Abstract
Purpose
Anaplastic oligodendrogliomas, pure (AO) and mixed (anaplastic oligoastrocytoma [AOA]), are chemosensitive, especially if codeleted for 1p/19q, but whether patients live longer after chemoradiotherapy is unknown.
Patients and Methods
Eligible patients with AO/AOA were randomly assigned to procarbazine, lomustine, and vincristine (PCV) plus radiotherapy (RT) versus RT alone. The primary end point was overall survival (OS).
Results
Two hundred ninety-one eligible patients were randomly assigned: 148 to PCV plus RT and 143 to RT. For the entire cohort, there was no difference in median survival by treatment (4.6 years for PCV plus RT v 4.7 years for RT; hazard ratio [HR] = 0.79; 95% CI, 0.60 to 1.04; P = .1). Patients with codeleted tumors lived longer than those with noncodeleted tumors (PCV plus RT: 14.7 v 2.6 years, HR = 0.36, 95% CI, 0.23 to 0.57, P < .001; RT: 7.3 v 2.7 years, HR = 0.40, 95% CI, 0.27 to 0.60, P < .001), and the median survival of those with codeleted tumors treated with PCV plus RT was twice that of patients receiving RT (14.7 v 7.3 years; HR = 0.59; 95% CI, 0.37 to 0.95; P = .03). For those with noncodeleted tumors, there was no difference in median survival by treatment arm (2.6 v 2.7 years; HR = 0.85; 95% CI, 0.58 to 1.23; P = .39). In Cox models that included codeletion status, the adjusted OS for all patients was prolonged by PCV plus RT (HR = 0.67; 95% CI, 0.50 to 0.91; P = .01).
Conclusion
For the subset of patients with 1p/19q codeleted AO/AOA, PCV plus RT may be an especially effective treatment, although this observation was derived from an unplanned analysis.
INTRODUCTION
Anaplastic oligodendroglioma (AO) is an uncommon brain cancer with distinctive histopathology; when copopulated with neoplastic astrocytes, a diagnosis of anaplastic oligoastrocytoma (AOA) is rendered.1 In addition to microscopic similarities, AO/AOA have a common molecular ancestry: Both harbor mutations of isocitrate dehydrogenase (IDH) and display the hypermethylation phenotype,2–4 which in AO is accompanied by frequent whole-arm losses of chromosomes 1p and 19q and mutations CIC, and in AOA, by mutations of TP53.5–8 Standard treatment for AO/AOA is surgical resection followed by radiotherapy (RT).9 With treatment, survival times are much longer for AO/AOA than for glioblastoma (GBM), a related cancer.9 Another distinction between AO/AOA and GBM is the role of chemotherapy in their initial management. For GBM, there is strong evidence of benefit from temozolomide chemotherapy given during and after RT.10 Currently, there is no such evidence for AO/AOA despite the widespread use of chemotherapy to treat these cancers.
Interest in chemotherapy for AO/AOA surfaced 25 years ago when responses to procarbazine, lomustine, and vincristine (PCV) were reported in small series of patients with recurrent tumors,11,12 which subsequently were confirmed in prospective trials13,14 and when reports describing the feasibility of pre-RT PCV surfaced.15,16 These findings led to randomized trials designed to clarify the role of PCV chemotherapy in AO/AOA. In this study by the Radiation Therapy Oncology Group (RTOG), an intensive PCV regimen was given before RT. In the European Organization for Research and Treatment of Cancer (EORTC) trial, standard-dose PCV was used after RT. Both began before the genetic features of AO/AOA were known, but nevertheless, tissues were collected for molecular studies. Soon thereafter, frequent 1p/19q codeletion was observed in oligodendrogliomas,5 and later, its potential prognostic and predictive utility was first described.17,18
Early results from RTOG 9402 and EORTC 26951 showed that progression-free survival (PFS) was prolonged by adding PCV to RT and that patients with codeleted tumors lived longer.19,20 In RTOG 9402, the progression-free survival benefit was only seen in the codeleted subset. Early results from 9402 did not identify a survival benefit after PCV plus RT in either codeleted or noncodeleted cases.19 Here we report the results of 9402 after long follow-up.
PATIENTS AND METHODS
Eligibility Criteria
Patients ≥ 18 years of age with AO or AOA were eligible. The criteria for anaplasia and mixed histology are described elsewhere.19 Diagnoses were confirmed by central review before randomization. Other criteria were postoperative Karnofsky performance status (KPS) ≥ 60 and adequate marrow and other organ function. Patients with other serious illnesses and pregnant patients were ineligible. All centers (n = 76) had institutional review board approval, and all patients consented.
Study Design and Treatment
Patients were stratified by age less than 50 versus ≥ 50 years, KPS 60 to 70 versus ≥ 80, and moderately anaplastic versus highly anaplastic19 then randomly assigned within 8 weeks to intensive PCV followed by immediate involved-field RT (experimental arm) or RT alone (control arm) in a permuted block design. Patients started treatment within 1 week of randomization. In the PCV arm, four cycles were given every 6 weeks before RT, as follows: lomustine 130 mg/m2 orally on day 1; procarbazine 75 mg/m2 orally daily, days 8 through 21; and vincristine 1.4 mg/m2 intravenously on days 8 and 29.19 There was no 2-mg limit on vincristine. Compliance was assessed by case reviews.
RT was prescribed as follows: 59.4 Gy in 33 fractions (1.8 Gy each), 5 days a week. Patients randomly assigned to PCV plus RT began RT within 6 weeks of the last chemotherapy dose. Megavoltage irradiation (≥ 4 MV) was used. RT was planned with gadolinium-enhanced magnetic resonance imaging (MRI) obtained within 2 weeks of randomization. The initial field was the T2-weighted abnormality plus a 2-cm margin (50.4 Gy in 28 fractions). The boost field was the enhanced T1-weighted abnormality plus a 1-cm margin (9 Gy in five fractions). After complete resection, the surgical cavity and T2 change with a 2-cm margin received 50.4 Gy; the cavity with a 1-cm margin got the boost. Quality assurance was gauged by case reviews.
The primary end point was overall survival (OS). PFS, toxicities, cognition, and quality of life were also compared. Anticonvulsants, antiemetics, and corticosteroids were prescribed as needed for symptom control. The status of chromosomes 1p and 19q was assessed by fluorescence in situ hybridization.18
Surveillance and Follow-Up
Baseline evaluations included blood counts, pulmonary and biochemistry screening, MRI or computed tomography (CT), neurologic assessment, and mini-mental status examination (MMSE).19,21 Quality of life (QOL) was also measured.22 During PCV, counts were checked weekly, and chemistries were checked before each cycle. During RT, patients were reviewed weekly. Follow-up MRI or CT scans were performed before each cycle and 4 to 6 weeks after RT; thereafter, scans were done at increasing intervals, and after 5 years, annually, or as needed. Assessments, MMSE, and QOL evaluations were scheduled similarly. Progression was defined using standard criteria.23 At progression, PCV was recommended for those randomly assigned to RT.19 Toxicity was graded using the National Cancer Institute Common Toxicity Criteria (version 1). For RT, RTOG acute morbidity criteria and RTOG/EORTC late morbidity criteria were used.
Statistical Methods
The study was designed to enroll 146 patients per arm, giving 80% power to detect a 50% increase in median OS from 3.8 years with RT to 5.7 years with PCV plus RT (hazard ratio [HR] for death, 0.67) with a type I error rate of 0.05. Survival was analyzed by the Kaplan-Meier method with two-sided log-rank statistics. OS was the time from randomization to death from any cause, and PFS was the time from randomization to progression or death. Analyses were performed on case-eligible (n = 291) and all-case (n = 299) bases with similar results. Only case-eligible results are presented. Cox proportional hazards models were fitted to adjust for stratification factors and confounding clinical and chromosomal variables. Likelihood ratio tests were performed to determine the increase in predictive ability of a given model after inclusion of chromosomal status and its interaction with treatment. χ2 tests were used to compare patient characteristics by treatment and chromosomal deletion status. All P values are two-tailed and have not been adjusted for multiple comparisons.
RESULTS
Patient Characteristics
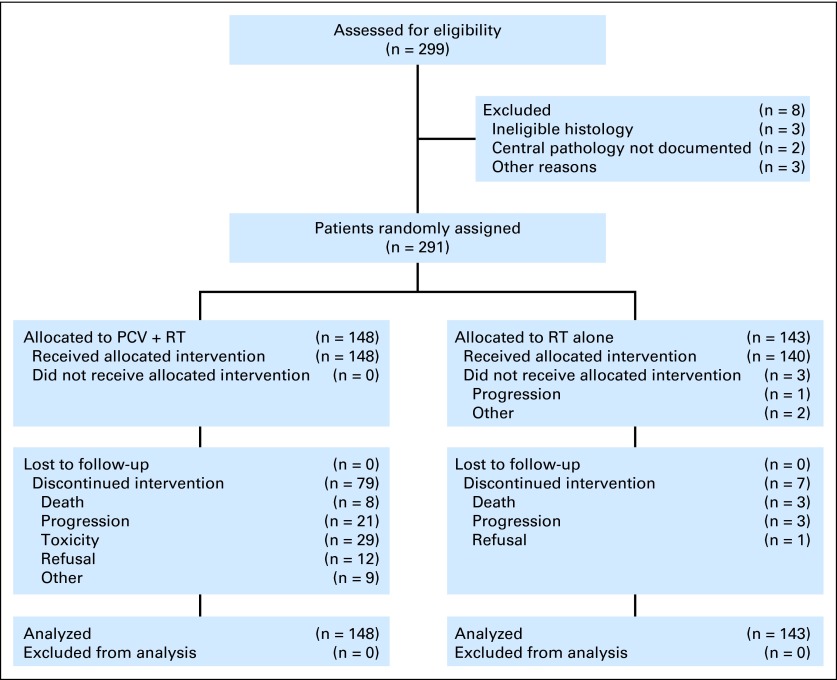
From 1994 to 2002, 291 eligible patients were randomized to PCV plus RT (n = 148) or RT (n = 143; Fig 1). Eight randomly assigned cases were ineligible: In the PCV plus RT arm, ineligibility was due to pregnancy (n = 1), missing KPS (n = 1), absent planning MRI (n = 1), and wrong diagnosis (n = 1). In the RT arm, no pathology review (n = 2) and wrong diagnosis (n = 2) were the reasons. Sixty-nine percent of patients were younger than 50 years, 88% had a resection, 90% had a KPS ≥ 80%, and 52% had an AO. Arms were balanced for clinical features, AO versus AOA, degree of anaplasia, and corticosteroid use (Table 1). The median duration of follow-up was 11.3 years (range, 0.5 to 16.8 years). The status of 1p or 19q could be ascertained in 91% of cases. (In 2006, at the time of initial reporting of 9402, 1p/19q data was available on only 70% of eligible cases because of insufficient tumor tissue, technical difficulties, or other reasons.19 In the intervening years, a concerted effort was made to retest and retrieve missing tissues.)
Fig 1.
CONSORT diagram. PCV, procarbazine, lomustine, and vincristine; RT, radiotherapy.
Table 1.
Clinical and Molecular Characteristics
| Characteristic | PCV/RT (n = 148) |
RT (n = 143) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 43 | 43 | ||
| Range | 18-75 | 19-76 | ||
| Age, years* | ||||
| < 50 | 102 | 69 | 99 | 69 |
| ≥ 50 | 46 | 31 | 44 | 31 |
| Sex | ||||
| Male | 90 | 61 | 84 | 59 |
| Female | 58 | 39 | 59 | 41 |
| Neurologic function | ||||
| No symptoms | 47 | 32 | 47 | 33 |
| Minor symptoms | 73 | 49 | 69 | 48 |
| Moderate (active) | 17 | 12 | 12 | 8 |
| Moderate (inactive) | 11 | 7 | 14 | 10 |
| Unknown | 0 | 0 | 1 | 1 |
| KPS* | ||||
| 60-70 | 15 | 10 | 15 | 10 |
| 80-100 | 133 | 90 | 128 | 90 |
| Surgery | ||||
| Total resection | 40 | 27 | 53 | 37 |
| Partial procedure | 85 | 57 | 75 | 52 |
| Biopsy only | 21 | 14 | 14 | 10 |
| No details | 2 | 1 | 1 | 1 |
| Tumor type | ||||
| AO | 77 | 52 | 73 | 51 |
| AOA (oligodendroglioma dominant) | 28 | 19 | 37 | 26 |
| AOA (neither element dominant) | 24 | 16 | 15 | 11 |
| AOA (astrocytoma dominant) | 19 | 13 | 18 | 13 |
| Tumor grade* | ||||
| Moderately anaplastic | 80 | 54 | 81 | 57 |
| Highly anaplastic | 68 | 46 | 62 | 43 |
| Multifocal tumor | ||||
| Yes | 15 | 10 | 10 | 7 |
| No | 132 | 89 | 131 | 92 |
| Unknown | 1 | 1 | 2 | 1 |
| Corticosteroids at baseline | ||||
| Yes | 92 | 62 | 79 | 55 |
| No | 56 | 38 | 64 | 45 |
| Chromosome 1p† | ||||
| Known | 134 | 128 | ||
| 1p deleted | 66 | 49 | 76 | 59 |
| 1p intact | 68 | 51 | 52 | 41 |
| Unknown | 14 | 15 | ||
| Chromosome 19q† | ||||
| Known | 135 | 129 | ||
| 19q deleted | 85 | 63 | 82 | 64 |
| 19q intact | 50 | 37 | 47 | 36 |
| Unknown | 13 | 14 | ||
| Chromosomes 1p & 19q† | ||||
| Known | 135 | 128 | ||
| Both deleted | 59 | 44 | 67 | 52 |
| One or neither deleted | 76 | 56 | 61 | 48 |
| Unknown | 13 | 15 | ||
Abbreviations: AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; KPS, Karnofsky performance status; PCV, procarbazine, lomustine, and vincristine; RT, radiotherapy.
Stratification factors at randomization.
No. and % of known.
Protocol Compliance
PCV was administered per protocol or acceptably in 95% of patients; the percentage receiving four, three, two, one, and no cycles was 54%, 22%, 9%, 12%, and 2%, respectively.19 PCV was stopped for progression or death in 17%, toxicity in 20%, and other reasons in 15%. RT was given per protocol or acceptably in 76% of PCV cases and 82% of controls; unacceptable variations occurred in 6% and 8%. RT was halted because of progression, death, or other reasons in 10% of PCV cases and 5% of controls. Lost to follow-up rates were 3% at 3 years and 10% at 10 years.
Survival by Treatment
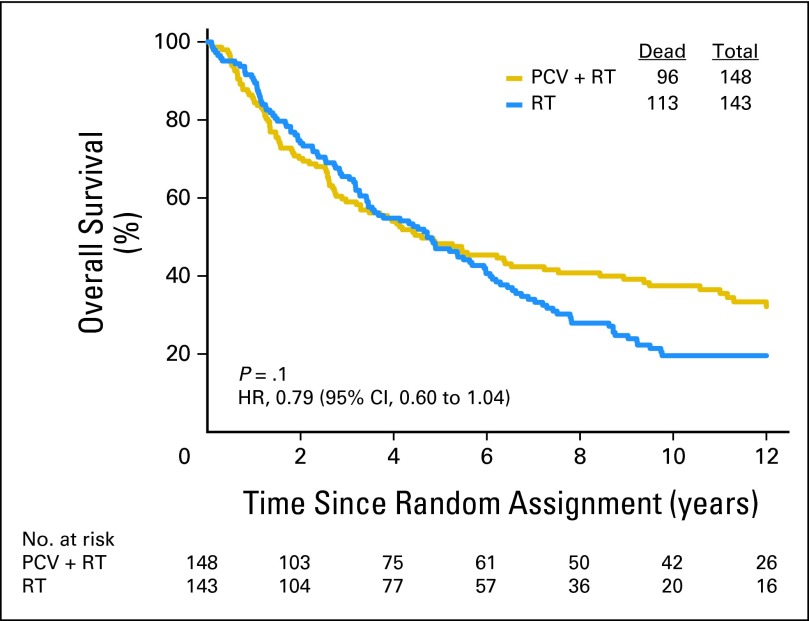
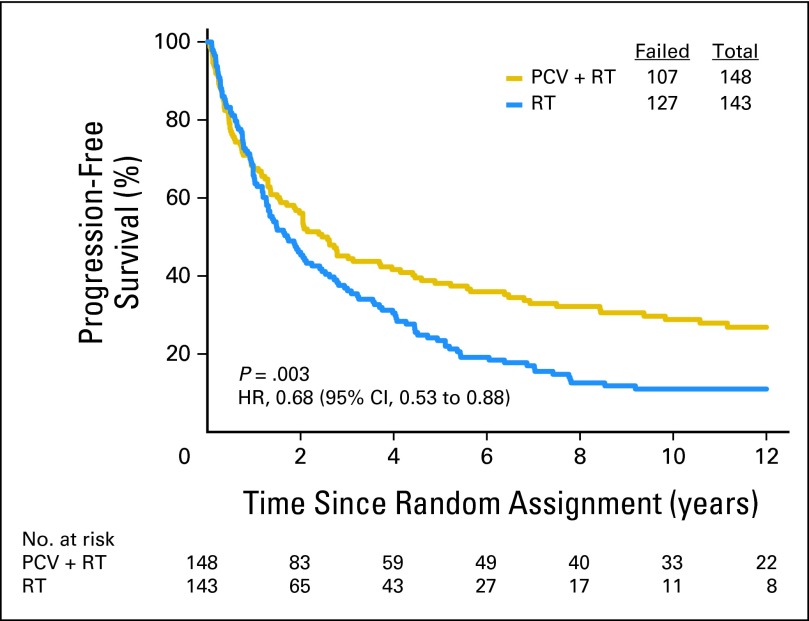
As of December 2011, 209 patients had died (72%). For the entire cohort, long-term follow-up revealed that PCV plus RT did not prolong the median survival time, which was 4.6 years after PCV plus RT versus 4.7 years after RT (Fig 2). The HR for death after PCV plus RT versus RT was 0.79 (95% CI, 0.60 to 1.04); log-rank P = .1. Baseline clinical features and treatment were evaluated in Cox proportional hazards models of OS. In stepwise multivariate analyses that considered age at diagnosis, sex, corticosteroid use, number of lesions, neurologic function, type of surgery, KPS, AO versus AOA, degree of anaplasia, treatment arm, and codeletion status, OS was prolonged by PCV plus RT (HR = 0.67; 95% CI, 0.50 to 0.91; P = .01; Appendix Table A1, online only). The likelihood ratio test for the interaction of treatment with codeletion status was not statistically significant, however. As previously reported and confirmed here, PCV plus RT prolonged PFS (Appendix Figs 1 through 5, online only).
Fig 2.
Kaplan-Meier estimates of overall survival by treatment group. The hazard ratio (HR) for survival of patients treated with procarbazine, lomustine, and vincristine (PCV) plus radiotherapy (RT) compared with RT alone was 0.79 (95% CI, 0.60 to 1.04; P = .1).
Deletion Status and Survival
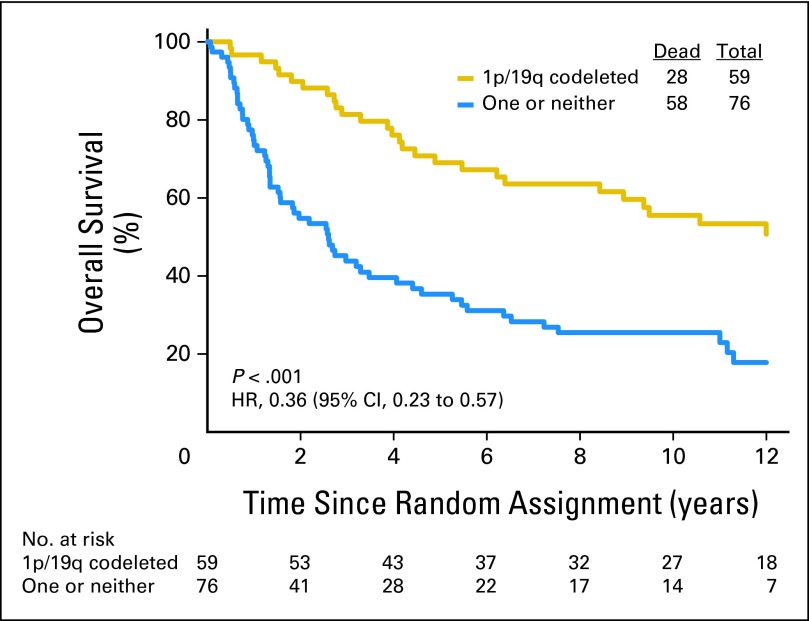
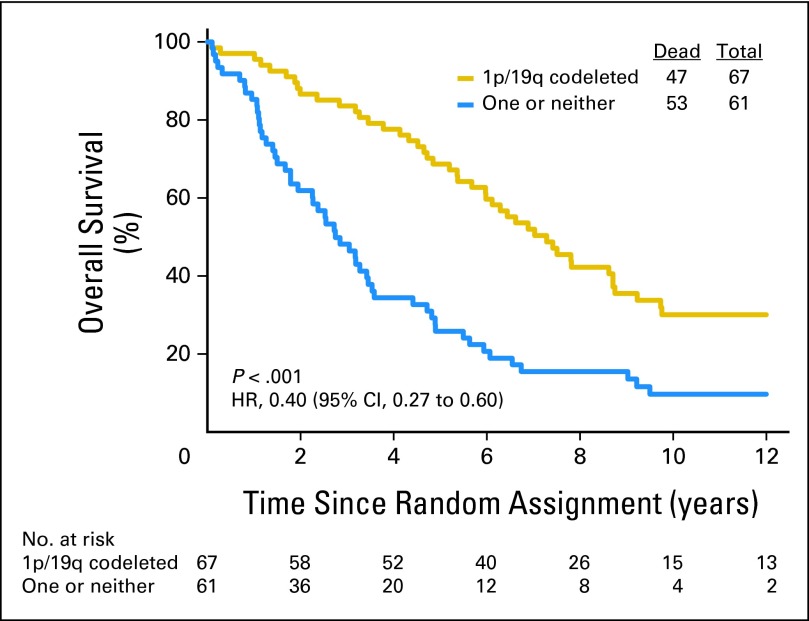
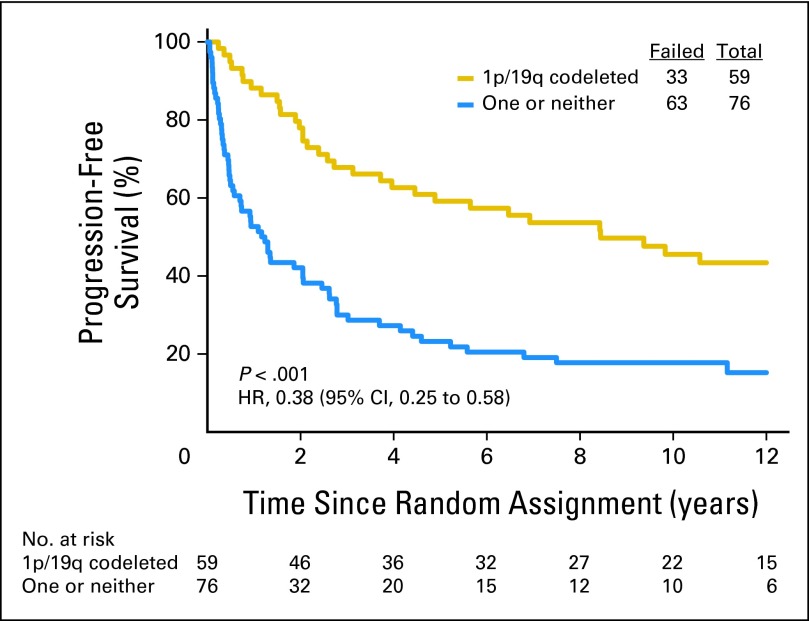
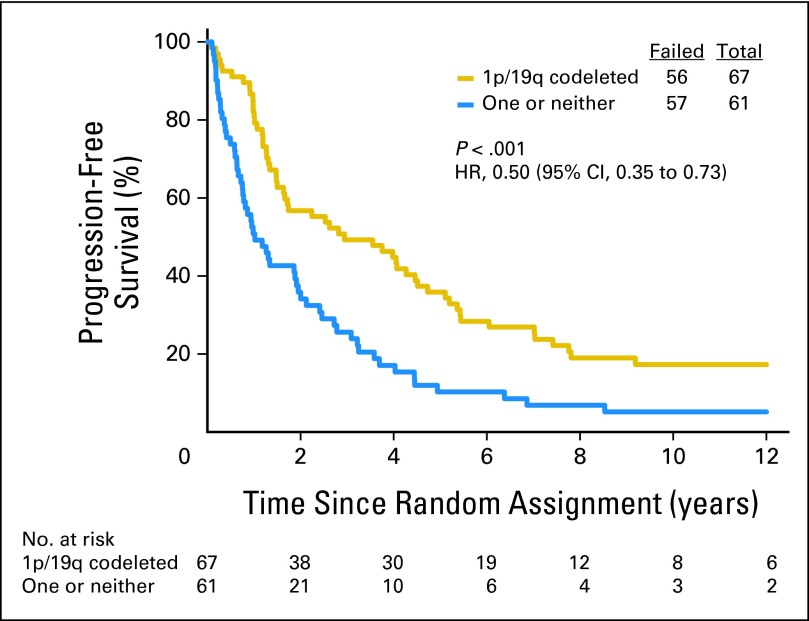
Data on 1p/19q status are summarized in Table 1. Loss of 1p was detected in 142 (54%) of 262 informative cases: 66 (49%) of 134 in the PCV plus RT group and 76 (59%) of 128 in the RT group. Loss of 19q was found in 167 (63%) of 264 informative cases: 85 (63%) of 135 in the PCV plus RT arm and 82 (64%) of 129 in the RT arm. Codeletion of 1p/19q was detected in 126 (48%) of 263 informative cases: 59 (44%) of 135 in the PCV plus RT arm and 67 (52%) of 128 in the RT arm. Codeletion was more frequent in AO than AOA (76% v 24%, P < .001) and in those not being treated with corticosteroids (49% v 34%, P = .01). Codeletion status was unknown in 28 cases (10%). Patients with codeleted tumors lived much longer than all others (PCV plus RT: 14.7 v 2.6 years, HR = 0.36, 95% CI, 0.23, 0.57, P < .001, Fig 3; RT: 7.3 v 2.7 years, HR = 0.40, 95% CI, 0.27 to 0.60, P < .001, Fig 4), and their PFS was also longer (PCV plus RT: 8.4 v 1.2 years, HR = 0.38, 95% CI, 0.25 to 0.58, P < .001; RT: 2.9 v 1.0 years, HR = 0.50, 95% CI, 0.35 to 0.73, P < .001). In data not shown, IDH status, an important prognostic factor,2 was balanced between the arms.
Fig 3.
Kaplan-Meier estimates of overall survival by genotype for procarbazine, lomustine, and vincristine plus radiotherapy arm. The hazard ratio (HR) for overall survival of patients with 1p/19q codeleted anaplastic oligodendroglioma (AO)/anaplastic oligoastrocytoma (AOA) compared with those with AO/AOA in whom one or neither allele was deleted was 0.36 (95% CI, 0.23 to 0.57; P < .001).
Fig 4.
Kaplan-Meier estimates of overall survival by genotype for radiotherapy arm. The hazard ratio (HR) for overall survival of patients with 1p/19q codeleted anaplastic oligodendroglioma (AO)/anaplastic oligoastrocytoma (AOA) compared with those with AO/AOA in whom one or neither allele was deleted was 0.40 (95% CI, 0.27 to 0.60; P < .001).
Treatment and Deletion Status
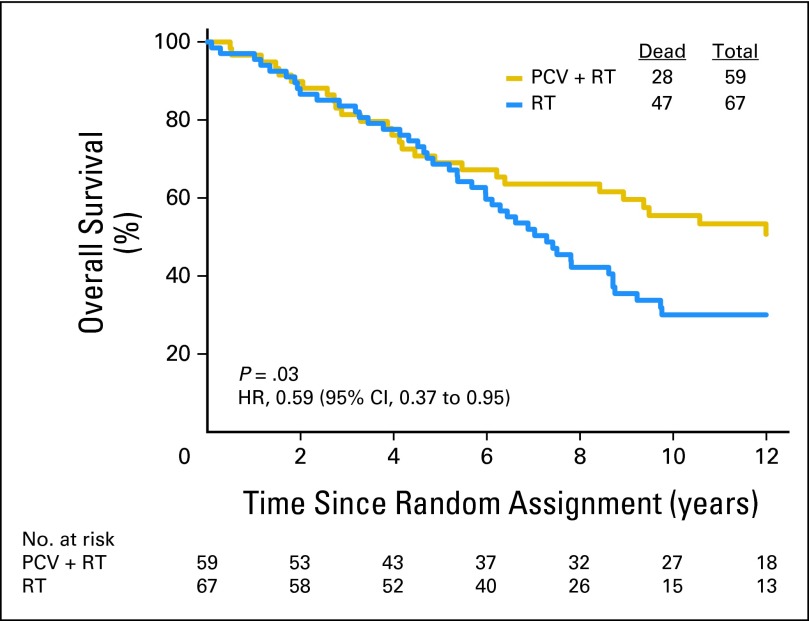
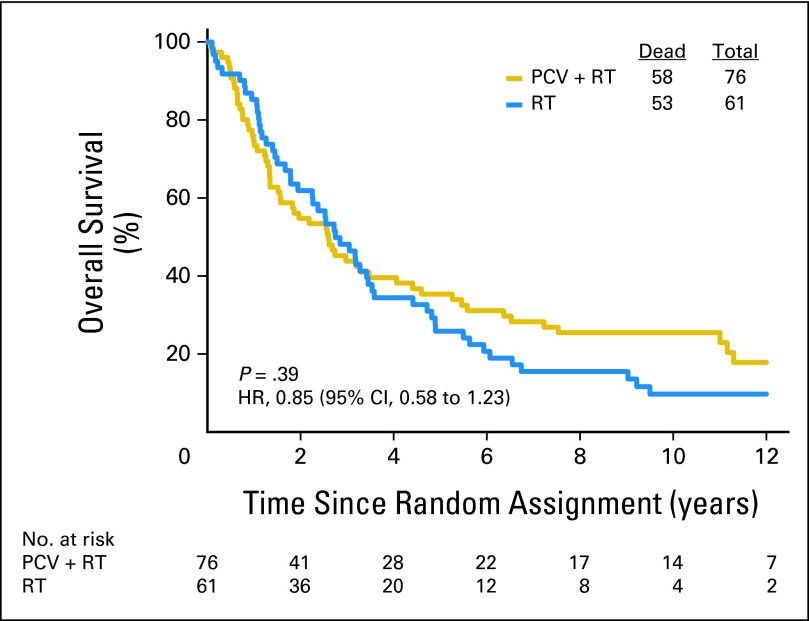
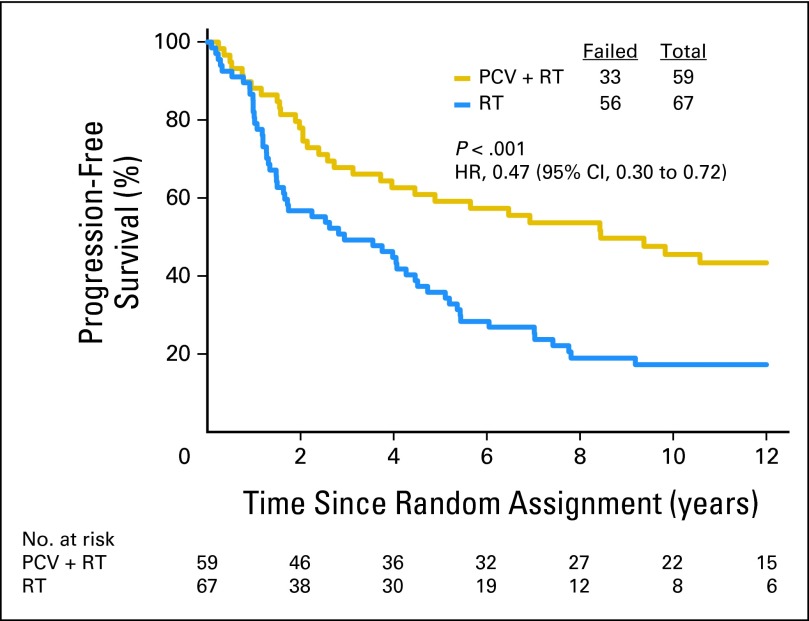
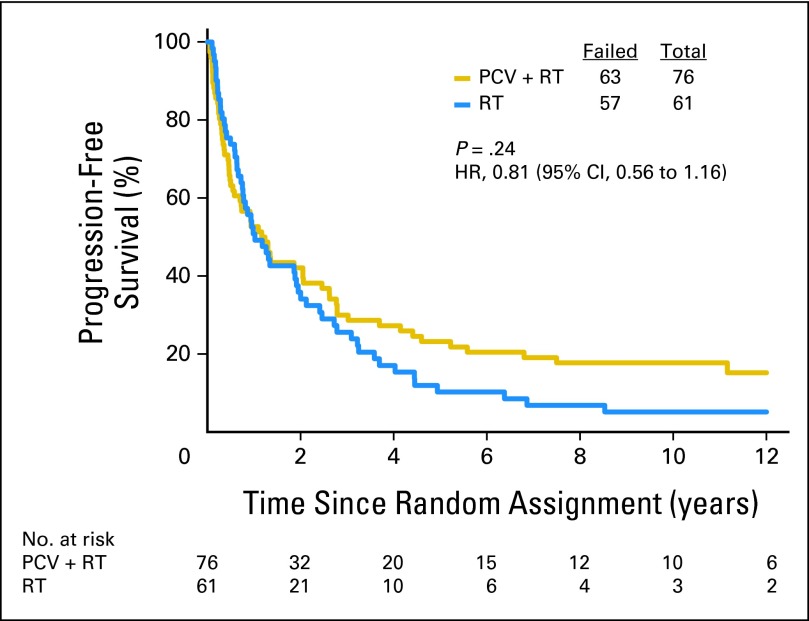
Fifty-nine patients with codeleted tumors were randomly assigned to PCV plus RT, and 67 were randomly assigned to RT. Long-term follow-up revealed that OS and PFS were longer after combined therapy. The median survival was 14.7 years in the PCV plus RT arm versus 7.3 years after RT (HR = 0.59; 95% CI, 0.37 to 0.95; P = .03; Fig 5). The median PFS was 8.4 years after PCV plus RT versus 2.9 years after RT alone (HR = 0.47; 95% CI, 0.30 to 0.72; P < .001). For patients with noncodeleted tumors, there were no median survival differences. Their median survival was 2.6 years after PCV plus RT versus 2.7 years after RT (HR = 0.85; 95% CI, 0.58 to 1.23; P = .39; Fig 6); their PFS was 1.2 years after PCV plus RT versus 1.0 year after RT (HR = 0.81; 95% CI, 0.56 to 1.16; P = .24).
Fig 5.
Kaplan-Meier estimates of overall survival by treatment for patients with 1p/19q codeleted anaplastic oligodendroglioma (AO)/anaplastic oligoastrocytoma (AOA). The hazard ratio (HR) for overall survival of patients with codeleted AO/AOA treated with procarbazine, lomustine, and vincristine (PCV) plus radiotherapy (RT) compared with those treated with RT alone was 0.59 (95% CI, 0.37 to 0.95; P = .03).
Fig 6.
Kaplan-Meier estimates of overall survival by treatment for patients with anaplastic oligodendroglioma (AO)/anaplastic oligoastrocytoma (AOA) in whom one or neither allele (1p or 19q) was deleted. The hazard ratio (HR) for overall survival of those with noncodeleted AO/AOA treated with procarbazine, lomustine, and vincristine (PCV) plus radiotherapy (RT) compared with those treated with RT alone was 0.85 (95% CI, 0.58 to 1.23; P = .39).
Clinical features and therapy were evaluated in Cox models of OS. For patients with codeleted tumors, PCV plus RT was significant in a model that considered corticosteroid use, number of lesions, type of surgery, and neurologic function (P < .1 in the univariate analysis) with treatment (adjusted HR = 0.51; 95% CI, 0.31 to 0.83; P = .007). For patients with noncodeleted tumors, treatment was not statistically significant in the Cox model.
Safety
Patients who were randomly assigned to PCV plus RT had more acute toxicities; the most frequent and serious toxicities were myelosuppression, cognitive or mood change, peripheral or autonomic neuropathy, vomiting, hepatic dysfunction, and allergic rash.19 Two early deaths were attributable to PCV-induced neutropenia. Serious immediate toxicities related to RT were uncommon, as were serious late toxicities. No instances of leukemia, severe dementia, or radionecrosis have been reported in either treatment arm. Both groups had similar MMSE and QOL scores until the last year of life, when scores declined equally rapidly.24
Treatment at Progression
Different treatments were prescribed at progression, including surgery, reirradiation, PCV, temozolomide, and investigational therapies. Surgery rates were similar between the treatment arms, 43% after PCV plus RT versus 56% after RT. However, rates of use of salvage chemotherapy were different: 41% after PCV plus RT versus 79% after RT (P < .001). In the codeleted subset, reoperative rates were again similar, 43% after PCV plus RT versus 54% after RT, but rates of chemotherapy were different, 57% after PCV plus RT versus 81% after RT (P = .04). Despite more intense therapy at progression, 1p/19q codeleted cases had inferior survival after RT alone.
DISCUSSION
RTOG 9402 tested the hypothesis that dose-intense PCV immediately before RT would prolong the lives of patients with AO/AOA compared with RT alone. When RTOG 9402 began, postoperative RT was the standard of care for all high-grade gliomas. Although therapy for GBM has since evolved, RT remains the standard for AO/AOA. Two aspects of RTOG 9402, dose-intense and pre-RT chemotherapy, were unusual at the time in brain cancer trials, but reflected schools of thought that were prevalent in oncology in the early 1990s when RTOG 9402 began: better tumor control with higher drug doses, therapeutic synergy when drugs accompany RT, and better drug delivery to nonirradiated tumors. Although the idea of dose-intensity has faded, the goal of combining traditional chemotherapeutics and targeted agents optimally with radiation treatment remains an important therapeutic concept. Indeed, daily TMZ with RT seems to have been a key step in the evolution of better treatment for GBM.10
Although the contribution of dose-intensity to the results of RTOG 9402 is unknown, and the sequencing of PCV/RT is likely less important than once thought,20,25 RTOG 9402 demonstrates the importance of precise diagnosis and long-term follow-up. In RTOG 9402, when histology was the sole metric for diagnostic accuracy, PCV plus RT did not afford a detectable survival benefit in the unadjusted analysis. With chromosomal testing, however, it became clear that patients with 1p/19q codeleted tumors had a doubling of survival after PCV plus RT. This interpretation is made cautiously, however, because RTOG 9402 was not powered for a subgroup analysis, and retrospective stratification by codeletion status was also unplanned. Furthermore, stricter histologic criteria, such as eliminating AOA cases, would not have obviated the need for chromosomal assessment because 29% of AOs had intact 1p or 19q alleles and 24% of AOAs were codeleted. Best results seem to have occurred when codeleted cases received PCV plus RT. The apparent doubling of survival in this subset was not detectable in 2006 when the median follow-up was 5 years and 1p/19q information was available on only 70% of participants.19 The implication that PCV plus RT may be a superior initial treatment for codeleted AO/AOA emerged with mature follow-up and was aided by thorough tissue retrieval.
When RTOG 9402 was planned, molecular heterogeneity, interactions with therapy, and consequences for clinical trials were not considered in designing randomized studies. This issue, described elsewhere,26 had the potential to obscure an important therapeutic effect in 9402 because AO/AOA is genetically heterogeneous: Some are 1p/19q codeleted, whereas others are 1p deleted only, 19q deleted only, or 1p and 19q intact. AO/AOA with codeletion has a distinctive biology. This knowledge is now incorporated into clinical trial design.
The results of RTOG 9402 suggest that chemoradiotherapy may be a highly effective treatment for patients with codeleted AO/AOA, but will PCV plus RT emerge as their new standard of care? Uncertainty arises because temozolomide is also effective for AO/AOA,27–29 and PCV is more toxic. Perhaps temozolomide plus RT, as prescribed for GBM,10 will be a more acceptable (if untested) new standard. Notwithstanding the lack of data on the relative efficacy of PCV and temozolomide, and a 1,000-patient retrospective study suggesting that PCV is highly effective for codeleted AO/AOA,30 clinicians may be reluctant to revisit PCV because it depletes marrow reserve and is sometimes debilitating.19 Indeed, two recent surveys suggest that oncologists prefer temozolomide or temozolomide plus RT for AO/AOA.31,32 Furthermore, experience from RTOG 9402, in which an early PFS benefit in codeleted cases19 became an OS benefit 6 years later, suggests that PFS may be an earlier end point for future AO/AOA trials. This is important because studies, like RTOG 9402, that take 20 years to complete are impractical.
Codeletion of chromosomes 1p/19q identifies a cancer that grows slowly, responds to chemotherapy, and is controlled for many years by PCV plus RT. Moreover, RTOG 9402 provides the strongest evidence to date that in the setting of PCV for AO/AOA, 1p/19q codeletion is both a predictive and prognostic biomarker. However, two additional observations suggest that the molecular elements that underlie the interaction of combined 1p/19q allelic loss with PCV plus RT are more complex than codeletion status alone. First, the survival curves for patients with codeleted tumors did not diverge for 5 years, long after RT with or without PCV, suggesting that not all patients with codeleted tumors benefited equally from PCV plus RT, or that some were effectively treated with salvage therapy at progression. Second, for patients with noncodeleted AO/AOA, the survival curves also diverged in favor of PCV plus RT, but this did not occur until after the median survival had been reached for this subset of cases. Whether this reflects errors in codeletion testing and incorrect assignment, benefit from PCV plus RT in the absence of visible codeletion, or other factors is unknown at this time. Furthermore, for the entire study cohort (codeleted and noncodeleted), the adjusted OS favored PCV plus RT at diagnosis. How do we interpret such findings, and how do they guide care and research?
Since 1p/19q codeletion status was not flawlessly predictive of benefit from PCV plus RT in either the codeleted or noncodeleted cases, clinicians are likely to be reluctant to make treatment decisions for their patients with AO/AOA based on 1p/19q information alone. Indeed, such was the experience in GBM, where O6-methyguanine-DNA methyltransferase promoter methylation status did not emerge as a predictive clinical test, especially because it failed to identify all patients with GBM who would have longer survival after temozolomide plus RT.33 Our data underscore that 1p/19q codeletion status is a marker, not a mechanism of sensitivity to PCV plus RT. Furthermore, 1p/19q codeletion is only one component of a cluster of molecular genetic alterations that define the AO/AOA cancer family; the other alterations include IDH mutations, promoter and histone hypermethylation, and mutations of CIC. In a recent sequencing analysis of AO, 1p/19q codeletion and mutations of IDH and CIC were closely associated.7 Currently, we are investigating the possibility that benefit from PCV plus RT can be predicted with so-called clinical-grade precision using a molecular approach that incorporates codeletion status with other biomarkers that characterize AO/AOA cancers.
Supplementary Material
Acknowledgment
We thank the 299 patients who participated in this multiyear clinical trial and their families. We also thank the many staff at Radiation Therapy Oncology Group, Southwest Oncology Group, North Central Cancer Treatment Group, National Cancer Institute of Canada Clinical Trials Group, and Eastern Cooperative Oncology Group; the members of the Pathology and Molecular Genetics Laboratories at the Mayo Clinic; and the nurses and data managers at the participating institutions, whose efforts were critical to the successful completion of this trial. We also thank David Macdonald, MD, David Louis, MD, and Susan Chang, MD, for the many insightful discussions that took place, and Andrew Lassman, MD, Samuel Wiebe, MD, and David Louis, MD, for reviewing the manuscript. We also thank the late Bernd Scheithauer, MD, who was the central pathologist.
Appendix
The following cooperative groups, institutions, and investigators conducted the trial: Radiation Therapy Oncology Group (RTOG): Akron City Hospital, Akron OH (William Demas, MD); Albert Einstein Medical Center, Philadelphia, PA (Sucha Asbell, MD); Christiana Care Health System, Newark, DL (Adam Raben, MD); Cleveland Clinic Foundation, Cleveland, OH (John Suh, MD); Community Clinical Oncology Programs–Columbus, OH (Larry Berk, MD), Greenville, SC (Jeannette Wilcox, MD), Kansas City, MI (William Stephenson, MD), Metro-MN (Paul Sperduto, MD), Ochsner Clinic (Carl Kardinal, MD), Southeast Cancer Control Consortium (James Atkins, MD) and Upstate Carolina (James Bearden, MD); Southern Nevada Cancer Research Foundation, Las Vegas, NV (Raul Meoz-Mendez, MD); Cross Cancer Institute, Edmonton, AB (Dorcas Fulton, MD) Dana-Farber Cancer Institute, Boston, MA (William Shipley, MD); Dartmouth Hitchcock Medical Center, Lebanon, NH (Eugen Hug, MD); Foundation for Cancer Research and Education, Phoenix, AZ (David Brachman, MD); Fox Chase Cancer Center, Philadelphia, PA (Andre Konski, MD); Hamilton Regional Cancer Center, Hamilton, ON (Anthony Whitton, MD); Hopital Notre-Dame du CHUM, Montreal, PQ (Karl Belanger, MD); Johns Hopkins Medical Center, Baltimore, MD (Ding-Jen Lee, MD); Louisiana State University Health Science Center, New Orleans, LA (Jill Gilbert, MD); London Regional Cancer Center, London, ON (Gregory Cairncross, MD); LDS Hospital, Salt Lake City, UT (William Sause, MD); Mayo Clinic, Rochester, MN (Jan Buckner, MD); McGill University, Montreal, PQ (Luis Souhami, MD); MD Anderson Cancer Center, Houston, TX (Ritsuko Komaki, MD); Medical College of Wisconsin, Milwaukee, WI (Elizabeth Gore, MD); New York University Medical Center, New York, NY (Michael Gruber, MD); Radiological Associates of Sacramento, CA (Christopher Jones, MD); SUNY Health Science Center, Brooklyn, NY (Marvin Rotman, MD); Thomas Jefferson University Medical Center, Philadelphia, PA (Walter Curran, MD); Tom Baker Cancer Centre, Calgary AB (Peter Forsyth, MD); University of Alabama Birmingham Medical Center, Birmingham AB (Ruby Meredith, MD, PhD); University of California Davis Medical Center, Davis CA (Janice Ryu, MD); University of California San Francisco, San Fransisco CA (Michael Prados, MD); University of Kentucky Medical Center, Louisville, KT (Roy Patchell, MD); University of Pennsylvania Medical Center, Philadelphia, PA (Peter Phillips, MD); University of Rochester, Rochester, NY (Paul Okunieff, MD); Wake Forest University Medical Center (Edward Shaw); Washington University, St Louis, MO (Jeff Michalski, MD) and Wayne State University, Detroit, MI (Lisa Rogers, MD). Southwest Oncology Group (SWOG): Cleveland Clinic Foundation, Cleveland, OH (Thomas Budd, MD); Community Clinical Oncology Programs–Columbus, Columbus, OH (Philip Kuebler, MD, PHD), Montana, Billings, MT (Patrick Cobb, MD), St Louis, St Louis, MO (Bethany Sleckman, MD), Northwest, Puget Sound, WA (Dustan Osborne, MD) and Wichita, Wichita, KA (Dennis Moore, MD); Henry Ford Hospital, Detroit, MI (Robert Chapman, MD); Loyola University, Chicago, IL (George Kovach, MD); Ohio State University, Columbus, OH (Paul Manuszak, MD); Providence Hospital, Southfield, MI (Howard Terebelo, MD); Puget Sound Oncology Consortium, Puget Sound, WA (Alex Spence, MD); University of Colorado, Denver, CO (Russell Tolley, MD); University of Michigan, Ann Arbor, MI (Laurence Baker, DO); University of New Mexico, Albuquerque, NM (Cheryl Willman, MD); University of Texas Southwestern, Dallas, TX (Karen Fink, MD, PhD), and University of Utah, Salt Lake City, UT (Thomas Warr, MD). North Central Cancer Treatment Group (NCCTG): Medcenter One Health Systems, Bismarck, ND (Edward Wos, DO); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA (Martin Wiesenfeld, MD); Iowa Oncology Research Association CCOP, Des Moines, IA (Roscoe Morton, MD); Michigan Cancer Consortium, Ann Arbor, MI (Philip Stella, MD), Meritcare Hospital CCOP, Fargo, ND (Preston Steen, MD), Geisinger Clinic & Medical Center CCOP, Danville, PA (Albert Bernath, MD); Illinois Oncology Research Association CCOP, Peoria, IL (John Kugler, MD) and Toledo Community Hospital Oncology Program CCOP, Toledo, OH (Paul Schaefer, MD); Sylvannia; Mayo Clinic and Mayo Foundation, Rochester, MN (Steven Alberts, MD) and CentraCare Clinic, St Cloud, MN (Harold Windschitl, MD). National Cancer Institute of Canada Clinical Trials Group (NCIC CTG): British Columbia Cancer Centre–Fraser Valley Centre, Surrey, BC (Alexander Agranovich, MD), Toronto Sunnybrook Regional Cancer Centre, Toronto, ON and University Health Network–Princess Margaret Hospital, Toronto, ON (Normand Laperierre, MD). Eastern Cooperative Oncology Group: Beth Israel Deaconess Hospital, Boston, MA (Michael Atkins, MD); Guthrie Clinic for Education and Research, Sayre, PA (Goran Broketa, MD); Hennepin County Medical Center, Minneapolis, MN (Daniel Schneider, MD); St Anthony's Medical Center, Rockford, IL (Laura Cisneros, MD); University of Florida Medical Center, Gainesville, FA (Robert Marsh, MD) and Western Michigan Cancer Center, Kalamazoo, MI (Raymond Lord III, MD).
Fig A1.
Kaplan-Meier estimates of progression-free survival by treatment group. HR, hazard ratio; PCV, procarbazine, lomustine, and vincristine; RT, radiotherapy.
Fig A2.
Kaplain-Meier estimates of progression-free survival by genotype for procarbazine, lomustine, and vincristine plus radiotherapy arm. HR, hazard ratio.
Fig A3.
Kaplan-Meier estimates by genotype for radiotherapy arm. HR, hazard ratio.
Fig A4.
Kaplan-Meier estimates of progression-free survival by treatment for patients with 1p/19q codeleted anaplastic oligodendroglioma/anaplastic oligoastrocytoma. HR, hazard ratio; PCV, procarbazine, lomustine, and vincristine; RT, radiotherapy.
Fig A5.
Kaplan-Meier estimates of progression-free survival for patients with anaplastic oligodendroglioma/anaplastic oligoastrocytoma in whom one or neither allele (1p or 19q) was deleted. HR, hazard ratio; PCV, procarbazine, lomustine, and vincristine; RT, radiotherapy.
Table A1.
Cox Proportional Hazards Model for Overall Survival
| Variable | P Value | Hazard Ratio | 95% CI |
|---|---|---|---|
| Assigned treatment, PCV+RT v RT alone | .0097 | 0.67 | 0.50 to 0.91 |
| Sex, female v male | .0055 | 0.64 | 0.47 to 0.88 |
| KPS, 80-100 v 60-70 | .0003 | 0.42 | 0.27 to 0.67 |
| Age, < 50 v ≥ 50 years | < .001 | 0.42 | 0.30 to 0.59 |
| Surgery, resection v biopsy | .0059 | 0.52 | 0.33 to 0.83 |
| Steroid, no v yes | .0259 | 0.69 | 0.50 to 0.96 |
| Multifocal disease, no v yes | .0009 | 0.41 | 0.24 to 0.70 |
| Tumor type, pure v mixed | .0023 | 0.58 | 0.41 to 0.83 |
| 1p/19q, both deleted v not both deleted | < .001 | 0.43 | 0.30 to 0.61 |
NOTE. Bolded values are favorable.
Abbreviations: KPS, Karnofsky performance status; PCV, procarbazine, lomustine, and vincristine; RT, radiotherapy.
Footnotes
Written on behalf of the Radiation Therapy Oncology Group, North Central Cancer Treatment Group, Southwest Oncology Group, National Cancer Institute of Canada Clinical Trials Group, and Eastern Cooperative Oncology Group.
The contents of this article are the sole responsibility of the authors and do not necessarily represent the views of the National Cancer Institute or Canadian Cancer Society.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00002569.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Gregory Cairncross, Edward Shaw
Administrative support: Walter Curran, Minesh Mehta
Provision of study materials or patients: Gregory Cairncross, Edward Shaw, Robert Jenkins, David Brachman, Jan Buckner, Karen Fink, Luis Souhami, Normand Laperriere
Collection and assembly of data: Gregory Cairncross, Meihua Wang, Edward Shaw, Robert Jenkins, Jan Buckner, Karen Fink, Luis Souhami, Minesh Mehta
Data analysis and interpretation: Gregory Cairncross, Meihua Wang, Edward Shaw, Robert Jenkins, Jan Buckner, Minesh Mehta
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported by Radiation Therapy Oncology Group Grants No. U10 CA21661 and U10 CA32115, North Central Cancer Treatment Group Grant No. U10 CA25224, Eastern Cooperative Oncology Group Grants No. CA17145 and CA21115, Southwest Oncology Group Grant No. CA32102, and Community Clinical Oncology Program Grant No. U10 CA37422, all from the National Cancer Institute. The study was also supported by a grant to the National Cancer Institute of Canada Clinical Trials Group from the Canadian Cancer Society.
REFERENCES
- 1.Louis DN, Ohgaki H, Weistler OD, et al., editors. 4th ed. Lyon, France: IARC Press; 2007. WHO Classification of Tumors of the Central Nervous System, [Google Scholar]
- 2.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2010;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reifenberger J, Reifenberger G, Liu L, et al. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol. 1994;145:1175–1190. [PMC free article] [PubMed] [Google Scholar]
- 6.Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333:1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226:7–16. doi: 10.1002/path.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourne TD, Schiff D. Update on molecular findings, management and outcome in low-grade gliomas. Nat Rev Neurol. 2010;6:695–701. doi: 10.1038/nrneurol.2010.159. [DOI] [PubMed] [Google Scholar]
- 9.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 10.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 11.Cairncross JG, Macdonald DR. Successful chemotherapy for recurrent malignant oligodendroglioma. Ann Neurol. 1988;23:360–364. doi: 10.1002/ana.410230408. [DOI] [PubMed] [Google Scholar]
- 12.Kim L, Hochberg FH, Thornton AF, et al. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for grade III and grade IV oligoastrocytomas. J Neurosurg. 1992;85:602–607. doi: 10.3171/jns.1996.85.4.0602. [DOI] [PubMed] [Google Scholar]
- 13.Cairncross G, Macdonald D, Ludwin S, et al. Chemotherapy for anaplastic oligodendroglioma: National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994;12:2013–2021. doi: 10.1200/JCO.1994.12.10.2013. [DOI] [PubMed] [Google Scholar]
- 14.van den Bent MJ, Kros JM, Heimans JJ, et al. Response rate and prognostic factors of recurrent oligodendroglioma treated with procarbazine, CCNU and vincristine chemotherapy: Dutch Neuro-Oncology Group. Neurology. 1998;51:1140–1145. doi: 10.1212/wnl.51.4.1140. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald DR, Gaspar LE, Cairncross JG. Successful chemotherapy for newly diagnosed aggressive oligodendroglioma. Ann Neurol. 1990;27:573–574. doi: 10.1002/ana.410270519. [DOI] [PubMed] [Google Scholar]
- 16.Paleologos NA, Macdonald DR, Vick NA, et al. Neoadjuvant procarbazine, CCNU, and vincristine for anaplastic and aggressive oligodendroglioma. Neurology. 1999;53:1141–1143. doi: 10.1212/wnl.53.5.1141. [DOI] [PubMed] [Google Scholar]
- 17.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 18.Smith JS, Perry A, Borell TJ, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- 19.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 20.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: A randomized European Organization for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Mackworth N, Fobair P, Prados MD. Quality of life self-reports from 200 brain tumor patients: Comparisons with Karnofsky performance scores. J Neurooncol. 1992;14:243–253. doi: 10.1007/BF00172600. [DOI] [PubMed] [Google Scholar]
- 23.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Cairncross G, Shaw E, et al. Cognition and quality of life after chemotherapy plus radiotherapy (RT) vs. RT for pure and mixed anaplastic oligodendrogliomas: Radiation Therapy Oncology Group trial 9402. Int J Radiat Oncol Biol Phys. 2010;77:662–669. doi: 10.1016/j.ijrobp.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 26.Betensky RA, Louis DN, Cairncross JG. Influence of unrecognized molecular heterogeneity on randomized clinical trials. J Clin Oncol. 2002;20:2495–2499. doi: 10.1200/JCO.2002.06.140. [DOI] [PubMed] [Google Scholar]
- 27.Chinot OL, Honore S, Dufour H, et al. Safety and efficacy of temozolomide in patients with recurrent anaplastic oligodendrogliomas after standard radiotherapy and chemotherapy. J Clin Oncol. 2001;19:2449–2455. doi: 10.1200/JCO.2001.19.9.2449. [DOI] [PubMed] [Google Scholar]
- 28.van den Bent MJ, Taphoorn MJ, Brandes AA, et al. Phase II study of first line chemotherapy with temozolomide in recurrent oligodendroglial tumors: The European Organization for the Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol. 2003;21:2525–2528. doi: 10.1200/JCO.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133–3138. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 30.Lassman AB, Iwamoto FM, Cloughesy TF, et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011;13:649–659. doi: 10.1093/neuonc/nor040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrey LE, Louis DN, Paleologos NA, et al. A survey of practice patterns for anaplastic oligodendroglioma. Neuro Oncol. 2007;9:314–318. doi: 10.1215/15228517-2007-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panageas KS, Iwamoto FM, Cloughesy TF, et al. Initial treatment patterns over time for anaplastic oligodendroglial tumors. Neuro Oncol. 2012;14:761–767. doi: 10.1093/neuonc/nos065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.