Abstract
An improved understanding of the factors that regulate the migration of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) would provide new insights into human heart development and suggest novel strategies to improve their electromechanical integration after intracardiac transplantation. Since nothing has been reported as to the factors controlling hESC-CM migration, we hypothesized that hESC-CMs would migrate in response to the extracellular matrix and soluble signaling molecules previously implicated in heart morphogenesis. To test this, we screened candidate factors by transwell assay for effects on hESC-CM motility, followed by validation via live-cell imaging and/or gap-closure assays. Fibronectin (FN) elicited a haptotactic response from hESC-CMs, with cells seeded on a steep FN gradient showing nearly a fivefold greater migratory activity than cells on uniform FN. Studies with neutralizing antibodies indicated that adhesion and migration on FN are mediated by integrins α-5 and α-V. Next, we screened 10 soluble candidate factors by transwell assay and found that the noncanonical Wnt, Wnt5a, elicited an approximately twofold increase in migration over controls. This effect was confirmed using the gap-closure assay, in which Wnt5a-treated hESC-CMs showed approximately twofold greater closure than untreated cells. Studies with microfluidic-generated Wnt5a gradients showed that this factor was chemoattractive as well as chemokinetic, and Wnt5a-mediated responses were inhibited by the Frizzled-1/2 receptor antagonist, UM206. In summary, hESC-CMs show robust promigratory responses to FN and Wnt5a, findings that have implications on both cardiac development and cell-based therapies.
Introduction
Human embryonic stem cell-derived cardiomyocytes (hESC-CMs) have attracted considerable interest as both a model for human heart development and a potential source for regenerating infarcted heart tissue. As described below, hESC-CMs exhibit significant spontaneous migratory activity in vitro. To our knowledge, this phenomenon has not been previously reported, nor is it known what signaling molecules might modulate their migration. While adult cardiomyocytes are not considered a particularly migratory cell type, the motility of immature cardiomyocytes such as hESC-CMs is not unexpected. Indeed, it is well established that a number of critical steps in heart development involve cardiomyocyte migration, including heart tube closure [1], muscularization of the outflow tract [2], as well as septation [3] and trabeculation [4] of the ventricles, but the chemotactic cues driving these processes remain incompletely defined. Promigratory factors have been identified for related cell types, including skeletal myoblasts [5] and adult cardiac progenitors [6,7], but it was unknown whether hESC-CMs would respond to these same factors.
An improved understanding of the conditions and signaling molecules that affect hESC-CM migration would have a signficant practical value. First, nearly all current knowledge regarding cardiomyocyte motility has come from developmental studies in nonhuman model systems. The hESC-CM system represents a unique opportunity to study this behavior in human cardiomyocytes. Second, while the transplantation of hESC-CMs improves contractile function in preclinical infarct models, our group has shown that the electromechanical integration of the hESC-CM grafts is limited in the injured hearts because many of the implants are isolated by scar tissue [8]. We speculate that, by stimulating their migration in vivo, one might be able to direct engrafted hESC-CMs toward the border zone, thereby increasing the likelihood of host–graft contact and electromechanical coupling.
To identify molecules that promote hESC-CM migration, we took a candidate factor approach and used the relatively high-throughput transwell assay to test molecules known to be involved in either cardiac morphogenesis [1,4,9–19] or the migration of myoblasts [5] or adult cardiac progenitors [6,7]. We then validated our transwell findings using the two-dimensional (2D) haptotaxis and chemotaxis assays, as well as the gap-closure assay. Based on these studies, we conclude that hESC-CMs sense and migrate in response to gradients of FN, an extracellular matrix (ECM) glycoprotein, and Wnt5a, a noncanonical Wnt ligand.
Materials and Methods
Reagents, antibodies, and immunostaining
Type 1 rat tail collagen (hereafter abbreviated as Col I), human plasma FN, and vitronectin (VN) were all purchased from Invitrogen (Grand Island, NY). Placental laminin (LN) was purchased from Sigma (St Louis, MO) and Type VI Col from BD Biosciences (San Jose, CA). For all coating procedures, FN, VN, LN, and Col VI were diluted in calcium-free phosphate-buffered saline (PBS), and Type 1 rat Col was diluted in 0.2 N acetic acid. Tissue culture plates were first coated overnight at 4°C with 0.1% polyethyleneimine (PEI; Sigma), rinsed three times with water, and then coated for 1 h at 37°C with the ECM protein at varying concentrations. For the gap closure and Wnt5a-stimulated live-cell microscopy assays, wells were coated with PEI, followed by FN at 2.5 μg/cm2 (10 μg/mL, 0.5 mL per well of a 24-well plate). All surfaces were rinsed with PBS, aspirated, and stored dry at 4°C until use.
Anti-integrin α-5 (#Ab23589; Abcam, Cambridge, MA) and anti-integrin α-V (#Ab16821; Abcam, Cambridge, MA) were used at 5 μg/mL for integrin blockade. For these studies, hESC-CM migration was quantified by immunostaining with rabbit anti-cardiac troponin T (cTnT) at 1:200 (#Ab91605; Abcam, Cambridge, MA). For all other transwell experiments and for quantification of cardiac purity, we used mouse anti-cTnT at 1:200 (#MS-295-P; Thermofisher, Waltham, MA).
L-cell-conditioned medium
Control L-cells and L-cells overexpressing Wnt5a [20] (ATCC, Manassas, VA) were cultured in 20 mL of Dulbecco's modified Eagle medium containing 10% fetal calf serum (FCS) and 1% penicillin/streptomycin per 150-cm2 dish. Once cells were confluent, FCS was reduced to 5%, and the medium was collected every 48 h for 6 days. The resultant conditioned medium (CM) was centrifuged at 3000g for 15 min at 4°C to pellet cell debris. Supernatants were stored at 4°C for up to 3 weeks.
hESC-CM derivation and purification
All experiments were approved by the University of Washington's Embryonic Stem Cell Research Oversight Committee and involved the H7 hESC line (Wicell Research Institute, NIH Stem Cell Registry #hESC-10-0061). In brief, undifferentiated hESCs [21] were expanded in mouse embryonic fibroblast CM supplemented with basic fibroblast growth factor [22]. To induce cardiogenesis, we treated compact monolayers of hESCs with serial activin A and BMP-4 as previously described [23,24] (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd). For the initial transwell screens of the ECM and soluble factors, we used nonselected hESC-CM populations of ∼60% cardiomyocyte purity [8,25]. For all other experiments, we used highly enriched hESC-CM preparations obtained via genetic selection. For this, we created a lentiviral vector in which regulatory elements from the striated muscle-specific muscle creatine kinase promoter [26] drive expression of the fluorescent protein, mCherry, and puromycin resistance (Supplementary Fig. S1B). We transduced the differentiating hESC-CM cultures with this vector on day 5 postinduction with activin A and then applied puromycin selection (2.5 μg/mL) on day 22 for 48 h. This protocol yielded cell populations that were 99% positive for both mCherry and the cardiac marker, cTnT, which were then used for migration studies (Supplementary Fig. S1C–E).
Transwell assay
For all transwell experiments, we used light-opaque transwell inserts with 8-μm-diameter pores and a growth area of 0.3 cm2 (BD Falcon Fluoroblok; BD Bioscience, San Jose, CA). For the ECM checkerboard experiments, we exposed each face of the membrane to 0.25, 2.5, or 25 μg/cm2 of dissolved proteins (which corresponded to a 75-μL volume of 1, 10, or 100 μg/mL ECM concentration) for 5 min at room temperature. At lower ECM concentrations, hESC-CMs typically failed to adhere. For all other transwell experiments, the top and the bottom of the membrane were both coated with 25 μg/cm2 FN for 5 min at room temperature. After coating, the membranes were aspirated, rinsed with PBS, and stored dry at 4°C until use. We seeded 7.5×105 hESC-CMs per transwell and allowed 14 h for initial adhesion before exposing the cells to a particular set of experimental conditions (eg, treatment with a candidate growth factor). To determine the number of cells present on either side of the membrane after the specified interval of time under these conditions, we fixed the membranes with 4% paraformaldehyde and then immunostained them with an antibody against cTnT (with detection by an Alexa-488-conjugated secondary antibody). A blinded observer used an Olympus IX71 inverted epifluorescence microscope to count the number of cTnT+ cardiomyocytes on either side of the membrane, using at least eight 10×fields. To account for any migration that may have occurred during the initial seeding period, we also immunostained the equivalently prepared membranes that were fixed at this timepoint and used these counts to correct the experimental groups. Migration across the transwell was then expressed either as the percentage of attached cardiomyocytes that had migrated to the bottom of the membrane [100×bottom/(top + bottom)] or as the ratio of migrated cardiomyocytes under treated versus control conditions (ie, fold over control).
Formation of surface-bound FN gradients
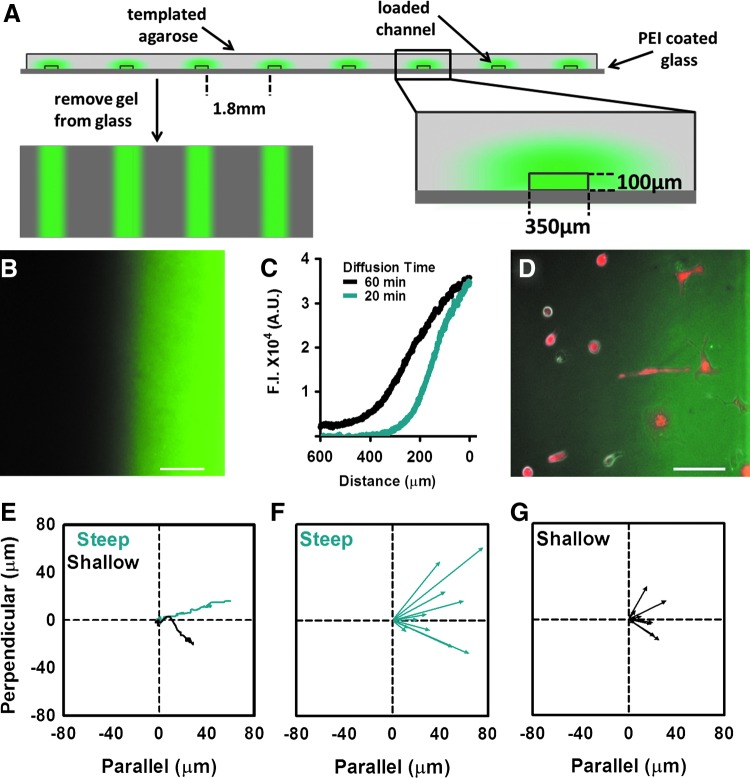
To generate reproducible gradients of FN on 2D glass surfaces, we employed the agarose diffusive printing method previously described by Mai et al. [27]. In brief, we used standard photolithographic techniques to fabricate a silicon wafer mold. To create the stamp, we poured a 3% agarose solution over the latter mold and allowed it to solidify for 30 min (Supplementary Fig. S2A, Step I). Next, we transferred the resultant patterned agarose stamp to a 0.1% PEI-coated glass-bottom dish (MatTek, Ashland, MA), punched holes on either side of the grid to access the channels, and then loaded the channels with 10 μL of Oregon 488-conjugated FN (Life Technologies, Grand Island, NY) at 850 μg/mL (Supplementary Fig. S2A, Step II). As it diffused through the gel, the fluorescent-tagged FN deposited onto the underlying glass surface (Fig. 3A, B, Supplementary Fig. S2B). Reproducible steep or shallow gradients of surface-bound FN were formed by allowing the molecule to diffuse through the gel for 20 or 60 min, respectively (Fig. 3C). The agarose stamp was then removed before cell seeding (Fig 3D).
FIG. 3.
hESC-CM migration on FN is dependent on the gradient slope. (A) Schematic showing the dimensions of the templated gel and the surface-bound gradient that results from this diffusive printing technique. (B) Representative image of a glass-bound gradient of fluorescent-tagged FN. (C) The steepness of the gradient was varied by changing the diffusion time of the protein through the agarose stamp and quantified using ImageJ analysis software [fluorescence intensity (F.I.) in arbitrary units (A.U.)]. (D) We calculated the net migration of genetically selected, mCherry+hESC-CMs on these gradients in the direction parallel and perpendicular to the gradient. Only cells within the linear range of the gradient were included in the analysis. Scale bar=100 μm. (E) Representative migration paths of hESC-CMs on steep (20 min, blue) and shallow (60 min, black) gradients. Displacement vectors of hESC-CMs on steep (F) and shallow (G) gradients of FN. N=11 cells per condition. Color images available online at www.liebertpub.com/scd
To quantitate the relative slope of the two surface-bound FN gradients, we used a Zeiss AxioObserver epifluorescence microscope equipped with an AxioCam CCD to image the Oregon 488–FN gradients formed with 20 or 60 min of diffusion time. The resultant fluorescent signal intensity was then analyzed using ImageJ software. The steep FN gradients (20 min of diffusion time) showed a fluorescence intensity slope of 123±7 arbitrary units (A.U.) per μm versus 77±4 A.U. per μm for the shallow gradients (60 min of diffusion) (P<0.01, n=3). We calibrated these fluorescent signals by comparison with surfaces coated with known concentrations of Oregon 488-FN and estimated that the steep and shallow gradients had slopes of ∼7.4 and 4.6 μg/cm2 FN per 100-μm distance, respectively. During live-cell microscopy experiments, we limited our analysis to cells in the linear portions of the FN gradient (ie, the first 200 μm of the steep gradient and the first 325 μm of the shallow gradient). This corresponded to the coating densities in the range of ∼2.5 to 15 μg/cm2 FN.
Live-cell migration analysis
To observe the migration of genetically selected mCherry + hESC-CMs on 2D surfaces, we used an automated inverted microscope (Nikon Eclipse Ti) equipped with a humidified environmental chamber (37°C, 5% CO2) and an Andor iXon + EM-CCD camera. Time-lapse images were acquired every 15 min and were analyzed with Nikon Elements Image Analysis Software by tracking the change in the position of the centroid of the cell at each time point. Cell speed was calculated as the path length (distance in μm) divided by time (hours). Vector diagrams were created by plotting the displacement of each cell from a common origin.
Gap-closure assay
We seeded genetically selected, mCherry + hESC-CMs into a commercially available tissue culture insert (Ibidi; catalog # 80209, Munchen, Germany) and cultured them until a confluent monolayer was formed (∼24 h). We then removed the insert, leaving a highly reproducible 500-μm-wide gap. Images were acquired both immediately after removal of the insert and after an additional 24 h of culture under control or Wnt5a-stimulated conditions (see Supplementary Online Video S1 for representative migratory behavior over a 24-h period). Gap closure was calculated by subtracting the gap area at the 24-h timepoint from the starting gap area. (measured using ImageJ software).
Chemotaxis assay of diffusive Wnt5a gradient
To determine the chemotactic response of hESC-CMs to Wnt5a, we used a commercially available, microfluidic-based chemotaxis device (iBidi 2D chemotaxis chamber; #80301, Munchen, Germany) as per the manufacturer's protocol. Based on a previously published characterization of this device, one would expect a linear gradient of Wnt5a to be formed within several hours and to remain stable for at least 48 h [28]. For our experiments, we coated the cell-viewing chamber of this device with FN (15 μg/cm2) for 1 h at 37°C and then rinsed multiple times with PBS. We then seeded genetically selected, mCherry+ hESC-CMs into the viewing chamber and allowed the cells to adhere overnight in control-CM. The next day, we loaded fresh control-CM and Wnt5a-CM into reservoirs located on either side of the cell-viewing chamber. Live-cell imaging was then performed as detailed above, allowing 12 h for the Wnt5a gradient to form and then tracking cardiomyocyte migration over the following 48 h.
Reverse transcriptase polymerase chain reaction (RT-PCR) assays
Genetically selected hESC-CMs were cultured on FN for 72 h and then were lysed for the isolation of total RNA (Qiagen RNeasy kit). mRNA was reverse-transcribed using the Superscript III first-strand cDNA synthesis kit and oligo(dT) primers (Invitrogen). PCR was performed for 30 cycles using previously validated primer sequences (see Supplementary Tables S1 and S2).
Statistics
Statistical analyses were performed using GraphPad Prism (La Jolla, CA) with the threshold for significance set at level P<0.05. Values are expressed as mean±standard error. To compare migration speed of hESC-CMs on the four different coating densities of each ECM protein, we used the Kruskal–Wallis method followed by the Dunn's post hoc for all pairwise comparisons. ECM transwell experiments were analyzed by one-way ANOVA, followed by Tukey's post hoc for all pairwise comparisons, and then Bonferroni-corrected. The initial transwell screen of soluble factors was analyzed using a one-way ANOVA followed by a Dunnett's post hoc comparing all experimental samples to control. For all other comparisons, a two-sample t-test was used.
Results
hESC-CMs migrate spontaneously on 2D surfaces
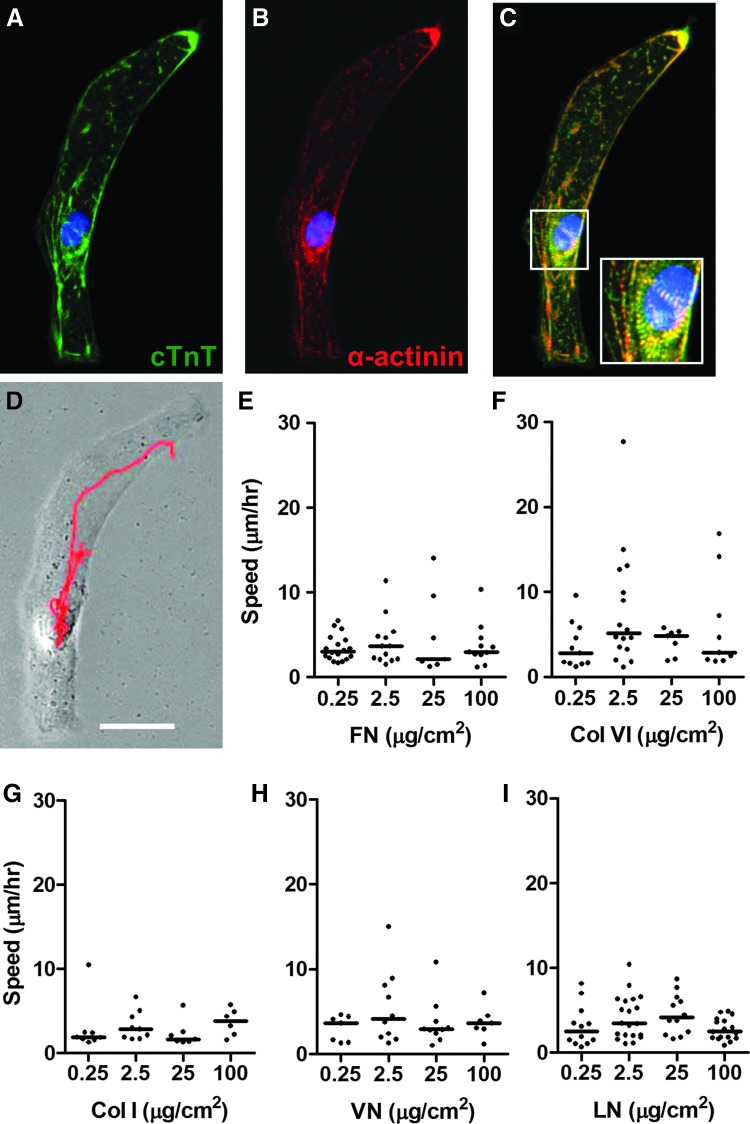
To demonstrate the migratory behavior of hESC-CMs in the absence of any exogenously applied cues, we used live-cell microscopy to image genetically selected, cTnT/α-actinin expressing hESC-CMs after 25 days of in vitro differentiation. For this, the cells were seeded at a low density into a glass-bottom dish coated with 0.1% PEI followed by FN at 2.5 μg/cm2, allowed to adhere for 16 h, and then monitored continuously for 24 h (Fig. 1A–D). Under these conditions, a majority of individual hESC-CMs exhibited random migration, although their migration speed varied considerably. Of note, this migration occurred despite the fact that the hESC-CMs maintained spontaneous contractile activity throughout (see Supplementary Online Video S2 for representative behavior). Having made the initial observation that hESC-CMs are motile, we sought to find an ECM molecule and optimal coating density that would induce the fastest rate of migration. For this, we again used live-cell microscopy to track the migration of hESC-CMs cultured on 2D surfaces coated with varying concentrations of FN, Col VI, Col I, VN, and LN. While there were some exceptionally fast cells on Col VI, hESC-CMs under most conditions migrated at a range of 2.5–10 μm/h with no significant difference in migration between the lower and higher concentrations of the ECM (Fig. 1E–I).
FIG. 1.
Human embryonic stem cell-derived cardiomyocytes (hESC-CMs) migrate spontaneously on two-dimensional (2D) surfaces. (A–C) Immunofluorescent images of the genetically selected, rhythmically contracting hESC-CMs also shown in Supplementary Video S2. Note that this cell expressed the cardiac markers cardiac troponin T (cTnT) (A, green) and α-actinin (B, red). The red trace in (D) represents the 24-h migration path of the centroid of this cell on fibronectin (FN) at 2.5 μg/cm2. Scale bar=50 μm. (E–I) Scatter plots showing the migration speed of individual hESC-CMs cultured on FN, collagen (Col) VI, Col I, vitronectin (VN), and laminin (LN) at the specified coating densities. Bar indicates median. N=7–20 cells per condition. Color images available online at www.liebertpub.com/scd
hESC-CMs exhibit a haptotactic response to FN and other ECM molecules
After confirming that hESC-CMs show random migration when cultured on surfaces coated with a uniform concentration of each ECM protein, we examined their behavior when exposed to concentration gradients of these molecules. For this, we employed the checkerboard transwell assay after Zigmond [29], coating the membranes with symmetric or asymmetric concentrations of FN, Col VI, Col I, VN, or LN. hESC-CMs were dispersed to single cells, seeded onto these transwells, and allowed to migrate for 16 h before fixation. We then quantified the absolute number of cTnT + cardiomyocytes on the bottom of each membrane and expressed migration as fold over control (symmetrically coated FN).
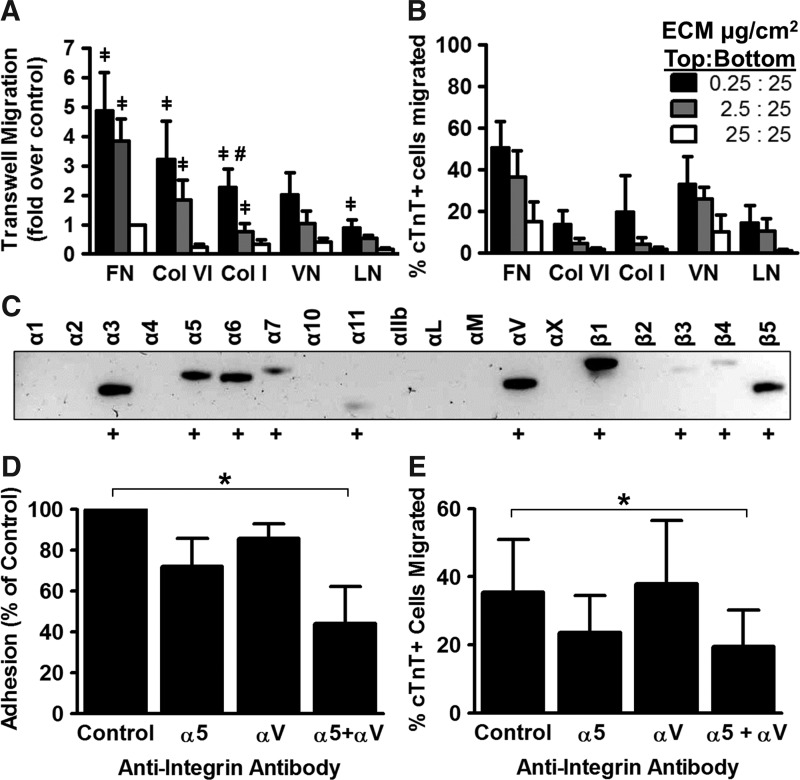
For all of the ECM molecules tested, the number of cells that migrated toward the higher concentration increased with the steepness of the ECM gradient across the membrane. The largest number of migrated cells was found when membranes were coated with FN at a 100-fold concentration difference from top to bottom (0.25 μg/cm2: 25 μg/cm2) with cells under these conditions showing nearly fivefold greater migration than cells on a symmetrically coated membrane (25 μg/cm2: 25 μg/cm2) (Fig. 2A). Moreover, cells on membranes with the 100-fold FN gradient exhibited nearly fivefold greater migration than those on membranes equivalently coated with LN. To correct for variation in the seeding efficiency between different ECM molecules and coating densities, we also calculated the percentage of the total number of cTnT+ hESC-CMs adherent to either side of the membrane that had migrated from top to bottom. This analysis yielded qualitatively similar results, with a trend toward increased migration with a steeper gradient of the ECM protein (Fig. 2B).
FIG. 2.
hESC-CMs exhibit a haptotactic response to FN. (A) We seeded hESC-CMs onto transwells coated on the top and bottom with varying ECM protein densities and then allowed the cells to migrate for 16 h. The absolute number of cTnT + cardiomyocytes detected on the bottom of each membrane was normalized to the symmetrically-coated FN control. 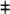 P<0.01 versus 25:25 μg/cm2, #P<0.01 versus 2.5:25 μg/cm2. N=4. (B) Same experimental samples as in (A), but with migration to the bottom here expressed as a percentage of the total number of cTnT+ cells attached to both sides of the membrane. (C) Integrin expression profile of genetically selected hESC-CMs by reverse transcriptase-polymerase chain reaction (RT-PCR). (D, E) hESC-CM adhesion (D) and migration (E) on FN-coated transwells are dependent on integrins α5 and αV. Neutralizing antibodies at 5 μg/mL. N=4, *P<0.05.
P<0.01 versus 25:25 μg/cm2, #P<0.01 versus 2.5:25 μg/cm2. N=4. (B) Same experimental samples as in (A), but with migration to the bottom here expressed as a percentage of the total number of cTnT+ cells attached to both sides of the membrane. (C) Integrin expression profile of genetically selected hESC-CMs by reverse transcriptase-polymerase chain reaction (RT-PCR). (D, E) hESC-CM adhesion (D) and migration (E) on FN-coated transwells are dependent on integrins α5 and αV. Neutralizing antibodies at 5 μg/mL. N=4, *P<0.05.
Adhesion and migration on FN are integrin α5 and αV dependent
We next wanted to determine the expression profile of integrins that might be involved in the migratory response to FN. Using RT-PCR on RNA isolated from genetically selected hESC-CMs, we found that hESC-CMs express α-integrins 3, 5–7, 11, and V and β-integrins 1, 3, 4, and 5 (Fig 2C, Supplementary Table S1). Noting that hESC-CMs express two of the known FN receptors, integrins α5 and αV, we sought to determine which receptor was required for hESC-CM migration on FN by quantifying adhesion and migration of hESC-CMs in the presence of neutralizing antibodies directed against integrins α5, αV, or both. We found that while neither antibody alone had a statistically significant effect on these parameters, simultaneous incubation with both anti-α5 and anti-αV antibodies resulted in a 55.8%±18.0% reduction in adhesion (Fig. 2D) and a 1.8-fold decrease in migration by the transwell assay (Fig. 2E).
hESC-CM migration on FN is dependent on the gradient slope
To better define the haptotactic response of hESC-CMs to FN, we performed live-cell imaging of genetically selected mCherry+ hESC-CMs cultured on surface-bound FN gradients of varying steepness. To create the surface-bound FN gradients, we applied agarose diffusive printing techniques [27] and showed that the gradient slope could be reproducibly varied by controlling the length of time that Oregon 488-conjugated FN was allowed to diffuse through the agarose gel (Supplementary Fig. S2, Fig. 3A, B). We then compared the behavior of mCherry+ hESC-CMs seeded onto steep versus shallow FN gradients (generated using 20 vs. 60 min of diffusion time, Fig. 3C). In all cases, the cells showed biased migration toward higher FN concentrations, but those exposed to a steeper FN gradient moved nearly a threefold greater distance in that direction than their counterparts on shallower gradients (40.3±6.3 μm vs. 15.6±2.8 μm in 24 h, P<0.01) (Fig. 3D–G), while there was no significant difference in the net movement perpendicular to the gradients.
To look for synergistic effects by ECM molecules on FN-mediated haptotaxis, we used live-cell microscopy to track hESC-CM migration on steep FN gradients to which we had added uniform Col VI at either 0.25 or 2.5 μg/cm2. We found that the cells on the FN gradient alone tended to migrate further than those on the gradient + Col VI, (40.9±12.3 vs. 21.3±6.2 and 25.8±6.4 μm in 24 h, respectively), although these differences did not reach statistical significance (Supplementary Fig. S3A–C). Given that there was no slowing of migration when the two ECMs were presented uniformly (Fig. S3D), a direct antagonistic effect seems unlikely to account for these findings.
Wnt5a is a promigratory factor for hESC-CMs
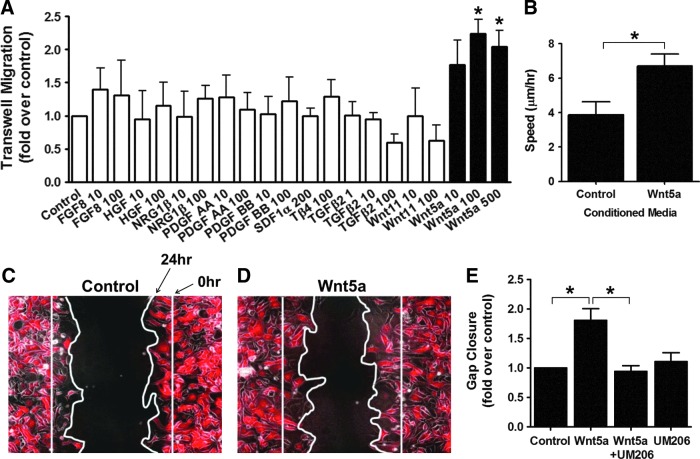
After determining the migratory responses of hESC-CMs to FN and other ECM proteins, we focused on the identification of soluble factors that might promote hESC-CM migration. For this, we initially used the high-throughput transwell assay to screen candidate signaling molecules that had been previously implicated in either heart morphogenesis or the migration of skeletal myoblasts or adult cardiac progenitors (see Supplementary Fig. S4A for specifics regarding the timing of growth factor treatment). Interestingly, among the 10 soluble factors tested, only Wnt5a mediated a statistically robust effect on hESC-CM migration. Indeed, when recombinant Wnt5a (100 and 500 ng/mL) was placed in the bottom chamber of the transwell, hESC-CM migration across the membrane doubled relative to control conditions (Fig. 4A).
FIG. 4.
Wnt5a is a promigratory factor for hESC-CMs. (A) We screened 10 soluble factors for effects on hESC-CM migration in the transwell assay. The only factor showing a significant promigratory effect was Wnt5a, which induced an approximately twofold increase in migration at concentrations of 100 and 500 ng/mL. N=4, *P<0.05. (B) During live-cell microscopy of hESC-CMs on unpatterned FN, Wnt5a accelerated random migration by approximately twofold. N=20, *P<0.05. (C, D) Representative images of hESC-CMs in the gap-closure assay after 24-h exposure to the control-CM (C) or Wnt5a-CM (D). (E) Wnt5a-treated cells migrated to cover an approximately twofold more surface area than control cells, a response that was inhibited by UM206 (100 nM). *P<0.05, N=4. Color images available online at www.liebertpub.com/scd
To further confirm the promigratory effects of Wnt5a on hESC-CMs, we used two independent migration assays to compare the medium conditioned by Wnt5a overexpressing (Wnt5a-CM) or wild-type L-cells (control-CM) [20]. First, we tracked the movement of genetically selected hESC-CMs by live-cell microscopy and found that myocytes treated with Wnt5a-CM had approximately twofold greater random migration than their counterparts in the control-CM (Fig. 4B). Second, to test for the effects on multicellular preparations of hESC-CMs, we used the gap-closure assay and found that migration into the gap was enhanced approximately twofold for cultures treated with Wnt5-CM versus control-CM (Fig. 4 C–E). Importantly, this effect was not accounted for by changes in cell size, retention, or proliferation (Supplementary Fig. S4B–D).
We also performed experiments to screen for synergistic effects or signaling interactions between Wnt5a and FN in our system. Although Wnt5a has been reported to alter the expression of integrins and FN in other cell types [30,31], we did not find any changes in expression of FN or its receptors (Integrins α5, αV, β1, and β3) in Wnt5a-stimulated hESC-CMs by quantitative RT-PCR (Supplementary Fig. S4E). To see if Wnt5a would augment the haptotactic response to FN, we again generated steep surface-bound gradients and tracked hESC-CM migration on these gradients in the presence and absence of Wnt5a (100 and 500 ng/μL). We detected no difference in migration relative to the FN gradient during 24 h of videomicroscopy between control and Wnt-stimulated cells (Supplementary Fig. S5).
Wnt5a promotes hESC-CM migration through a frizzled-dependent pathway
By RT-PCR, we found that hESC-CMs express multiple receptors and coreceptors for Wnts, including Frizzled (Fzd) proteins 1–4, Fzd 6–9, LRP5, LRP6, Ror2, and Ryk (Supplementary Fig. S4F, Supplementary Table S2). Based on insights from cardiac development [32] and migration studies in other cell types [33–35], we hypothesized that the promigratory effects of Wnt5a on hESC-CMs are mediated by Fzd2. To test this hypothesis, we performed the gap-closure assay in the presence of the Fzd1/2 peptide antagonist, UM206 (100 nM) [36], and found that it completely inhibited the effects of Wnt5a stimulation (Fig. 4E).
Wnt5a is chemotactic for hESC-CMs
The preceding data demonstrate that Wnt5a exerts a chemokinetic effect on hESC-CMs. To determine whether Wnt5a also acts as a chemoattractive factor, we first employed the checkerboard transwell assay, in which Wnt5a- or control-CM was variably placed on the top or bottom of the membrane. Consistent with a chemotactic effect, we found that the placement of Wnt5a-CM in the lower chamber of the transwell resulted in a 2.2-fold increase in hESC-CM migration to the bottom of the membrane, while its location in the upper chamber or symmetric Wnt5a-CM on the top and bottom had no effect (Fig 5A). As before, this effect of Wnt5a-CM was completely inhibited in the presence of UM206.
FIG. 5.
Wnt5a is chemotactic for hESC-CMs. (A) Checkerboard assay in which Wnt5a-CM was placed on either the top or bottom of the membrane in the presence or absence of UM206 (100 nM). Wnt5a promoted hESC-CM migration only when placed in the bottom chamber, indicating a chemotactic effect. This response was inhibited by UM206. N=4 *P<0.05, **P<0.01. (B) Representative migration paths for hESC-CMs under control conditions (black) and during exposure to a Wnt5a gradient (red). Displacement vectors of hESC-CMs in the control CM (C) and Wnt5a CM (D) gradients. N=21–24 cells per condition. Color images available online at www.liebertpub.com/scd
To more directly confirm the chemotactic activity of Wnt5a, we used a commercially available microfluidic-based device to expose hESC-CMs to a gradient of Wnt5a-CM during live-cell imaging for 48 h. Interestingly, hESC-CMs exposed to a Wnt5a gradient moved 5.7 times farther in the direction parallel to the gradient (ie, toward higher Wnt5a) than did cells under control conditions (42.0±7.8 μm vs. 7.4±9.4 μm, P<0.01, N=20–23). The two conditions showed no difference in the net movement perpendicular to the gradient (see Fig. 5B for representative paths of hESC-CMs with and without exposure to a Wnt5a gradient, and Fig. 5C & D for the corresponding displacement vectors).
Discussion
While other investigators have described the migration of both immature cardiomyocytes in the developing heart [2–4,16,37] and putative cardiac progenitors in the postnatal heart [6,7], similar motility has not been previously reported in cardiomyocytes derived from pluripotent stem cells. Here we used highly purified populations of hESC-CMs and found that these cells can migrate at speeds in the range of 5–10 μm/h. While much slower than highly migratory cell types (eg, neutrophils) [38], such values are comparable to those previously reported for Chinese hamster ovary cells, a commonly used cell type in migration studies and one that obviously lacks sarcomeric machinery [39]. Indeed, our finding that hESC-CMs migrate while simultaneously exhibiting rhythmic contraction raises interesting questions as to how cytoskeletal reorganization and myofilament cross-bridge cycling remain compartmentalized. This same issue has been raised with regard to beating cardiomyocytes undergoing mitosis [40].
In the present study, we focused on identifying signaling pathways that might regulate hESC-CM migration and identified two promigratory factors: FN, an ECM glycoprotein, and noncanonical Wnt5a. We showed by transwell and live-cell microscopy that the haptotactic response to FN can be enhanced by increasing the slope of the surface-bound gradient and that migration and adhesion on FN involve both integrins α5 and αV. Both of these integrins were previously shown to be expressed by hESC-CMs in vitro [41]. Wnt5a was the only soluble factor in our screens that promoted hESC-CM migration, and it appears to exert both chemokinetic and chemoattractant effects. Interestingly, both the chemokinetic and chemotactic responses to Wnt5a can be blocked by UM206, suggesting that they are dependent on Fzd1 and/or 2.
Our findings with hESC-CMs cultured on various ECM substrates are also pertinent to cardiac development. In the developing chick heart, FN is expressed in an anterior-to-posterior gradient, which has been implicated in the directed migration of precardiac mesodermal cells anteriorly and to the midline [16]. Subsequent studies in the mouse [42] and zebrafish [1] have confirmed the importance of FN in the formation of the primary heart tube, but its precise role in guiding cellular migration in these models remains unclear. Although we are unaware of any direct evidence of a FN gradient during closure of the human heart tube, our data suggest that this molecule could act as an effective guidance cue for early human cardiomyocytes.
It is also worth noting that we observed the fastest migration speeds (up to 35 μm/h) with hESC-CMs cultured on Col VI. We included this ECM molecule in our screens, because its gene lies on chromosome 21, and it has been suggested by some as a potential culprit in the congenital heart defects associated with Down syndrome [14,43,44]. Col VI has been reported to be overexpressed in the atrioventricular region in trisomy 21 [14], and it has been speculated that this results in aberrant cell migration and cardiac malformation [14,45]. In future work, it would be of interest to revisit selected experiments from this study using induced pluripotent stem cells from normal subjects and patients with congenital heart disease. By comparing the migratory responses of wild-type and diseased cardiomyocytes (or wild-type cardiomyocytes to the ECM from diseased fibroblasts), we can explore the hypothesis that perturbed cardiomyocyte migration contributes to some instances of structural heart disease [3,46].
Our finding that Wnt5a is chemoattractive for hESC-CMs is compatible with prior studies of normal and abnormal cardiac development in model organisms. Wnt5a is known to be expressed in the cushion mesenchyme at the time of myocardialization [17,46,47], which places this signal at a suitable location to guide the migration of cardiomyocytes into the adjacent cushion to muscularize the outflow track septum. Transgenic mice lacking Wnt5a [17] or the Wnt receptors Fzd1 or Fzd2 [32] all show defective outflow tract septation. Interestingly, the loss of other components of the noncanonical Wnt signaling pathway, including Ror2, Vangl2, and Dishevelleds 2 or 3, also results in septal defects [18,46,48]. When taken collectively with our own findings using human cardiomyocytes, these studies raise the possibility that aberrant Wnt5a signaling and defective cardiomyocyte migration could contribute to dysmorphogenesis in humans. Interestingly, while the best evidence for Wnt5a-induced cardiomyocyte migration in nonhuman models comes from studies of outflow tract development [17,18,20,32,46–49], nearly all hESC-CMs showed Wnt5a responsiveness in our live cell-imaging studies. Our hESC-CM preparations have been previously shown to include multiple cardiac subtypes [25], implying that Wnt5a-mediated migration is not limited to a minor subset of hESC-CMs.
In addition to being a model for human heart development, hESC-CMs have attracted significant interest as a potential cell source for remuscularizing infarcted heart tissue. In recent work, our laboratory found that hESC-CMs can couple with the host myocardium after transplantation into the infarcted myocardium, but many hESC-CM implants remain electrically isolated by intervening scar tissue [8]. The present study suggests a number of novel strategies for improving their electromechanical integration. For example, by providing a suitable chemokinetic signal (eg, Wnt5a), one might stimulate the random migration of hESC-CMs in vivo and increase the likelihood of the host–graft or graft–graft contact. Support for this possibility comes from our experiments in the gap-closure assay, in which Wnt5a-stimulated hESC-CMs covered an ∼250-μm distance within a 24-h period. It might also be possible to harness the chemoattractant properties of Wnt5a by injecting Wnt5a-eluting controlled release microspheres [50,51] into the infarct border zone (perhaps with guidance by electroanatomic mapping). If this intervention resulted in an appropriately inductive Wnt5a gradient, hESC-CM grafts isolated in scar tissue might be drawn toward viable host muscle and/or better-coupled graft implants in the border zone. Migration might be also facilitated by the delivery of hESC-CMs within FN- or Col VI-based hydrogels [52,53].
Of course, any of the preceding strategies would have to take into account the biological activity and the patterns of endogenous expression of the factors employed. For example, endogenous Wnt5a has been implicated in myofibroblast differentiation and migration during early infarct repair [36]. FN and Col VI expression is known to be upregulated in the early postinfarct heart, where they are thought to help facilitate inflammation and ventricular remodeling [54,55]. FN signaling may also promote undesirable effects such as cardiac fibrosis [56] or cardiomyocyte hypertrophy [57]. We may be able to avoid such off-target effects by limiting delivery of the cells and the exogenous factor to the already-established infarct scar or by using appropriate synthetic biomaterials (eg, hydrogels with incorporated FN functional domains [52]).
Our findings in the present study are well supported by prior work, suggesting that FN and Wnt5a act as guidance cues for early cardiomyocytes in model organisms. However, because we took a candidate molecule approach in this study, we cannot rule out the possibility that other factors (or combinations of factors) might also promote hESC-CM migration. Moreover, while we deliberately focused here on hESC-CMs at a stage of maturation comparable to that used in our prior in vitro and transplantation studies [8,25], we have also observed cardiomyocyte motility in cultures after 100 days of in vitro maturation (data not shown). While beyond the scope of the present work, it would be of obvious interest to investigate in the future how motility is affected by parameters, including their stage of maturation and electrophysiological phenotype [25,58,59].
Supplementary Material
Acknowledgments
We are grateful to Dr. Ron Seifert, Scott Lundy, and Ben Van Biber for their technical assistance. This work was supported by the U.S. National Institutes of Health grants R01-HL064387, P01-HL094374, and U01-HL099997 (to MAL).
Author Disclosure Statement
MAL is a founder, consultant, and equity holder in BEAT Biotherapeutics.
References
- 1.Trinh LA. Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 2.van den Hoff MJ. Moorman AF. Ruijter JM. Lamers WH. Bennington RW. Markwald RR. Wessels A. Myocardialization of the cardiac outflow tract. Dev Biol. 1999;212:477–490. doi: 10.1006/dbio.1999.9366. [DOI] [PubMed] [Google Scholar]
- 3.Hakim ZS. DiMichele LA. Doherty JT. Homeister JW. Beggs HE. Reichardt LF. Schwartz RJ. Brackhan J. Smithies O. Mack CP. Taylor JM. Conditional deletion of focal adhesion kinase leads to defects in ventricular septation and outflow tract alignment. Mol Cell Biol. 2007;27:5352–5364. doi: 10.1128/MCB.00068-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J. Bressan M. Hassel D. Huisken J. Staudt D. Kikuchi K. Poss KD. Mikawa T. Stainier DY. A dual role for ErbB2 signaling in cardiac trabeculation. Development. 2010;137:3867–3875. doi: 10.1242/dev.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corti S. Salani S. Del Bo R. Sironi M. Strazzer S. D'Angelo MG. Comi GP. Bresolin N. Scarlato G. Chemotactic factors enhance myogenic cell migration across an endothelial monolayer. Exp Cell Res. 2001;268:36–44. doi: 10.1006/excr.2001.5267. [DOI] [PubMed] [Google Scholar]
- 6.Zakharova L. Mastroeni D. Mutlu N. Molina M. Goldman S. Diethrich E. Gaballa MA. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87:40–49. doi: 10.1093/cvr/cvq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bock-Marquette I. Saxena A. White MD. Dimaio JM. Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 8.Shiba Y. Fernandes S. Zhu WZ. Filice D. Muskheli V. Kim J. Palpant NJ. Gantz J. Moyes KW, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy II. Railo A. Rapila R. Hast T. Sormunen R. Tavi P. Rasanen J. Vainio SJ. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc Res. 2010;85:100–109. doi: 10.1093/cvr/cvp254. [DOI] [PubMed] [Google Scholar]
- 10.Zhou W. Lin L. Majumdar A. Li X. Zhang X. Liu W. Etheridge L. Shi Y. Martin J, et al. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartram U. Molin DG. Wisse LJ. Mohamad A. Sanford LP. Doetschman T. Speer CP. Poelmann RE. Gittenberger-de Groot AC. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- 12.Bouchey D. Argraves WS. Little CD. Fibulin-1, vitronectin, and fibronectin expression during avian cardiac valve and septa development. Anat Rec. 1996;244:540–551. doi: 10.1002/(SICI)1097-0185(199604)244:4<540::AID-AR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Hilenski LL. Terracio L. Borg TK. Myofibrillar and cytoskeletal assembly in neonatal rat cardiac myocytes cultured on laminin and collagen. Cell Tissue Res. 1991;264:577–587. doi: 10.1007/BF00319047. [DOI] [PubMed] [Google Scholar]
- 14.Gittenberger-de Groot AC. Bartram U. Oosthoek PW. Bartelings MM. Hogers B. Poelmann RE. Jongewaard IN. Klewer SE. Collagen type VI expression during cardiac development and in human fetuses with trisomy 21. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1109–1116. doi: 10.1002/ar.a.10126. [DOI] [PubMed] [Google Scholar]
- 15.Klewer SE. Krob SL. Kolker SJ. Kitten GT. Expression of type VI collagen in the developing mouse heart. Dev Dyn. 1998;211:248–255. doi: 10.1002/(SICI)1097-0177(199803)211:3<248::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Linask KK. Lash JW. Precardiac cell migration: fibronectin localization at mesoderm-endoderm interface during directional movement. Dev Biol. 1986;114:87–101. doi: 10.1016/0012-1606(86)90385-4. [DOI] [PubMed] [Google Scholar]
- 17.Schleiffarth JR. Person AD. Martinsen BJ. Sukovich DJ. Neumann A. Baker CV. Lohr JL. Cornfield DN. Ekker SC. Petryk A. Wnt5a is required for cardiac outflow tract septation in mice. Pediatr Res. 2007;61:386–391. doi: 10.1203/pdr.0b013e3180323810. [DOI] [PubMed] [Google Scholar]
- 18.Oishi I. Suzuki H. Onishi N. Takada R. Kani S. Ohkawara B. Koshida I. Suzuki K. Yamada G. Schwabe GC. Mundlos S. Shibuya H. Takada S. Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 19.Hutson MR. Zhang P. Stadt HA. Sato AK. Li YX. Burch J. Creazzo TL. Kirby ML. Cardiac arterial pole alignment is sensitive to FGF8 signaling in the pharynx. Dev Biol. 2006;295:486–497. doi: 10.1016/j.ydbio.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 20.Chen W. ten Berge D. Brown J. Ahn S. Hu LA. Miller WE. Caron MG. Barak LS. Nusse R. Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 21.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 22.Xu C. Inokuma MS. Denham J. Golds K. Kundu P. Gold JD. Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 23.Laflamme MA. Chen KY. Naumova AV. Muskheli V. Fugate JA. Dupras SK. Reinecke H. Xu C. Hassanipour M, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 24.Xu C. Police S. Hassanipour M. Li Y. Chen Y. Priest C. O'Sullivan C. Laflamme MA. Zhu WZ, et al. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen Med. 2011;6:53–66. doi: 10.2217/rme.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu WZ. Xie Y. Moyes KW. Gold JD. Askari B. Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–786. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salva MZ. Himeda CL. Tai PW. Nishiuchi E. Gregorevic P. Allen JM. Finn EE. Nguyen QG. Blankinship MJ, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- 27.Mai J. Fok L. Gao H. Zhang X. Poo MM. Axon initiation and growth cone turning on bound protein gradients. J Neurosci. 2009;29:7450–7458. doi: 10.1523/JNEUROSCI.1121-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zantl R. Horn E. Chemotaxis of slow migrating mammalian cells analysed by video microscopy. Methods Mol Biol. 2011;769:191–203. doi: 10.1007/978-1-61779-207-6_13. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond SH. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977;75:606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olivares-Navarrete R. Hyzy SL. Hutton DL. Dunn GR. Appert C. Boyan BD. Schwartz Z. Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta Biomater. 2011;7:2740–2750. doi: 10.1016/j.actbio.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuga LJ. Ben-Yehudah A. Kovkarova-Naumovski E. Oriss T. Gibson KF. Feghali-Bostwick C. Kaminski N. WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am J Respir Cell Mol Biol. 2009;41:583–589. doi: 10.1165/rcmb.2008-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H. Smallwood PM. Wang Y. Vidaltamayo R. Reed R. Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development. 2010;137:3707–3717. doi: 10.1242/dev.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto S. Fumoto K. Okamoto T. Kaibuchi K. Kikuchi A. Binding of APC and dishevelled mediates Wnt5a-regulated focal adhesion dynamics in migrating cells. EMBO J. 2010;29:1192–1204. doi: 10.1038/emboj.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishita M. Enomoto M. Yamagata K. Minami Y. Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol. 2010;20:346–354. doi: 10.1016/j.tcb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto H. Oue N. Sato A. Hasegawa Y. Matsubara A. Yasui W. Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–2046. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 36.Laeremans H. Hackeng TM. van Zandvoort MA. Thijssen VL. Janssen BJ. Ottenheijm HC. Smits JF. Blankesteijn WM. Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation. 2011;124:1626–1635. doi: 10.1161/CIRCULATIONAHA.110.976969. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi JK. Lou X. Alexander JM. Sugizaki H. Delgado-Olguin P. Holloway AK. Mori AD. Wylie JN. Munson C, et al. Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat Commun. 2011;2:187. doi: 10.1038/ncomms1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandeville JT. Ghosh RN. Maxfield FR. Intracellular calcium levels correlate with speed and persistent forward motion in migrating neutrophils. Biophys J. 1995;68:1207–1217. doi: 10.1016/S0006-3495(95)80336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhoads DS. Guan JL. Analysis of directional cell migration on defined FN gradients: role of intracellular signaling molecules. Exp Cell Res. 2007;313:3859–3867. doi: 10.1016/j.yexcr.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahuja P. Perriard E. Perriard JC. Ehler E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J Cell Sci. 2004;117:3295–3306. doi: 10.1242/jcs.01159. [DOI] [PubMed] [Google Scholar]
- 41.van Laake LW. van Donselaar EG. Monshouwer-Kloots J. Schreurs C. Passier R. Humbel BM. Doevendans PA. Sonnenberg A. Verkleij AJ. Mummery CL. Extracellular matrix formation after transplantation of human embryonic stem cell-derived cardiomyocytes. Cell Mol Life Sci. 2010;67:277–290. doi: 10.1007/s00018-009-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George EL. Baldwin HS. Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- 43.Davies GE. Howard CM. Farrer MJ. Coleman MM. Bennett LB. Cullen LM. Wyse RKH. Burn J. Williamson R. Kessling AM. Genetic-variation in the Col6a1 region is associated with congenital heart-defects in trisomy-21 (Downs-Syndrome) Ann Hum Genet. 1995;59:253–269. doi: 10.1111/j.1469-1809.1995.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 44.Davies GE. Howard CM. Farrer MJ. Coleman MM. Cullen LM. Williamson R. Wyse RK. Kessling AM. Unusual genotypes in the COL6A1 gene in parents of children with trisomy 21 and major congenital heart defects. Hum Genet. 1994;93:443–446. doi: 10.1007/BF00201672. [DOI] [PubMed] [Google Scholar]
- 45.Delom F. Burt E. Hoischen A. Veltman J. Groet J. Cotter FE. Nizetic D. Transchromosomic cell model of Down syndrome shows aberrant migration, adhesion and proteome response to extracellular matrix. Proteome Sci. 2009;7:31. doi: 10.1186/1477-5956-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips HM. Murdoch JN. Chaudhry B. Copp AJ. Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 47.van den Hoff MJ. Moorman AF. Wnt, a driver of myocardialization? Circ Res. 2005;96:274–276. doi: 10.1161/01.RES.0000157574.65952.b8. [DOI] [PubMed] [Google Scholar]
- 48.Gao C. Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 49.Sinha T. Wang B. Evans S. Wynshaw-Boris A. Wang J. Disheveled mediated planar cell polarity signaling is required in the second heart field lineage for outflow tract morphogenesis. Dev Biol. 2012;370:135–144. doi: 10.1016/j.ydbio.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim JJ. Hammoudi TM. Bratt-Leal AM. Hamilton SK. Kepple KL. Bloodworth NC. McDevitt TC. Temenoff JS. Development of nano- and microscale chondroitin sulfate particles for controlled growth factor delivery. Acta Biomater. 2011;7:986–995. doi: 10.1016/j.actbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinney MA. McDevitt TC. Emerging strategies for spatiotemporal control of stem cell fate and morphogenesis. Trends Biotechnol. 2013;31:78–84. doi: 10.1016/j.tibtech.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosh K. Ren XD. Shu XZ. Prestwich GD. Clark RA. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12:601–613. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 53.Seidlits SK. Drinnan CT. Petersen RR. Shear JB. Suggs LJ. Schmidt CE. Fibronectin-hyaluronic acid composite hydrogels for three-dimensional endothelial cell culture. Acta Biomater. 2011;7:2401–2409. doi: 10.1016/j.actbio.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 54.Dobaczewski M. Gonzalez-Quesada C. Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naugle JE. Olson ER. Zhang X. Mase SE. Pilati CF. Maron MB. Folkesson HG. Horne WI. Doane KJ. Meszaros JG. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;290:H323–H330. doi: 10.1152/ajpheart.00321.2005. [DOI] [PubMed] [Google Scholar]
- 56.Bujak M. Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen H. Huang XN. Stewart AF. Sepulveda JL. Gene expression changes associated with fibronectin-induced cardiac myocyte hypertrophy. Physiol Genomics. 2004;18:273–283. doi: 10.1152/physiolgenomics.00104.2004. [DOI] [PubMed] [Google Scholar]
- 58.He JQ. Ma Y. Lee Y. Thomson JA. Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 59.Mummery C. Ward-van Oostwaard D. Doevendans P. Spijker R. van den Brink S. Hassink R. van der Heyden M. Opthof T. Pera M, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 60.Howlett A. Humana Press; Totowa, NJ: 1999. Integrin Protocols. [Google Scholar]
- 61.Okoye UC. Malbon CC. Wang HY. Wnt and Frizzled RNA expression in human mesenchymal and embryonic (H7) stem cells. J Mol Signal. 2008;3:16. doi: 10.1186/1750-2187-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.