Abstract
Purpose
The National Surgical Adjuvant Breast and Bowel Project trial C-08 was designed to investigate the safety and efficacy of adding bevacizumab to fluorouracil, leucovorin, and oxaliplatin (FOLFOX6) for the adjuvant treatment of patients with stage 2-3 colon cancer. Our report summarizes the primary and secondary end points of disease-free and overall survival, respectively, with 5 years median follow-up time.
Patients and Methods
Patients received modified FOLFOX6 once every 2 weeks for a 6-month period (control group) or modified FOLFOX6 for 6 months plus bevacizumab (5 mg/kg) once every 2 weeks for a 12-month period (experimental group). The primary end point of the study was disease-free survival (DFS) and overall survival (OS) was a secondary end point.
Results
Of 2,673 analyzed patients, demographic factors were well-balanced by treatment. With a median follow-up of 5 years, the addition of bevacizumab to mFOLFOX6 did not result in an overall significant increase in DFS (hazard ratio [HR], 0.93; 95% CI, 0.81 to 1.08; P = .35). Exploratory analyses found that the effect of bevacizumab on DFS was different before and after a 1.25-year landmark (time-by-treatment interaction P value <.0001). The secondary end point of OS was no different between the two study arms for all patients (HR, 0.95; 95% CI, 0.79 to 1.13; P = .56) and for those with stage 3 disease (HR, 1.0; 95% CI, 0.83 to 1.21; P = .99).
Conclusion
Bevacizumab for 1 year with modified FOLFOX6 does not significantly prolong DFS or OS in stage 2-3 colon cancer. We observed no evidence of a detrimental effect of exposure to bevacizumab. A transient effect on disease-free survival was observed during bevacizumab exposure in the study's experimental arm.
INTRODUCTION
The combined use of fluorouracil, leucovorin, and oxaliplatin for 6 months constitutes the standard of care for the adjuvant treatment of patients with colon cancer. In two large randomized studies, this combination was established as superior to single-agent therapy using leucovorin-modulated fluorouracil.1,2 The National Surgical Adjuvant Breast and Bowel Project (NSABP) trial C-07 and the Multicenter International Study of Oxaliplatin/Fluorouracil-Leucovorin in the Adjuvant Treatment of Colon Cancer trials both demonstrated that 3-year DFS was significantly prolonged in patients with stage 2 or 3 colon cancer by the addition of oxaliplatin to fluorouracil and leucovorin, with hazard ratios of 0.80 and 0.77, respectively. Bevacizumab, a humanized monoclonal antibody directed against circulating vascular endothelial growth factor (VEGF), has demonstrated clinical benefit in patients with advanced colorectal cancer when combined with fluorouracil, leucovorin, and oxaliplatin in both untreated and previously treated patients. A study published by Giantonio et al3 randomly assigned 577 previously treated patients with colorectal cancer to fluorouracil, leucovorin, and oxaliplatin (FOLFOX) with or without bevacizumab. This trial showed a significant increase in response rate (8.6% v 22.7%), progression-free survival (4.7 v 7.3 months), and overall survival (OS; 10.8 v 12.9 months) for patients treated without and with the addition of bevacizumab, respectively. A second randomized study of 1,401 previously untreated patients with advanced disease failed to demonstrate a significant difference in response rate (38% v 38%) or OS (19.9 v 21.3 months) with the addition of bevacizumab to FOLFOX; however, the addition of bevacizumab did result in a significant improvement in progression-free survival from 8.0 to 9.4 months (P = .0023).4 These data, coupled with apparent clinical benefit associated with the addition of bevacizumab to oxaliplatin-based chemotherapy regimens from nonrandomized registry studies, underpin the rationale for testing the benefit of adding bevacizumab to FOLFOX chemotherapy in the adjuvant colon cancer setting.5,6
The primary goal of NSABP C-08 is to test the potential benefit and safety associated with the addition of bevacizumab to modified FOLFOX6 (using oxaliplatin at a dose of 85 mg/m2) in the adjuvant colon cancer setting. This report summarizes the updated disease-free survival (DFS), OS, and late adverse events associated with the addition of bevacizumab to standard chemotherapy in the adjuvant treatment of patients with stage 2 and 3 colon cancer. The safety profile and early DFS associated with the use of bevacizumab in combination with chemotherapy as used in NSABP C-08 has been reported.7,8
PATIENTS AND METHODS
Study Population
This study was approved by institutional review committees with assurances approved by the Department of Health and Human Services, in accordance with the Helsinki Declaration. Written informed consent was required for participation.
Patients meeting the eligibility criteria with stage 2 or 3 colon adenocarcinoma were stratified by number of positive lymph nodes and institution and were then randomly assigned 1:1 to receive either modified FOLFOX6 for 6 months or modified FOLFOX6 for 6 months plus bevacizumab for 12 months beginning concurrently with chemotherapy. This was an open-label study with no blinding of treatment assignment for patients, physicians, or investigators. Patients with Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 were randomly assigned within a window of 21 to 50 days following surgical removal of the primary tumor, and treatment was begun within 1 week of random assignment. Patients were excluded for any history of cerebral vascular accident, transient ischemic attack, symptomatic peripheral vascular disease, or an arterial thrombotic episode such as a myocardial infarction within the 12 months before randomization.
Regimens
The modified FOLFOX6 (mFOLFOX6) regimen includes leucovorin 400 mg/m2 intravenous (IV) on day 1, fluorouracil 400 mg/m2 IV bolus day 1 followed by 2,400 mg/m2 IV over 46 hours, and oxaliplatin 85 mg/m2 IV day 1. Bevacizumab is given in the experimental arm at a dose of 5 mg/kg IV day 1. All therapy is given once every 2 weeks for 12 doses (for a period of 6 months) or, for bevacizumab, 26 doses (for a period of 1 year).
Statistical Considerations
DFS was the primary end point defined as colon cancer recurrence, second primary cancer of any type, or death from any cause. Secondary end points included survival and toxicity related to study therapy. Random assignment was performed centrally using a biased-coin minimization algorithm.9
Protocol C-08 was designed to have 90% power to detect a 23.4% reduction in the hazard of DFS event when 592 DFS events were observed (approximately 3 years' median follow-up). Time to an event was measured from randomization. All P values will be two-sided and evaluated as significant at the .05 level. Time-to-event plots were prepared using the Kaplan-Meier method.10 Hazard ratios (HR) were calculated from Cox models,11 and P values for time to event are from the log-rank test stratified for nodal status (N0, N1, N2).12 Proportions were compared by Fisher's exact method.13 The primary analysis of DFS is based on the intention-to-treat principle14 excluding only patients with no follow-up and patients not at risk for the primary end point at the time of randomization (metastases or positive surgical margins). OS analyses include all patients with follow-up. The time-treatment interaction was tested by adding a time-varying term for the product of the treatment indicator and an indicator for DFS time more than 1.25 years to a Cox model already including the treatment indicator and stratified by nodal status.15
RESULTS
Patients
This analysis uses data collected by June 30, 2011, by which time 743 DFS and 442 OS events had occurred. During the 2-year period between September 2004 and October 2006, 2,710 patients were accrued to the study; 1,356 to the control and 1,354 to the experimental arms (Fig 1). A total of 15 patients (1.1%) and 17 patients (1.3%) in the control and experimental arms, respectively, had no clinical follow-up and were excluded from the OS analysis. Thus, there were 2,678 (1,341 in the control group and 1,337 in the experimental group) patients evaluated for OS. There were an additional three patients in the control arm and two in the bevacizumab arm found to have unclear margins or metastatic disease at entry so that they were not at risk for recurrence, leaving 2,673 patients (1,338 in the control arm and 1,335 in the bevacizumab arm) evaluable for DFS. Patient characteristics are well balanced by treatment arm (Table 1). The median follow-up was 4.9 years. Slightly more than half the patients were younger than 60 years and approximately 15% were older than 70 years. There was an equal gender distribution. Stage 2 patients constituted approximately 25% of the total.
Fig 1.
CONSORT diagram. DFS, disease-free survival; mFF6, modified fluorouracil, leucovorin, and oxaliplatin for 6 months; mFF6 + Bev, mFF6 for 6 months plus bevacizumab for 12 months; OS, overall survival.
Table 1.
Patient Characteristics (analyzed patients) of NSABP C-08
| Characteristic | mFF6 (n = 1,338) |
mFF6+Bev (n =. 1,335) |
Total (N = 2,673) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||
| < 50 | 332 | 24.8 | 342 | 25.6 | 674 | 25.2 |
| 50-59 | 447 | 33.4 | 435 | 32.6 | 882 | 33.0 |
| 60-69 | 347 | 25.9 | 366 | 27.4 | 713 | 26.7 |
| ≥ 70 | 212 | 15.8 | 192 | 14.4 | 404 | 15.1 |
| Sex | ||||||
| Female | 673 | 50.3 | 669 | 50.1 | 1,342 | 50.2 |
| Male | 665 | 49.7 | 666 | 49.9 | 1,331 | 49.8 |
| Race | ||||||
| White | 1,172 | 87.6 | 1,161 | 87 | 2,333 | 87.3 |
| Black | 101 | 7.5 | 114 | 8.5 | 215 | 8 |
| Other | 50 | 3.7 | 43 | 3.2 | 93 | 3.5 |
| Multiracial | 2 | 0.1 | 1 | 0.1 | 3 | 0.1 |
| Unknown | 13 | 1.0 | 16 | 1.2 | 29 | 1.1 |
| ECOG performance status | ||||||
| 0 (fully active) | 1,089 | 81.4 | 1,075 | 80.6 | 2,164 | 81.0 |
| 1 (no strenuous activity) | 249 | 18.6 | 259 | 19.4 | 508 | 19.0 |
| Nodal stage | ||||||
| N0 (node negative) | 332 | 24.8 | 334 | 25.0 | 666 | 24.9 |
| N1 (1-3 positive nodes) | 611 | 45.7 | 607 | 45.5 | 1,218 | 45.6 |
| N2 (≥ 4 positive nodes) | 395 | 29.5 | 394 | 29.5 | 789 | 29.5 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; mFF6, modified fluorouracil, leucovorin, and oxaliplatin for 6 months; mFF6 + Bev, mFF6 for 6 months plus bevacizumab for 12 months; NSABP, National Surgical Adjuvant Breast and Bowel Project.
Toxicity
Toxicity information related to patients enrolled onto NSABP C-08 has been published,7 and no additional toxicity signals have been noted. The addition of bevacizumab to mFOLFOX6 was not associated with an increase in mortality. Grade ≥ 3 toxicities that occurred significantly more frequently included hypertension, wound complications, pain, and proteinuria. To evaluate whether toxicities associated with bevacizumab changed during the course of the 1-year bevacizumab exposure, we compared toxicities that occurred during the first 6 months with those occurring during the last 6 months of bevacizumab, excluding patients who had the same toxicity during the first 6 months. Grade 3-4 bevacizumab-associated toxicities with an incidence of at least 1% occurred less frequently during the last 6 months of exposure (hypertension, 7% v 5.5%; pain, 6.6% v 4.1%; arterial thrombotic episodes, 1% v 0.6%). To examine toxicities associated with having been previously exposed to bevacizumab within the context of our study, we compared toxicities generally associated with the use of bevicizumab between the two arms during the 9-month therapy-free interval beginning 3 months after completion of all therapy. Toxicity data were not collected beyond 1 year after therapy completion. As listed in Table 2, toxicities were few and equally distributed between the two arms of the study. None of the noted differences was found to be statistically significant.
Table 2.
Grade ≥ 3 Toxicities Generally Associated With Bevacizumab During the 9-Month Period Beginning 3 Months After Completion of All Therapy
| Toxicity | mFF6 | mFF6+Bev |
|---|---|---|
| Hypertension | 0.6 | 0.7 |
| Pain | 1.1 | 1.1 |
| Proteinuria | 0.1 | 0 |
| ATE | 0.1 | 0.5 |
| VTE | 0.4 | 0.2 |
| Hemorrhage | 0.3 | 0.3 |
Abbreviations: ATE, arterial thrombotic event; mFF6, modified fluorouracil, leucovorin, and oxaliplatin for 6 months; mFF6 + Bev, mFF6 for 6 months plus bevacizumab for 12 months; VTE, venous thrombotic event.
DFS
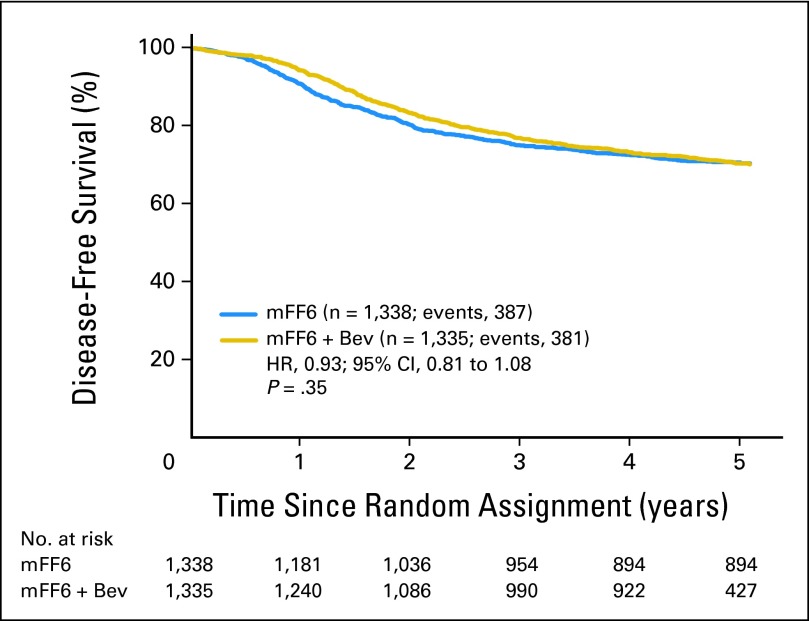
As shown in Figure 2, the addition of bevacizumab to mFOLFOX6 did not result in an overall significant increase in DFS (HR, 0.93; 95% CI, 0.81 to 1.08; P = .35). The point estimates for 3-year DFS were 77.9% and 75.1% for the experimental and control arms, respectively. The effect of bevacizumab treatment did not vary by stage (interaction P = .60). For patients with stage 2 disease, 3-year DFS was 87.3% and 85.4% for the experimental and control arms, respectively (HR, 0.86; 95% CI, 0.59 to 1.25; P = .43) and for patients with stage 3 disease, 73.5% and 71.7% for the experimental and control arms, respectively (HR, 0.95; 95% CI, 0.82 to 1.11; P = .55). Outcomes were similar for the end point of time to recurrence (ignoring second primary cancer and death unrelated to recurrence).
Fig 2.
Disease-free survival (DFS). Overall DFS for patients treated with modified fluorouracil, leucovorin, and oxaliplatin (mFF6) alone for 6 months or mFF6 for 6 months plus bevacizumab for 12 months (mFF6 + Bev). HR, hazard ratio.
We previously published the time-varying treatment effect.8 Now, with 5 years median follow-up, this effect remains highly statistically significant (time-treatment interaction P < .0001), supporting a different effect of bevacizumab during and immediately following its discontinuation compared with its later effects. The HR before the 15-month landmark strongly favored bevacizumab (HR, 0.61; 95% CI, 0.48 to 0.78; P < .0001), whereas this benefit was entirely lost subsequently (HR, 1.19; 95% CI, 0.99 to 1.42; P = .059).
OS
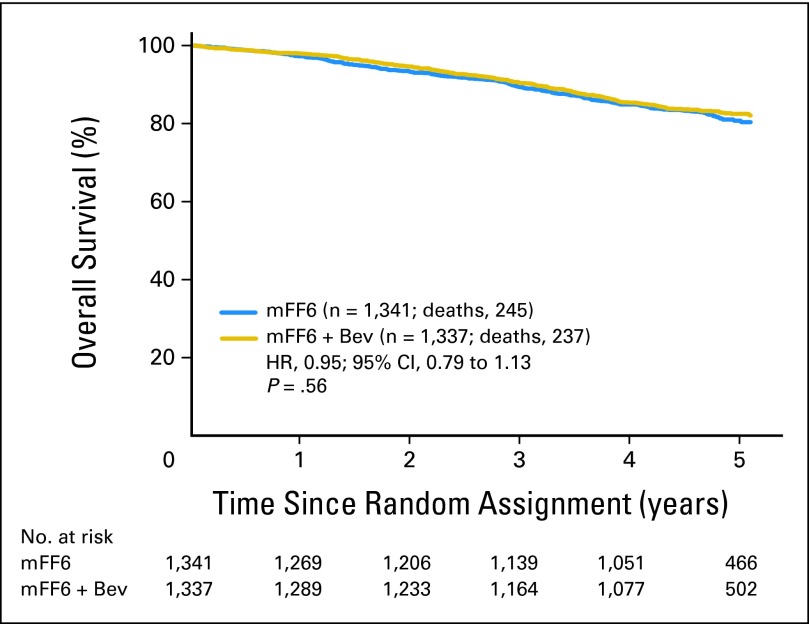
As shown in Figure 3, the addition of bevacizumab to mFOLFOX6 resulted in no significant difference in OS (HR, 0.95; 95% CI, 0.79 to 1.13; P = .56). The point estimates for 5-year OS were 82.5% and 80.7% for the experimental and control arms, respectively. The effect of bevacizumab on OS did not vary significantly with time as it did for DFS (time-treatment interaction P = .08).
Fig 3.
Overall survival for all patients treated with modified fluorouracil, leucovorin, and oxaliplatin (mFF6) alone for 6 months or mFF6 for 6 months plus bevacizumab for 12 months, (mFF6 + Bev). HR, hazard ratio.
For patients with stage 2 disease, the 5-year OS estimates were 94.3% and 90.3% for the experimental and control arms, respectively (HR, 0.62; 95% CI, 0.36 to 1.08; P = .093). Stage 3 patients had 5-year OS estimates of 78.7% and 77.6% for the experimental and control arms, respectively (HR, 1.00; 95% CI, 0.83 to 1.21; P = .99). A test for a stage-treatment interaction on OS was not significant (P = .11).
Survival After Recurrence
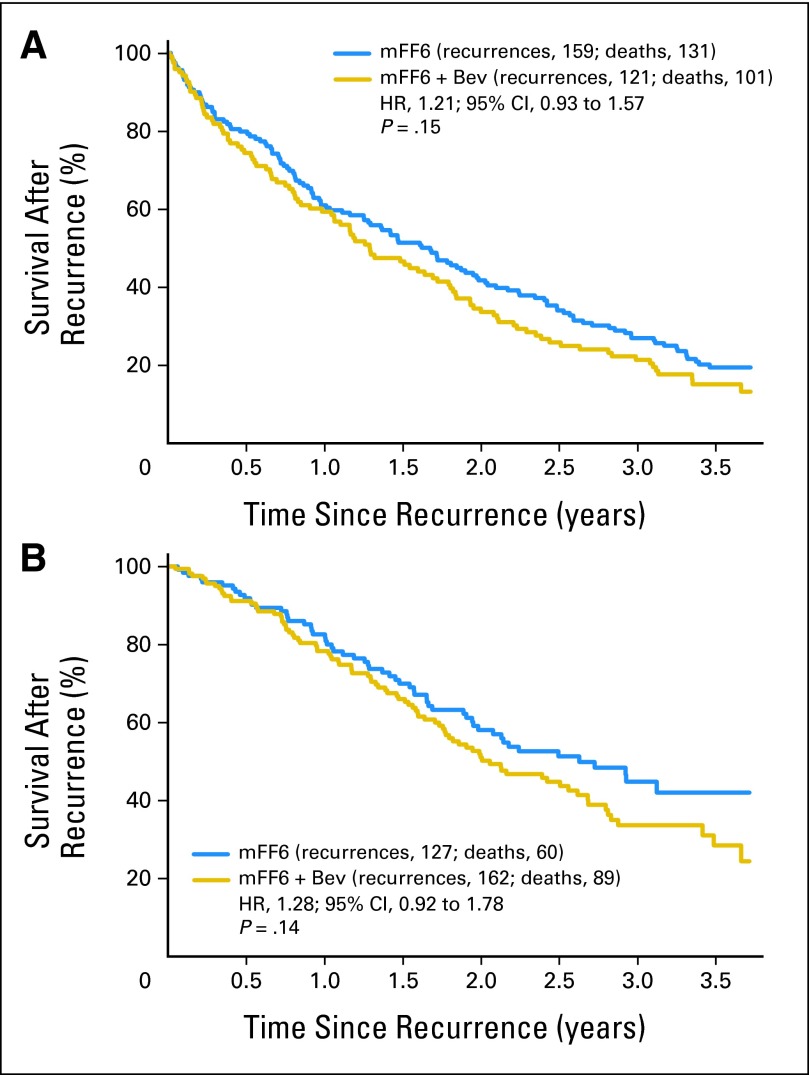
Cancer recurred in 286 and 283 patients in the control and bevacizumab-containing arms, respectively. Of these patients, 191 and 190 have died in each arm, respectively. Although not statistically significant (P = .1), patients in the bevacizumab arm appeared to have a more rapid rate of death relative to those whose disease recurred in the control arm (HR, 1.15; 95% CI, 0.94 to 1.41). To explore whether the decrement in survival after relapse may be related to ongoing or recently completed bevacizumab exposure, we investigated survival after relapse within 18 months of study entry when the exposure to bevicizumab would be expected to be greatest (Fig 4A) and beyond 18 months after study entry when the exposure to bevacizumab would be expected to be negligible (Fig 4B). As shown in Figure 4, the effect of bevacizumab on survival after relapse did not seem to vary by the timing of the relapse (before or after 18 months from study entry; HR, 1.21 v 1.28, respectively).
Fig 4.
Survival after recurrence for patients relapsing (A) within the first 18 months after study entry and (B) relapsing 18 months after study entry. HR, hazard ratio; mFF6, modified fluorouracil, leucovorin, and oxaliplatin for 6 months; mFF6 + Bev, mFF6 for 6 months plus bevacizumab for 12 months.
DISCUSSION
The NSABP C-08 study was designed to investigate the use of anti-VEGF therapy for the adjuvant treatment of stage 2 and 3 colon cancer. The addition of 1 year of bevacizumab to 6 months of modified FOLFOX6 chemotherapy in this setting did not improve either DFS or OS. Although there was a hint of a possible effect on OS in stage 2 (P = .093), the HR confidence limits were wide and the stage-treatment interaction was not significant (P = .11), suggesting that this may be a result of chance variation. However, with 5 years' median follow-up, there continues to be a highly significant time-by-treatment interaction with DFS such that the effect of bevacizumab was favorable before a 15-month landmark but detrimental subsequently, resulting in no positive effect overall. However, it should be noted that because there was a 6-month difference in the duration of therapy between the study arms and because the scanning intervals were not mandated by the protocol, the timing of post-therapy scans was different between the arms such that patients on the control arm were scanned earlier than patients in the experimental arm (mean, 1.09 months). The biased timing of imaging is confounded with the estimated DFS times such that definitive conclusions concerning the DFS differences across the arms at the early time points cannot be justified. However, bias-adjusted analyses presented in our previous publication suggest these differences cannot be entirely explained by the imaging bias. The presence of time-dependent differences in DFS in the AVANT16 study support the noted time-dependent differences in DFS as nonartifactual. Given the suggestion from the recently disclosed data from the AVANT study16 that bevacizumab exposure may result in a harmful effect on outcomes, it is important to note that there was no evidence of a detrimental effect of bevicizumab on any of the end points investigated in NSABP C-08, including DFS and OS. These data support the conclusion that although bevacizumab may delay recurrence, its use does not prevent recurrence.
We observed that patients in the bevacizumab-containing arm who relapsed after adjuvant treatment seemed to have a higher rate of death than did those patients treated with chemotherapy alone. Although this difference did not reach statistical significance, a similar observation was reported in the AVANT trial.16 Hypotheses to explain this phenomenon include the possibility that bevacizumab changes the biology of the disease to a more aggressive phenotype. Several publications using preclinical murine models have suggested that exposure to anti-VEGF therapy may result in the development of a more aggressive tumor phenotype with a greater propensity for growth and metastatic spread.17–21 However, in this case, we would have expected to see a detrimental effect on OS in the bevacizumab-treated patient group, which was not observed. An alternative explanation is that bevacizumab is less effective and/or less frequently used in relapsed patients previously exposed to bevacizumab in the adjuvant setting.22 A decrement in OS would also be expected if this hypothesis were true. Finally, it is plausible that bevacizumab may interfere with the sensitivity of computed tomography scanning owing to its effects on tumor vascularity and permeability and thereby potentially delay the detection of a small recurrence. However, we found no evidence for a change in survival after relapse in patients who relapsed early, in whom bevacizumab would be expected to have its greatest effect on scanning sensitivity compared with those who relapse beyond 18 months from study entry, for whom any effects of bevacizumab would be expected to be minimal (Fig 4).
The failure of bevacizumab to enhance the outcomes associated with FOLFOX chemotherapy calls into question our traditional paradigm of adjuvant colon drug development, namely, to consider agents for testing in the adjuvant setting only after they are found to be beneficial in the treatment of patients with advanced disease. The addition of bevacizumab to oxaliplatin-based chemotherapy has been shown to be beneficial for patients with advanced colorectal cancer both as initial and subsequent therapy.3,4 Similarly, cetuximab and irinotecan have been shown to have beneficial effects in patients with advanced disease, yet have not been shown to be associated with benefit in the adjuvant colon setting.22–25 These experiences along with the present bevacizumab observations suggest that we should not assume that therapy associated with benefit in the metastatic setting will necessarily translate into benefit in the adjuvant setting.
On the basis of our results, which showed a lack of benefit associated with the use of bevacizumab for 1 year along with 6 months of FOLFOX chemotherapy, bevacizumab should not be used for the management of patients with stage 2 or 3 colon cancer in the adjuvant setting.
Supplementary Material
Acknowledgment
We thank Barbara C. Good, PhD, for editorial assistance.
Footnotes
Supported by Public Health Service Grants No. U10-CA-12027, U10-CA-69651, U10-CA-37377, and U10-CA-69974 from the National Cancer Institute, US Department of Health and Human Services.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00096278.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter underconsideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Carmen J. Allegra, Genentech (C); Greg Yothers, Genentech (C); Norman Wolmark, Genentech (U) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Carmen J. Allegra, Greg Yothers, Michael J. O'Connell, Saima Sharif
Administrative support: Greg Yothers, Saima Sharif
Collection and assembly of data: Carmen J. Allegra, Greg Yothers, Saima Sharif
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med . 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 2.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol . 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 3.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol . 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [Errata: J Clin Oncol 26:3110, 2008 and J Clin Oncol 27:653, 2009] [DOI] [PubMed] [Google Scholar]
- 5.Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: The BRiTE observational cohort study. Oncologist . 2009;14:862–870. doi: 10.1634/theoncologist.2009-0071. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: The BEAT study. Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 7.Allegra CJ, Yothers G, O'Connell MJ, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol . 2009;27:3385–3390. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing the efficacy of bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol . 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White SJ, Freedman LS. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer . 1978;37:849–857. doi: 10.1038/bjc.1978.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc . 1958;53:457–481. [Google Scholar]
- 11.Cox DR. Regression models and life tables. J Royal Stat Soc Series B . 1972;34:187–220. [Google Scholar]
- 12.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep . 1966;50:163–170. [PubMed] [Google Scholar]
- 13.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J Royal Stat Soc . 1922;85:87–94. [Google Scholar]
- 14.Lachin JM. Statistical considerations in the intent-to-treat principle. Control Clin Trials . 2000;21:167–189. doi: 10.1016/s0197-2456(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 15.Klein JP, Moeschberger ML. New York, NY: Springer; 2003. Survival Analysis: Techniques for Censored and Truncated Data. [Google Scholar]
- 16.De Gramont A, Van Custem E, Tabernero J, et al. AVANT: Results from a randomized, three-arm multinational phase III study to investigate bevacizumab with either XELOX or FOLFOX4 versus FOLFOX4 alone as adjuvant treatment for colon cancer. J Clin Oncol. 2011;29 (suppl 4; abstr 362) [Google Scholar]
- 17.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell . 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell . 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loges S, Mazzone M, Hohensinner P, et al. Silencing or fueling metastasis with VEGF inhibitors: Antiangiogenesis revisited. Cancer Cell . 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds AR, Hart IR, Watson AR, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med . 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 21.Ellis LM, Reardon DA. Cancer: The nuances of therapy. Nature . 2009;458:290–292. doi: 10.1038/458290a. [DOI] [PubMed] [Google Scholar]
- 22.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III coloncancer: Results of CALGB 89803. J Clin Oncol . 2007;25:3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 23.Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecanversus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802) Ann Oncol . 2009;20:674–680. doi: 10.1093/annonc/mdn680. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer:PETACC-3. J Clin Oncol . 2009;27:3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 25.Alberts SR, Sargent DJ, Smyrk TC, et al. Adjuvant mFOLFOX6 with or without cetuxiumab (Cmab) in KRAS wild-type (WT) patients (pts) with resected stage III colon cancer (CC): Results from NCCTG Intergroup Phase III Trial N0147. J Clin Oncol. 2010;28:262s. (suppl; abstr CRA3507) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.