer and Leukemia Group B joined the trial on December 15, 2001. The objectives of trial S0016 were to compare the PFS, OS, overall response rate, complete remission (CR) rate, toxicities, and rate of human antimouse antibody (HAMA) formation after frontline treatment of advanced FL with these regimens. The mature results of this trial, with a median follow-up of 4.9 years, comprise our report.
Fig 1.
Progression-free survival (PFS) and overall survival (OS) of 90 eligible patients with advanced-stage follicular non-Hodgkin lymphoma treated with six cycles of cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy followed by tositumomab/131I-tositumomab on Southwest Oncology Group protocol S9911. FU, follow-up.
PATIENTS AND METHODS
Eligibility
Patients older than 18 years with biopsy-proven, untreated, bidimensionally measurable bulky stage II or stage III-IV FL (grade 1, 2, or 3) expressing CD20 were eligible if they had a performance status of 0 to 2, granulocytes ≥ 1,500 cells/μL, and platelets ≥ 100,000/μL. Bulky adenopathy was defined as more than 10 cm or greater than one third the thoracic diameter. Patients were excluded if they received any prior therapy, were HIV-positive, had CNS involvement, were pregnant or lactating, or had serious cardiac disease or a prior malignancy (except nonmelanoma skin cancer or in situ cervical cancer). Investigators were counseled not to enroll asymptomatic, low-tumor-burden patients for whom watchful waiting would be appropriate, but the registering physician was ultimately responsible for deciding if treatment was necessary. All patients submitted written informed consent in accordance with institutional and federal guidelines.
Pathology Review
All diagnostic biopsies were reviewed centrally by expert pathologists of SWOG to confirm the diagnosis of FL in accordance with WHO 2008 morphologic, immunophenotypic, and genetic criteria. Patients with greater than 25% diffuse architecture and more than 15 centroblasts per high power field were considered to have diffuse large B-cell lymphoma and were excluded. Excisional biopsies or core-needle biopsies large enough to show follicular architecture were mandatory; fine-needle aspirates or marrow biopsies alone were inadequate. CD20 expression demonstrated by flow cytometry or immunoperoxidase staining was required.
Baseline Studies
All patients received a physical examination, had their patient history recorded, and received tests for complete blood cell count, β2 microglobulin, lactate dehydrogenase, chest radiograph or computed tomography (CT) scan, abdominal and pelvic CT, and bone marrow biopsy. Blood chemistries, thyroid-stimulating hormone levels, urinalysis, and electrocardiography were also performed. Patients with heart disease were only eligible if the cardiac ejection fraction was normal. The protocol was amended on December 15, 2006, to require serum submission for centralized HAMA testing using a commercial enzyme-linked immunosorbent assay (UBI MIGIWEL HAMA kit, United Biotech, Mountain View, CA) on days 0, 133, 200, 365, and 596. Patients beyond day 596 at the time of amendment submitted a single HAMA specimen at their next study assessment.
Study Design and Protocol Treatment
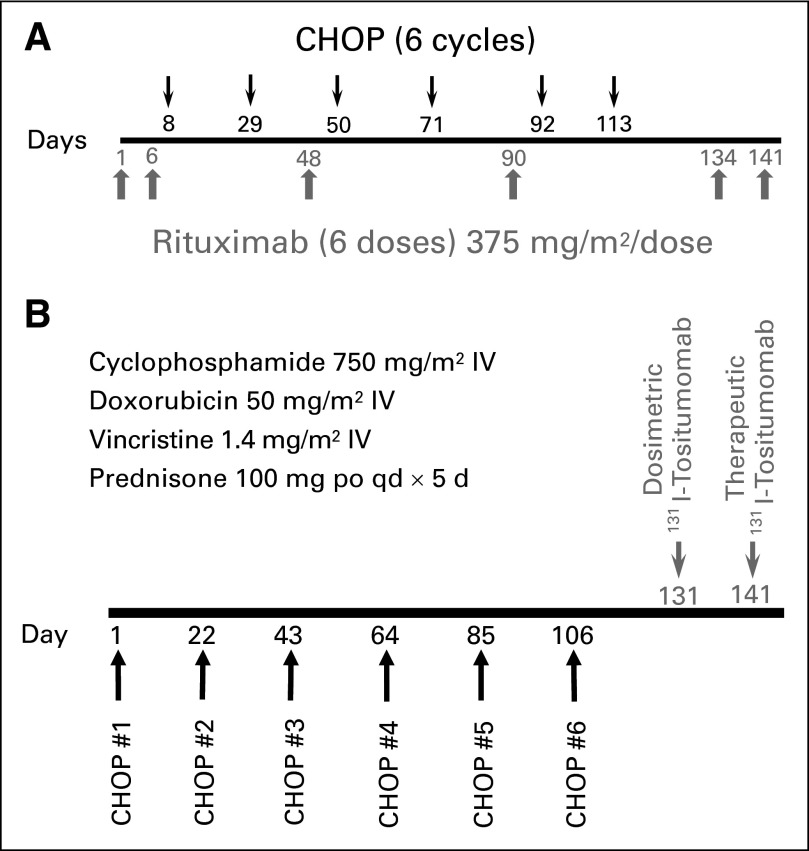
All patients received CHOP every 21 days for six cycles with supportive care and dose reductions as published.7–8 The CHOP-alone arm was closed on December 15, 2002, after 17 patients were accrued because of emerging data that demonstrated the addition of six doses of rituximab to CHOP17 and to other induction chemotherapy regimens18–21 markedly improves PFS and OS in FL. Patients on the CHOP-R arm were treated as described by Czuczman et al16 with 375 mg/m2 of rituximab on days 1, 6, 48, 90, 134, and 141 and with CHOP chemotherapy on days 8, 29, 50, 71, 92, and 113 (Fig 2A). Patients on the CHOP-RIT arm received RIT 4 to 8 weeks after the sixth cycle of CHOP, as published for S9911 (Fig 2B).7–8 The therapeutic infusion of tositumomab/131I-tositumomab was administered 7 to 14 days after the dosimetric infusion with 450 mg of tositumomab followed by 35 mg of 131I-tositumomab, labeled with enough 131I calculated to deliver a 0.75 Gy whole-body dose (0.65 Gy if the platelet count was 100,000-149,999/μL). Thyroid protection was as previously described.7 Patients were removed from protocol treatment for progressive disease, unacceptable toxicity, or patient preference.
Fig 2.
Design of Southwest Oncology Group Protocol S0016. (A) Schema for patients randomly assigned to six cycles of cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy with six doses of rituximab (CHOP-R). (B) Schema for patients randomly assigned to six cycles of cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy followed by consolidation with tositumomab/131I-tositumomab radioimmunotherapy (CHOP-RIT). IV, intravenous.
A dynamic allocation scheme was used to randomly assign patients to the arms of the study at registration and was stratified for ß2 microglobulin level (> institutional upper limit of normal v ≤ institutional upper limit of normal).
Assessment of Clinical Responses
Data were centrally reviewed and clinical responses (partial response, CRu, or CR) were assigned by the SWOG statistical center and principal investigator based on criteria from two international workshops.22–23 Remission and survival status were assessed 200 and 365 days after therapy initiation and every 6 months until death and whenever symptoms or signs of lymphoma recurrence developed. Day 200 was chosen as the initial restaging time point because it allowed evaluation of patients on both arms at least 4 weeks after completion of therapy. At each time point, a patient history, physical examination, blood counts, and CT scans were required. Fluorodeoxyglucose positron emission tomography was not required but, when available, was used to assess remissions as described in the “Revised response criteria for malignant lymphoma.”24 Bone marrow aspiration and biopsy were required on day 200 if positive at baseline and on day 365 for all patients who had not progressed.
Ascertainment of Secondary Malignancies
The development of myelodysplasia (MDS), acute myeloid leukemia (AML), or other secondary malignancies was assessed by investigators and trial coordinators at each protocol-mandated follow-up timepoint to ensure these serious adverse events were recorded. Furthermore, National Cancer Institute guidelines in the protocol required that all AML, MDS, and acute lymphoblastic leukemia be reported within 30 days to SWOG and to the Investigational Drug Branch of the National Cancer Institute.
Statistical Considerations
Approximately 500 eligible patients randomly assigned over 4.5 years with 2 additional years of follow-up were required to have power of 0.86 to detect an improvement in PFS of CHOP-RIT over CHOP-R, corresponding to a hazard ratio of 1.50 based on a one-sided .025 level stratified log-rank test and assuming exponential PFS distributions. For these calculations, it was projected that the hazard rate for CHOP-R was 0.175 (2-year PFS of 70%), based on data from the phase II trial SWOG S9800. OS and PFS curves were generated using Cox regression and plotted by the Kaplan and Meier method.25–26 PFS was defined as the time from registration to the first observation of progression or death as a result of any cause. Our study was monitored throughout accrual and follow-up by the SWOG Data and Safety Monitoring Committee, with formal interim analyses after 50% and 75% of eligible patients were randomly assigned. The cutoff date for all analyses was July 25, 2011. All response and survival data (PFS and OS) are reported on a modified intent-to-treat basis, in which only patients known to be ineligible were excluded. Patients who received no treatment are excluded from the evaluation of adverse events.
RESULTS
Patient Characteristics
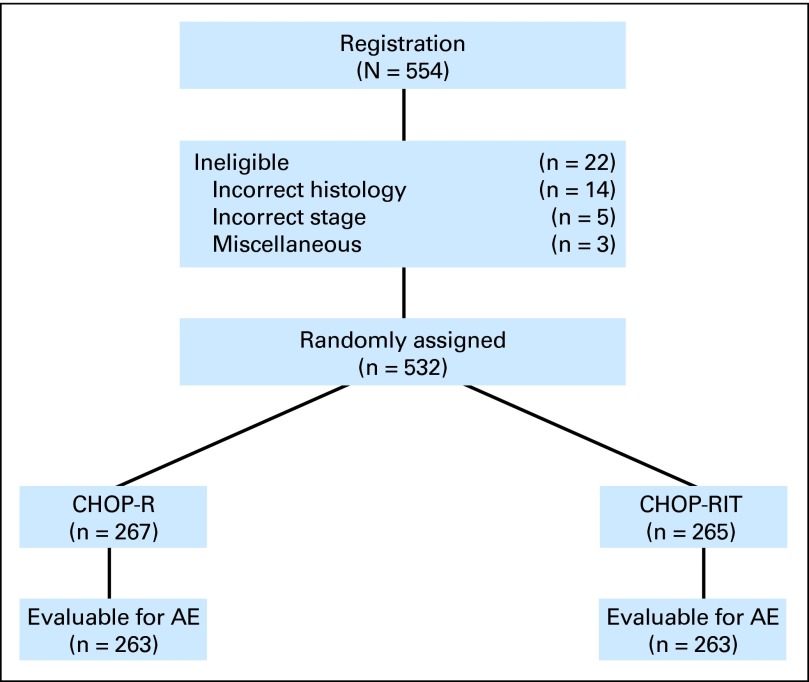
Between March 1, 2001 and September 15, 2008, 554 patients were randomly assigned to CHOP-R (279 patients) or CHOP-RIT (275 patients; Fig 3). Of the registered patients, 430 patients were enrolled by SWOG, 121 by Cancer and Leukemia Group B, and three by Eastern Cooperative Oncology Group. Of the enrolled patients, 532 were eligible; 267 patients were assigned to CHOP-R and 265 to CHOP-RIT. Twenty-two patients were ineligible, including 14 with incorrect histology based on central review, five with incorrect stage, and three for miscellaneous reasons. Treatment was completed as planned by 496 patients (93%), including 254 patients (95%) on CHOP-R and 242 patients (91%) on CHOP-RIT. On the CHOP-R arm, 13 patients (5%) did not complete treatment because of adverse events (four patients), death (one patient), or other reasons (seven patients). In the CHOP-RIT group, 23 patients (9%) did not complete treatment, because of adverse events (four patients), refusal (eight patients), disease progression (two patients), death (two patients), or other reasons (seven patients). Patient characteristics were well balanced between the two arms (Table 1). The β2 microglobulin was elevated in 53% of patients receiving CHOP-R and 54% receiving CHOP-RIT, indicating that more than half the enrolled patients exhibited this adverse prognostic factor. Approximately a quarter of the patients had B symptoms (CHOP-R, 29%; CHOP-RIT, 26%) and bulky masses more than 10 cm (CHOP-R, 24%; CHOP-RIT, 26%). Centralized review of pathology resulted in revising the grade of disease in 41 patients, including 35 for whom the local designation of grade 3 FL was changed to grade 1 to 2 FL. Central review showed grade 3 FL in 9% of patients in the CHOP-RIT arm and 8% in the CHOP-R arm. An alternative categorization of grade by whether the lymphoma was predominantly grade 3 rendered similar results (CHOP-RIT arm, 8%; CHOP-R arm, 8%). Stage IV lymphoma was present in 59% of patients treated with CHOP-R compared with 64% receiving CHOP-RIT, and 69% of patients on each arm had intermediate- or high-risk Follicular Lymphoma International Prognostic Index (FLIPI) scores (Table 1).
Fig 3.
CONSORT diagram illustrating patient flow on Southwest Oncology Group protocol S0016. AE, adverse event; CHOP-R, cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy with rituximab; CHOP-RIT, cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy followed by tositumomab/131I-tositumomab radioimmunotherapy.
Table 1.
Characteristics of Patients Randomly Assigned on Southwest Oncology Group Protocol S0016
| Characteristic | CHOP-R (%) | CHOP-RIT (%) |
|---|---|---|
| Median age, years | ||
| CHOP-R | 54.5 | |
| CHOP-RIT | 53.3 | |
| Male sex | 53 | 55 |
| White race | 90 | 90 |
| Elevated β2M | 53 | 54 |
| B symptoms | 29 | 26 |
| Bulk > 10 cm | 24 | 26 |
| Hemoglobin < 12 gm/dL | 11 | 11 |
| BM involvement | 56 | 56 |
| Follicular grade 3 | 8 | 9 |
| Stage | ||
| II | 4 | 2 |
| III | 37 | 34 |
| IV | 59 | 64 |
| FLIPI risk | ||
| Low | 30 | 31 |
| Intermediate | 47 | 43 |
| High | 22 | 26 |
Abbreviations: BM, bone marrow; CHOP-R, cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab; CHOP-RIT, cyclophosphamide, doxorubicin, vincristine, prednisone with tositumomab/iodine I-131 tositumomab radioimmunotherapy; FLIPI, Follicular Lymphoma International Prognostic Index.
Clinical Responses
Objective remissions were documented in 224 (84%) of 267 eligible patients treated with CHOP-R and in 223 (84%) of 265 patients with CHOP-RIT. Complete remissions were detected in 108 patients (40%) in the CHOP-R arm and 120 patients (45%) in the CHOP-RIT arm (P = .30). Forty-nine patients were not assessable for response, largely owing to missing follow-up studies (eg, neck CT scans) As a sensitivity analysis, if these 49 patients are excluded from the denominator, then the overall response rate was 93% in the CHOP-R arm and 92% in the CHOP-RIT arm, and CR rates were 45% for CHOP-R and 49% for CHOP-RIT.
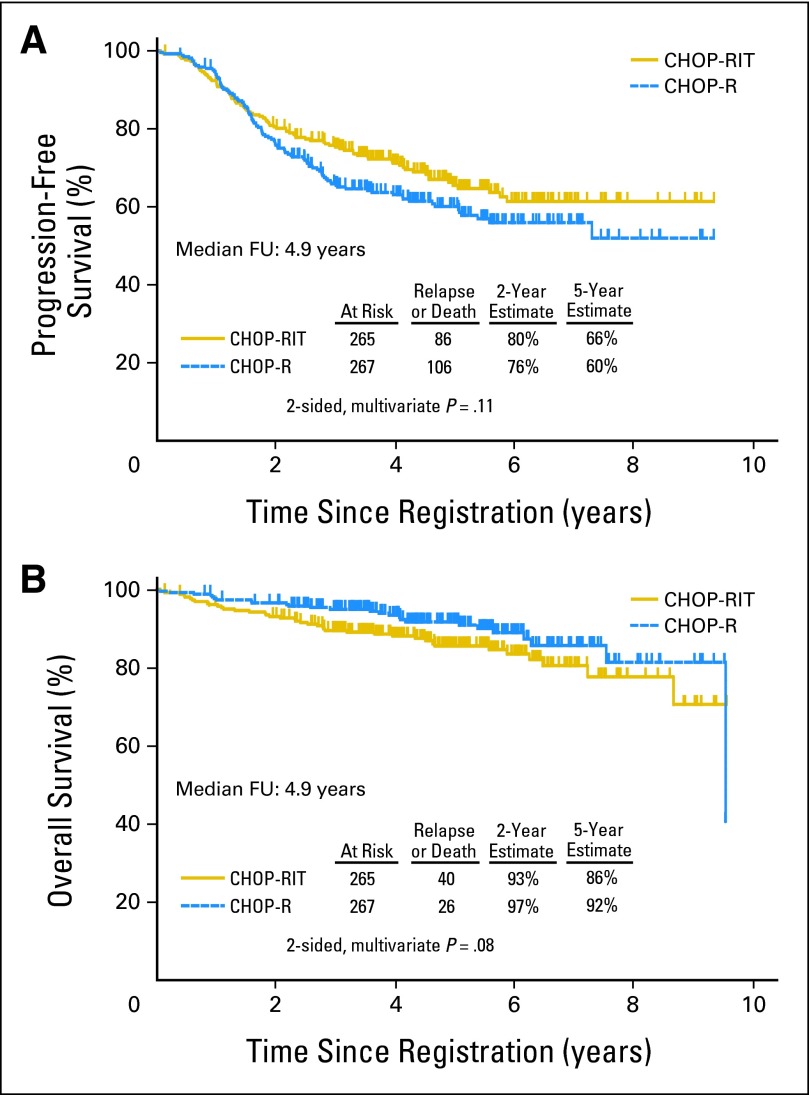
PFS and OS
After a median follow-up of 4.9 years, the estimated 2-year and 5-year PFS were 80% and 66%, respectively in the CHOP-RIT arm and 76% and 60%, respectively, in the CHOP-R arm (two-sided P = .11; one-sided P = .05; Fig 4A). Thus, there was no difference in PFS meeting the protocol-specified significance level of one-sided P = .021. Most (80%) of the progression events occurred in the first 3 years. The 2-year and 5-year OS rates were 93% and 86%, respectively, in the CHOP-RIT arm and 97% and 92%, respectively, in the CHOP-R arm (Fig 4B; P = .08). Estimates of 5-year PFS were 66% with CHOP-RIT and 60% with CHOP-R, and estimates of 5-year OS were 86% with CHOP-RIT and 92% with CHOP-R. To date, 40 patients in the CHOP-RIT arm and 26 patients in the CHOP-R arm have died. The major cause of death in both arms was lymphoma progression, including 22 (85%) of 26 patients in the CHOP-R arm and 28 (70%) of 40 patients in the CHOP-RIT arm (P = .47). All causes of death are listed in Appendix Table A1 (online only).
Fig 4.
(A) Progression-free and (B) overall survival of 532 patients with advanced-stage follicular lymphoma randomly assigned to either six cycles of cyclophosphamide, doxorubicin, vincristine, prednisone plus rituximab (CHOP-R) or six cycles of cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy followed by consolidation with tositumomab/131I-tositumomab radioimmunotherapy (CHOP-RIT). FU, follow-up.
Prognostic Factor Analysis
Cox proportional hazards multivariable regression analysis was performed to identify clinical features associated with PFS and OS.26 Elevated serum-β2M, elevated lactate dehydrogenase (LDH) level, and higher FLIPI index27 were all strongly associated with worse PFS and OS (Table 2). A combined model using LDH and β2M was generated, with patients coded as high risk if they had both elevated LDH and elevated β2M; medium risk if one factor was elevated; and low risk if neither was elevated. This model was a particularly strong predictor of patient outcomes and compared favorably to the FLIPI index. An exploratory, retrospective, unplanned subgroup analysis based on these factors was performed that suggests that low-risk patients may have a superior PFS if treated with CHOP-RIT and high-risk patients may have a superior OS if treated with CHOP-R. A more detailed prognostic factor analysis is ongoing and will be reported separately.
Table 2.
Prognostic Factor Analysis for Southwest Oncology Group Protocol S0016: Prognostic Factor Analysis
| Model | HR | 95% CI | P |
|---|---|---|---|
| Outcome, PFS | |||
| LDH alone | 1.59 | 1.17 to 2.17 | .003 |
| Serum-β2 M alone | 1.70 | 1.27 to 2.28 | .0004 |
| Serum-β2 M and LDH* | 2.24 | 1.51 to 3.31 | < .0001 |
| FLIPI* | 2.41 | 1.63 to 3.56 | < .0001 |
| Outcome, OS | |||
| LDH alone | 2.46 | 1.49 to 4.07 | .0004 |
| Serum-β2 M alone | 2.22 | 1.31 to 3.76 | .003 |
| Serum-β2 M and LDH* | 3.95 | 2.01 to 7.78 | < .0001 |
| FLIPI* | 3.81 | 1.92 to 7.54 | .0001 |
Abbreviations: FLIPI, Follicular Lymphoma International Prognostic Index; HR, hazard ratio; LDH, lactate dehydrogenase; PFS, progression-free survival; OS, overall survival.
HRs show comparisons between high- versus low-risk groups.
Toxicities
Six patients received little (≤ 1 day) or no treatment and are not evaluable for toxicity (CHOP-R, four patients; CHOP-RIT, two patients). Therefore, 263 patients in each arm are evaluable for toxicity. Adverse events were similar on the two arms and appeared dominated by the effects of CHOP chemotherapy rather than the immunotherapeutic components (Table 3). Grade 3 to 4 neutropenia was seen in 48% of patients with CHOP-R compared with 51% on CHOP-RIT. Grade 3 to 4 thrombocytopenia was more common with RIT (18% v 2%; P < .0001), whereas febrile neutropenia was more common with CHOP-R (16%) than with CHOP-RIT (10%; P = .05). There was a trend toward more infection with CHOP-R than CHOP-RIT (23% v 17%) though this did not reach statistical significance (P = .10). There was, as expected, more grade 1 to 2 thyroid dysfunction in the CHOP-RIT group compared with CHOP-R (7% v 3%, respectively) and more HAMA formation (CHOP-RIT, n = 47; 17%; v CHOP-R, n = 41; 2%), though neither trend was statistically significant (P = .07 and P = .06, respectively). Platelet transfusions were given to seven patients receiving CHOP-RIT and one patient receiving CHOP-R (P = .07).
Table 3.
Grades 3-5 Adverse Events Observed on Southwest Oncology Group Protocol S0016
| Adverse Events(grade ≥ 3) | CHOP-R(n = 263) |
CHOP-RIT(n = 263) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Neutropenia | 127 | 48 | 135 | 51 | .54 |
| Thrombocytopenia | 6 | 2 | 46 | 18 | < .0001 |
| Anemia | 7 | 3 | 8 | 3 | 1.00 |
| Infection | 61 | 23 | 45 | 17 | .10 |
| Febrile neutropenia | 42 | 16 | 26 | 10 | .05 |
| Gastrointestinal | 20 | 8 | 24 | 9 | .64 |
| Cardiovascular | 19 | 7 | 9 | 3 | .08 |
| Fatigue | 11 | 4 | 9 | 3 | .82 |
| Grade 4 nonhematologic | 14 | 5 | 11 | 4 | .68 |
| Treatment-related deaths | 1 | < 1 | 4 | 2 | .37 |
| Second malignancies | 23 | 9 | 22 | 8 | 1.00 |
| AML/MDS | 3 | 1 | 8 | 3 | .22 |
Abbreviations: AML/MDS, acute myeloid leukemia/myelodysplastic syndrome; CHOP-R, cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab; CHOP-RIT, cyclophosphamide, doxorubicin, vincristine, prednisone with tositumomab/iodine I-131 tositumomab radioimmunotherapy.
Grade 4 nonhematologic toxicities were rare (approximately 5% in both arms). Secondary malignancies were equally frequent in the two groups (CHOP-R, 23 patients; 9%; CHOP-RIT, 22 patients; 8%; Appendix Table A2). There were more cases of MDS/AML in the CHOP-RIT arm (8 patients; 3%) compared with the CHOP-R arm (three patients; 1%), but this difference was not statistically significant (P = .22). There was one treatment-related death as a result of neutropenic infection with CHOP-R, and four treatment-related deaths in the CHOP-RIT arm (one as a result of AML, one MDS, one acute lymphoblastic leukemia, and one respiratory failure; P = .37).
DISCUSSION
Both regimens used in this trial produced outstanding outcomes in advanced FL, with more than 60% of patients estimated to be progression-free 5 years after treatment and relatively flat slopes in the PFS curves after 3 years (Fig 4A). Overall survival was similarly impressive, with 80% of patients estimated to be alive 8 years after treatment with either regimen (Fig 4B). The excellent PFS and OS rates observed for the CHOP-RIT arm were predicted by the preceding phase II study (SWOG S9911; Fig 2)7–8; however, the PFS and OS rates in the CHOP-R arm exceeded expectations. It must be acknowledged that the relatively young median age of patients (54 years) and the moderate FLIPI profile might be partially responsible for the outstanding PFS and OS rates observed, however, the results are nevertheless impressive when compared with earlier SWOG trials conducted with the same eligibility criteria.27a Toxicities were similar on both arms of the study and were dominated by hematologic events, as is typical of CHOP therapy.7,17,28–30 The immunotherapy components seemed to cause only minor incremental toxicity, with slightly more severe neutropenia in patients treated with CHOP-R (Table 3), as previously reported.17 RIT caused more thrombocytopenia, as well as trends toward more hypothyroidism and HAMA formation, as expected.7–8 The greatest concern with RIT is its potential to induce secondary malignancies, especially MDS/AML.31–32 It is reassuring that there was no difference in secondary malignancies overall (CHOP-R, 23 patients; 9% v CHOP-RIT, 22 patients; 8%) and that the trend toward more MDS/AML with CHOP-RIT (eight patients; 3% v three patients; 1%) was not statistically significant (P = .22), although further follow-up is necessary.
It is instructive to compare the results of this trial with those of FIT (First-Line Indolent Trial), in which patients with advanced FL were randomly assigned to consolidation with two doses of rituximab plus 90Y-ibritumomab tiuxetan or no consolidation.14 Induction chemotherapy was not standardized, with 9% of patients receiving chlorambucil, 26% receiving CVP, 43% receiving CHOP-like regimens, 5% receiving fludarabine, and only 16% receiving rituximab-containing combinations. A statistically significant improvement in PFS was observed with RIT in the FIT trial (36 months) compared with no consolidation (13 months; P < .0001). No differences in OS were observed.14 The S0016 trial differs substantially from the FIT trial. First, all patients on S0016 received the same chemotherapy regimen, namely CHOP. Furthermore, in S0016 both arms received immunotherapy, consisting of six doses of rituximab with CHOP-R, compared with consolidation with 131I-tositumomab with CHOP-RIT. S0016 patients were treated more intensively than FIT patients and had longer PFS (> 60 months in both arms, compared with 33 months on the best arm of the FIT trial).14 S0016 does not show a statistically significant difference in either PFS or OS after a median follow-up of 4.9 years. Yet the excellent results from both arms suggest that a single therapeutic dose of 131I-tositumomab administered as consolidation to patients with no rituximab exposure may be an alternative to six doses of rituximab integrated with CHOP induction chemotherapy, particularly because the CHOP-RIT regimen required significantly less infusion time than CHOP-R (by a total of approximately 18.5 hours).
Several unanswered questions remain concerning optimal front-line therapy of FL. One is whether a combined strategy of CHOP-R followed by RIT would be superior to the two regimens studied in S0016. A second question is how maintenance rituximab following induction therapy6,33–34 would compare with RIT consolidation. Third, it is conceivable that the best strategy might be to combine CHOP-R induction, RIT consolidation, and rituximab maintenance as in a recent SWOG pilot trial (S0801; Jonathan Friedberg; unpublished data). Finally, the outstanding results of recent immunochemotherapy regimens such as S9911 (10-year PFS of 60%; Fig 2), S0016 (≥ 60% PFS at 5-10 years; Fig 4A), and other recent immunochemotherapy studies6 suggest that it may be time to challenge the common view that advanced FL will inevitably relapse. The slopes of the PFS curves on these trials suggest that many patients older than 60 years with FL treated with modern immunochemotherapy regimens may never experience recurrences in their lifetimes.
Supplementary Material
Acknowledgment
We thank Jeri Jardine, Scott Kurruk, Nancy Press, and the Southwest Oncology Group operations and statistical offices for data management and administrative assistance conducting this trial and preparing this article. We also acknowledge the generous support of GlaxoSmithKline, who provided tositumomab and iodine/131iodine tositumomab for this trial, as well as a research grant covering nuclear medicine costs and human antimouse antibody testing.
Appendix
Table A1.
Causes of Death Observed on Protocol S0016 by Treatment Arm
| Causes of Death | CHOP-R | CHOP-RIT |
|---|---|---|
| Relapsed lymphoma | 22 | 28 |
| Acute myeloid leukemia | 0 | 2 |
| Pancreatic cancer | 1 | 1 |
| Rectal cancer | 1 | 0 |
| Gastric cancer | 0 | 1 |
| Bladder cancer | 0 | 1 |
| Lung cancer | 0 | 1 |
| Stroke | 1 | 0 |
| Infection | 1 | 1 |
| Pulmonary hypertension | 0 | 1 |
| Perforated gastric ulcer | 0 | 1* |
| Drug overdose | 0 | 1 |
| Unknown | 0 | 2 |
| Total | 26 | 40 |
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CHOP-R, cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab; CHOP-RIT, cyclophosphamide, doxorubicin, vincristine, prednisone with tositumomab/iodine I-131 tositumomab radioimmunotherapy.
One patient randomly assigned to the CHOP-RIT arm developed a gastric ulcer that perforated while receiving CHOP chemotherapy (attributed to prednisone). He declined further protocol therapy, never received 131I-tositumomab, and eventually died of apparent inanition related to the perforated ulcer.
Table A2.
Secondary Malignancies Observed on Protocol S0016 by Treatment Arm
| Secondary Malignancies | CHOP-R | CHOP-RIT |
|---|---|---|
| Nonmelanoma skin cancer | 5 | 4 |
| Melanoma | 2 | 1 |
| Myelodysplastic syndrome | 2 | 4 |
| Acute myeloid leukemia | 1 | 4* |
| Acute lymphoblastic leukemia | 0 | 1 |
| Bladder cancer | 0 | 2 |
| Breast cancer | 3 | 0 |
| Lung cancer | 2 | 1 |
| Pancreatic adenocarcinoma | 3 | 1 |
| Prostate adenocarcinoma | 2 | 1 |
| Gastric adenocarcinoma | 0 | 1 |
| Rectal carcinoma | 1 | 0 |
| Thyroid | 1 | 0 |
| Head and neck cancer | 0 | 2 |
| Diffuse large B-cell lymphoma | 1 | 0 |
| Multiple myeloma | 1 | 0 |
| Total | 23† | 22 |
Abbreviations: CHOP-R, cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab; CHOP-RIT, cyclophosphamide, doxorubicin, vincristine, prednisone with tositumomab/iodine I-131 tositumomab radioimmunotherapy.
One CHOP-RIT patient who was initially diagnosed with myelodysplasia that later evolved to AML is counted only once with AML.
One CHOP-R patient had both myelodysplastic syndrome and a nonmelanoma skin cancer.
Footnotes
Supported in part by the following PHS Cooperative Agreement Grants No. CA32102, CA38926, CA11083, CA27057, CA13612, CA35431, CA20319, CA35090, CA35261, CA46282, CA45560, CA58861, CA35281, CA67575, CA35176, CA45377, CA35128, CA76429, CA22433, CA63844, CA12644, CA04919, CA45450, CA35119, CA35178, CA58416, CA67663, CA46441, CA35192, CA37981, CA76447, CA52654, CA16385, CA073590, CA45808, CA58882, CA14028, CA58723, CA74811, CA31946 (to the Southwest Oncology Group) awarded by the National Cancer Institute; NCI R01 CA076287 (to O.W.P.), and in part by a grant from GlaxoSmithKline to the Southwest Oncology Group.
See accompanying editorial on page 294 and article on page 308; listen to the podcast by Dr Leonard at www.jco.org/podcasts
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00006721.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy,please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Oliver W. Press, Roche/Genentech (C), Spectrum (C); Jonathan W. Friedberg, Genentech (C); Myron S. Czuczman, Genentech (C), Spectrum Pharmaceuticals (C); Ajay K. Gopal, Seattle Genetics (C); Bruce D. Cheson, Roche/Genentech (C); Richard I. Fisher, Roche (C) Stock Ownership: None Honoraria: Oliver W. Press, Roche/Genentech, Spectrum; Ajay K. Gopal, Millennium/Takeda, Seattle Genetics; David G. Maloney, Genentech, GlaxoSmithKline, Roche Research Funding: Mark S. Kaminski, GlaxoSmithKline; Ajay K. Gopal, Abbott, Cephalon, Eli Lilly, Emergent BioSolutions, Gilead Sciences, GlaxoSmithKline, Piramal, SBio, Seattle Genetics, Spectrum Pharmaceuticals; David G. Maloney, Genentech Expert Testimony: None Other Remuneration: Mark S. Kaminski, Royalties from patents on CD20 Radioimmunotherapy
AUTHOR CONTRIBUTIONS
Conception and design: Oliver W. Press, Joseph M. Unger, Jonathan W. Friedberg, Michael LeBlanc, David G. Maloney, Bruce D. Cheson, Thomas P. Miller, Richard I. Fisher
Administrative support: Richard I. Fisher
Provision of study materials or patients: Oliver W. Press, Jonathan W. Friedberg, Myron S. Czuczman, Ajay K. Gopal, David G. Maloney, Bruce D. Cheson, Shaker Dakhil, Thomas P. Miller
Collection and assembly of data: Oliver W. Press, Joseph M. Unger, Lisa M. Rimsza, Myron S. Czuczman, Mark S. Kaminski, Rita M. Braziel, Catherine M. Spier, Ajay K. Gopal, David G. Maloney, Shaker Dakhil, Thomas P. Miller
Data analysis and interpretation: Oliver W. Press, Joseph M. Unger, Lisa M. Rimsza, Jonathan W. Friedberg, Myron S. Czuczman, Mark S. Kaminski, Ajay K. Gopal, David G. Maloney, Bruce D. Cheson, Thomas P. Miller, Richard I. Fisher
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Press OW. Follicular lymphoma. Williams' Hematology (ed 8) In: Kaushansky K, Lichtman M, Beutler E, et al., editors. New York, NY: McGraw-Hill Medical Publishing Division; 2009. p. 1565. [Google Scholar]
- 2.Fisher RI, LeBlanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol . 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 3.Swenson WT, Wooldridge JE, Lynch CF, et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol . 2005;23:5019–5026. doi: 10.1200/JCO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Fayad L, Cabanillas F, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas M.D. Anderson Cancer Center. J Clin Oncol . 2006;24:1582–1589. doi: 10.1200/JCO.2005.03.3696. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: First report of the national LymphoCare study. J Clin Oncol . 2009;27:1202–1208. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet . 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 7.Press OW, Unger JM, Braziel RM, et al. A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood . 2003;102:1606–1612. doi: 10.1182/blood-2003-01-0287. [DOI] [PubMed] [Google Scholar]
- 8.Press OW, Unger JM, Braziel RM, et al. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin's lymphoma: Five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol . 2006;24:4143–4149. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- 9.Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med . 2005;352:441–449. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 10.Link BK, Martin P, Kaminski MS, et al. Cyclophosphamide, vincristine, and prednisone followed by tositumomab and iodine-131-tositumomab in patients with untreated low-grade follicular lymphoma: Eight-year follow-up of a multicenter phase II study. J Clin Oncol . 2010;28:3035–3041. doi: 10.1200/JCO.2009.27.8325. [DOI] [PubMed] [Google Scholar]
- 11.Zinzani PL, Rossi G, Franceschetti S, et al. Phase II trial of short-course R-CHOP followed by 90Y-ibritumomab tiuxetan in previously untreated high-risk elderly diffuse large B-cell lymphoma patients. Clin Cancer Res . 2010;16:3998–4004. doi: 10.1158/1078-0432.CCR-10-0162. [DOI] [PubMed] [Google Scholar]
- 12.Zinzani PL, Tani M, Fanti S, et al. A phase II trial of CHOP chemotherapy followed by yttrium 90 ibritumomab tiuxetan (Zevalin) for previously untreated elderly diffuse large B-cell lymphoma patients. Ann Oncol . 2008;19:769–773. doi: 10.1093/annonc/mdm560. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs SA, Swerdlow SH, Kant J, et al. Phase II trial of short-course CHOP-R followed by 90Y-ibritumomab tiuxetan and extended rituximab in previously untreated follicular lymphoma. Clin Cancer Res . 2008;14:7088–7094. doi: 10.1158/1078-0432.CCR-08-0529. [DOI] [PubMed] [Google Scholar]
- 14.Morschhauser F, Radford J, Van Hoof A, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol . 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 15.Witzig TE, Fishkin P, Gordon LI, et al. Treatment recommendations for radioimmunotherapy in follicular lymphoma: A consensus conference report. Leuk Lymphoma . 2011;52:1188–1199. doi: 10.3109/10428194.2011.570396. [DOI] [PubMed] [Google Scholar]
- 16.Czuczman MS, Grillo-López AJ, White CA, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol . 1999;17:268–276. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- 17.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood . 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 18.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood . 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 19.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol . 2008;26:4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 20.Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: An East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25:1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 21.Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: Results of the GELA-GOELAMS FL2000 study. Blood . 2008;112:4824–4831. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI Sponsored International Working Group. J Clin Oncol . 1999;17:1244–8452. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol . 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011;29:1844–1854. doi: 10.1200/JCO.2010.32.5225. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc . 1958;53:457–481. [Google Scholar]
- 26.Cox DR. Regression models and life tables (with discussion) J Roy Statist Soc Serv B . 1972;34:187–220. [Google Scholar]
- 27.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood . 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 27a.Velasquez WS, Lew D, Grogan TM, et al. Combination of fludarabine and mitoxantrone in untreated stages III and IV low-grade lymphoma: S9501. J Clin Oncol . 2003;21:1996–2003. doi: 10.1200/JCO.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 28.Dana BW, Dahlberg S, Nathwani BN, et al. Long-term follow-up of patients with low-grade malignant lymphomas treated with doxorubicin-based chemotherapy or chemoimmunotherapy. J Clin Oncol . 1993;11:644–651. doi: 10.1200/JCO.1993.11.4.644. [DOI] [PubMed] [Google Scholar]
- 29.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med . 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 30.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol . 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 31.Bennett JM, Kaminski MS, Leonard JP, et al. Assessment of treatment-related myelodysplastic syndromes and acute myeloid leukemia in patients with non-Hodgkin lymphoma treated with tositumomab and iodine 131I tositumomab. Blood . 2005;105:4576–4582. doi: 10.1182/blood-2004-12-4690. [DOI] [PubMed] [Google Scholar]
- 32.Witzig TE, White CA, Gordon LI, et al. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-hodgkin's lymphoma. J Clin Oncol . 2003;21:1263–1270. doi: 10.1200/JCO.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 33.Vidal L, Gafter-Gvili A, Salles G, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: An updated systematic review and meta-analysis of randomized trials. J Natl Cancer Inst. 2011;103:1799–1806. doi: 10.1093/jnci/djr418. [DOI] [PubMed] [Google Scholar]
- 34.Martinelli G, Schmitz SF, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol . 2010;28:4480–4484. doi: 10.1200/JCO.2010.28.4786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.