Abstract
Studies over 5 decades have examined ABO blood groups and risk of pancreatic cancer in Western, Asian, and other populations, though no systematic review has been published. We studied data from 908 pancreatic cancer cases and 1,067 population controls collected during December 2006–January 2011 in urban Shanghai, China, and reviewed the literature for all studies of this association. Random-effects meta-analysis provided summary odds ratio estimates according to blood group and by populations endemic versus nonendemic for cytotoxin-associated gene A (CagA)-positive Helicobacter pylori. In our Shanghai study, versus group O, only ABO group A was associated with risk (odds ratio (OR) = 1.60, 95% confidence interval (CI): 1.27, 2.03). In 24 pooled studies, group A showed increased risk in both CagA-nonendemic and -endemic populations (ORpooled = 1.40, 95% CI: 1.32, 1.49). In nonendemic populations, groups B and AB were also associated with higher risk (OR = 1.38, 95% CI: 1.16, 1.64; and OR = 1.52, 95% CI: 1.24, 1.85, respectively). However, in CagA-endemic populations, groups B and AB were not associated with risk (OR = 1.05, 95% CI: 0.92, 1.19; and OR = 1.13, 95% CI: 0.92, 1.38, respectively). These population differences were significant. One explanation for contrasts in associations of blood groups B and AB between CagA-endemic and -nonendemic populations could involve gastric epithelial expression of A versus B antigens on colonization behaviors of CagA-positive and CagA-negative H. pylori strains.
Keywords: ABO blood group system, Asia, case-control studies, meta-analysis, pancreatic neoplasms
Pancreatic cancer is one of the most aggressive cancers. Incidence of the disease has increased dramatically in China over the last 30 years. In urban Shanghai, the average annual age-adjusted incidence rate (per 100,000 people) rose from 3.7 in men and 3.2 in women in 1973 to 11.2 in men and 10.9 in women in 2000 (1). Most patients are diagnosed at late stages, and the prognosis of pancreatic cancer is dismal, with median survival of less than 6 months and overall 5-year survival of less than 5% (2). Cigarette smoking and family history of cancer are 2 known risk factors but do not explain much of the disease's etiology (3, 4).
An association between ABO blood group and risk of pancreatic cancer was considered more than 50 years ago in the context of explorations between blood groups and various malignant diseases (5). After publication of a few reports, interest largely waned until the PanScan genome-wide association study identified the single nucleotide polymorphism (SNP) rs505922, which is highly linked to ABO groups O versus non-O, as its top hit (6). Studies of pancreatic cancer and ABO groups have been fairly consistent in identifying blood group A in association with increased risk, but variable as to whether blood groups B or AB are also associated with risk, particularly in Asian populations, where colonization by cytotoxin-associated gene A (CagA)-positive strains of Helicobacter pylori is common. We carried out a large population-based case-control study of pancreatic cancer in urban Shanghai, China, and sought to address ABO blood group associations with pancreatic cancer in CagA-endemic and -nonendemic populations through meta-analysis of results from the Shanghai study along with all available published studies.
MATERIALS AND METHODS
Study population
Between December 2006 and January 2011, subjects were enrolled in a population-based case-control study of pancreatic cancer in urban Shanghai, China. All participants were Shanghai residents aged 35–79 years. Potential cases were identified through an “instant case reporting system” of networked hospital staff in the 37 major Shanghai hospitals in which the majority of individuals with pancreatic cancer are diagnosed and obtain care. In total, 1,237 newly diagnosed patients with pancreatic cancer were reported to the Shanghai Cancer Institute over the 49-month enrollment period. Of these, 149 had died, were unable to be contacted, or refused to participate; the remaining 1,088 (88%) were recruited into the study. All relevant hospital records, pathology reports and slides, and imaging materials were collected for subsequent review of case eligibility by an expert panel of clinicians and pathologists organized specifically for this study. Among the 1,088 patients, 198 were excluded because of diagnoses of benign tumors or of nonpancreatic or nonadenocarcinoma primaries, leaving 890 confirmed cases of pancreatic cancer for analysis. During the 49 months of patient accrual, representative population controls were randomly selected from the files of the Shanghai Residents Registry according to categories of frequency matching by age group and sex. The Shanghai Residents Registry enumerates all permanent residents of Shanghai. Contact was attempted for 1,653 candidates. Among those selected, 94 were found to have been diagnosed with malignant diseases and 30 were deceased (both categories ineligible). An additional 462 refused to participate. The remaining 1,067 (70%) were recruited as controls. The study was approved by the institutional human subjects review boards of the Shanghai Cancer Institute and Yale University.
Assessment of ABO blood group
At interview, subjects provided signed informed consent, after which they were interviewed in person by trained study staff. Upon completion of the questionnaire interview, saliva samples were obtained from 84% of subjects with Oragene DNA collection kits (DNA Genotek, Inc., Kanata, Ontario, Canada) and venipuncture blood samples were obtained from 80% of subjects, yielding biospecimens for genetic analysis from a total of 847 cases and 975 controls.
Following interviews, blood samples (packed on ice) and Oragene saliva samples were immediately transported to the Shanghai Cancer Institute specimen processing laboratory, where they were promptly centrifuged, aliquotted into standard components, and frozen at −80°C. After the study received export approval by the Chinese National Office for the Management of Human Genetic Resources, aliquot samples were shipped via air courier to our laboratories at Yale University (New Haven, Connecticut). DNA was extracted from the saliva samples according to the manufacturer's directions and stored at −80°C. For analysis of samples when saliva specimens were unavailable or did not work after repeated genotyping attempts, DNA was extracted from buffy coat aliquots by standard methods with MagNA Pure Compact nucleic acid isolation kits (Roche Diagnostics Co., Indianapolis, Indiana).
The common ABO blood groups were determined by genotyping 2 known functional SNPs, rs8176719 and rs8176746, with custom TaqMan genotyping assays (Applied Biosystems, Inc., Foster City, California) following the manufacturer's instructions. The rs8176719 variant encodes the exon 6 261G deletion, which results in the common O allele, whereas rs8176746 encodes exon 7 C796A, which is 1 of the 7 standard ABO variants that distinguish A alleles from B alleles. Minor groove binder (MGB) probes and primers and assay conditions were as described elsewhere (7). Because rs8176746 is 7 nucleotide bases from a second variant, rs8176747 (G803C), the probes and primers for rs8176746 simultaneously determine the presence of both SNPs or neither SNP. These 2 SNPs are extremely highly linked, and discordant pairings create very rare ABO genotypes (8). Phased haplotypes of the rs8176719 and rs8176746 alleles were determined (Web Appendix 1, available at http://aje.oxfordjournals.org/). To validate the assays, we genotyped 30 buffy coat DNA samples (5 each of AA, AO, BB, BO, AB, and OO) from our Connecticut pancreas cancer case-control study (9) as described here and compared with their hemagglutination phenotype results (A, B, AB, and O), showing 100% concordance.
Meta-analysis
The Ovid Medline and PubMed databases were searched for articles published from database inception through September 30, 2012. Our search strategy included only the terms “ABO” or “AB0” and “cancer” or “carcinoma” or “adenocarcinoma” in any text field of the databases. This search identified 463 distinct publications that were individually examined for studies of human subjects in which frequencies or estimated relative risks of the ABO blood groups were reported for pancreatic cancer cases and controls. We reviewed the bibliographies of all relevant publications to identify possible additional studies for inclusion. Studies were considered regardless of publication language, location, or population. We generally followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist as it applies to reviews of observational studies (10).
We considered as eligible only articles with original study data. Data from secondary analyses or meta-analyses were not included. Where multiple papers described substantially the same subjects, we included only the largest or most informative results. For articles that provided adjusted odds ratio or relative risk estimates and 95% confidence intervals, we used those data as published. Various patient characteristics, such as tumor location or grade, subject sex, age at diagnosis, body mass index, smoking status, and others, have been examined according to ABO blood group and have not shown appreciable differences (11–13), nor has patient survival differed substantially by ABO group (14, 15). Thus, for studies providing only raw ABO blood-group frequencies, we calculated and used unadjusted odds ratios and their confidence intervals. One otherwise eligible study (16) that used individual case-control matching reported too few cases with blood group O (n = 1) and thus was not included in our analyses. Descriptions of the individual studies are noted in the Results section, and detailed discussion of possible overlaps among the Shanghai and Heidelberg studies is presented in Web Appendix 1.
For the combined study analyses, fixed-effects model Cochran's Q heterogeneity P values were extreme for all of the blood groups (P ≤ 0.016); thus, we used random-effects models that included a study heterogeneity variance component according to the method of DerSimonian and Laird (17). In each analysis, studies having fewer than 2 pancreatic cancer cases or controls were omitted. This accounts for some slight variation in total numbers of subjects analyzed across the various blood groups and genotypes. One study that provided blood group frequencies separately for black and white subjects was analyzed as separate data sets (18). The study by Macafee (19) combined a small number of individuals with blood types B and AB together into 1 category, and because AB frequency is generally much lower than B frequency in Western populations, we considered all of the combined subjects as group B. We did not weight individual study odds ratios by any measures of study quality. All summary analysis calculations were performed in Microsoft Excel (Microsoft Corp., Redmond, Washington). Odds ratio estimates were verified by unconditional logistic regression adjusted for study. To evaluate possible publication bias, all forest plots of results list the studies ordered according to decreasing association variance, as funnel plots. All P values are 2-sided.
We examined possible heterogeneity of results according to various study subclassifications, including East Asian versus Western and CagA-positive H. pylori–endemic versus –nonendemic study populations. CagA-positive–endemic and –nonendemic populations were so classified according to criteria discussed by Pounder and Ng (20), Perez-Perez et al. (21), and Epplein et al. (22). To determine statistical significance of the comparisons, the separate study subgroups were assumed to be independent, and thus the differences in pooled log odds ratios normally distributed, with variances given by the sum of the variances of the subgroup pooled log estimates.
RESULTS
Shanghai study
In total, 847 confirmed pancreatic cancer cases and 975 population controls were recruited into the study and provided biospecimens. Saliva samples were genotyped for 656 cases and 949 controls, and buffy coat specimens were genotyped for 191 cases and 26 controls. Saliva genotyping of 1 case sample and 4 control samples was unsuccessful for both ABO SNPs after 3 attempts each, yielding successful genotyping of 846 cases and 971 controls. Among the subjects for whom buffy coat specimens were genotyped were 45 with saliva specimens that had failed genotyping of 1 or both SNPs. Thus, the success fraction for genotyping the saliva samples for the 2 SNPs was 1,605/(1,605 + 5 + 45) = 97%. To confirm genotyping accuracy, we repeated genotyping in blinded fashion of 77 saliva and 6 buffy coat DNA samples for rs8176746 and 69 saliva and 6 buffy coat DNA samples for rs8176719. All of the repeat genotypes were identical to the original results. Regarding age, sex, education, alcohol intake, and tobacco use, characteristics were similar between interviewed cases and controls, between genotyped cases and controls, and between genotyped subjects and total subjects (Web Table 1).
Tables 1 and 2 show the genotype and phenotype distributions of the ABO groups in cases and controls. The odds ratio for all non-O blood groups as a whole compared with group O was 1.31 (95% confidence interval (CI): 1.07, 1.61). Analyses limited to cases with pancreatic ductal adenocarcinoma as proven by direct examination of pathology specimens produced similar odds ratios (for group A, odds ratio (OR) = 1.51, 95% CI: 1.08, 2.12; for group B, OR = 0.98, 95% CI: 0.68, 1.42; for group AB, OR = 1.19, 95% CI: 0.75, 1.91; and for all non-O groups combined, OR = 1.24, 95% CI: 0.92, 1.67).
Table 1.
ABO Blood Group Genotypes of Pancreatic Cancer Patients and Population Control Subjects, Urban Shanghai, China, 2006–2011
| rs8176746 × rs8176719 Genotypea | No. of Cases | Genotype Frequency in Cases, % | No. of Controls | Genotype Frequency in Controls, % | Phenotype A Alleles | Phenotype B Alleles | Phenotype AB Alleles | Phenotype O Alleles |
|---|---|---|---|---|---|---|---|---|
| AA × GG | 31 | 3.7 | 43 | 4.4 | BB | |||
| AA × GD | 1 | 0.1 | 1 | 0.1 | BOb | |||
| AC × GG | 90 | 10.6 | 110 | 11.3 | AB | |||
| AC × GDc | 184 | 21.7 | 232 | 23.9 | BO | |||
| CC × GG | 62 | 7.3 | 55 | 5.7 | AA | |||
| CC × GD | 257 | 30.4 | 222 | 22.9 | AO | |||
| CC × DD | 221 | 26.1 | 308 | 31.7 | OO |
a The rs8176719 alleles are exon 6 261G (“G”) and 261delG (“D”). The rare genotypes AA × DD and AC × DD did not occur among cases or controls. (See footnote b.)
b The AD haplotype corresponds to the rare O24, O40, or O41 alleles (8).
c The AC × GD genotype denotes either haplotypes AG and CD (the common BO alleles, respectively) or AD and CG, which haplotype is rare in the Han Chinese population.
Table 2.
ABO Phenotype Odds Ratios for Pancreatic Cancer With Respect to Group O, Urban Shanghai, China, 2006–2011
| Phenotype | Phenotype Frequency, % |
Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| In Cases | In Controls | |||
| A (alleles AA, AO) | 37.7 | 28.5 | 1.60 | 1.27, 2.03 |
| B (alleles BB, BO) | 25.5 | 28.4 | 1.09 | 0.85, 1.40 |
| AB (alleles AB) | 10.6 | 11.3 | 1.14 | 0.82, 1.58 |
| O (alleles OO) | 26.1 | 31.7 | 1 | Referent |
Meta-analysis
Web Table 2 shows characteristics of the present study and the 23 other independent studies identified in review of the literature (5, 7, 9, 12, 15, 18, 19, 23–38) as eligible for inclusion in our combined analyses. Data in Vogel and Krüger (39) were not used because primary data from each of the original studies were available. In total, data from 10,415 cases and 869,044 controls were used in the present analyses.
Most studies included in the meta-analysis determined ABO phenotype by antiserum hemagglutination methods. These methods are sufficiently standard and widely used in large-scale blood banking that, where studies omitted description of their ABO determination method, we assumed it to be seroagglutination. Five recent studies determined ABO germline genotypes. Three of these (15, 27, 36) involved genotyping rs505922, in very high linkage disequilibrium with rs8176719, which as a homozygote deletion, underlies all of blood group O, save for some rare O variant groups. The remaining 2 studies (Nakao et al. (7) and the present study) genotyped rs8176719 directly. Three of the genotyping studies (Nakao et al. (7), Wolpin et al. (27), and the present study) additionally genotyped rs8176746, which identifies groups A, B, and AB (Table 1). These 3 studies allow examination of AA, AO, BB, and BO genotypes in addition to the 4 phenotypes. Regardless of the genotyping method, all of the studies except that by Greer et al. (35) had case and control allele frequencies in Hardy-Weinberg equilibrium (all P values > 0.28). The 700,000 controls in the report by Greer et al. (35) had P = 0.009 which, when considering multiple comparisons across 24 studies, is still within acceptable Hardy-Weinberg proportions.
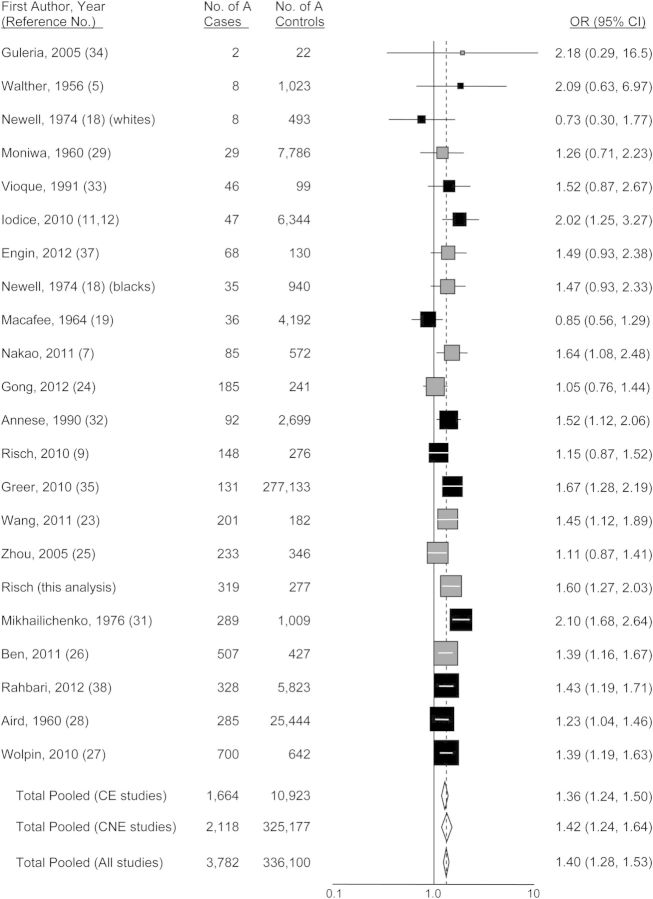
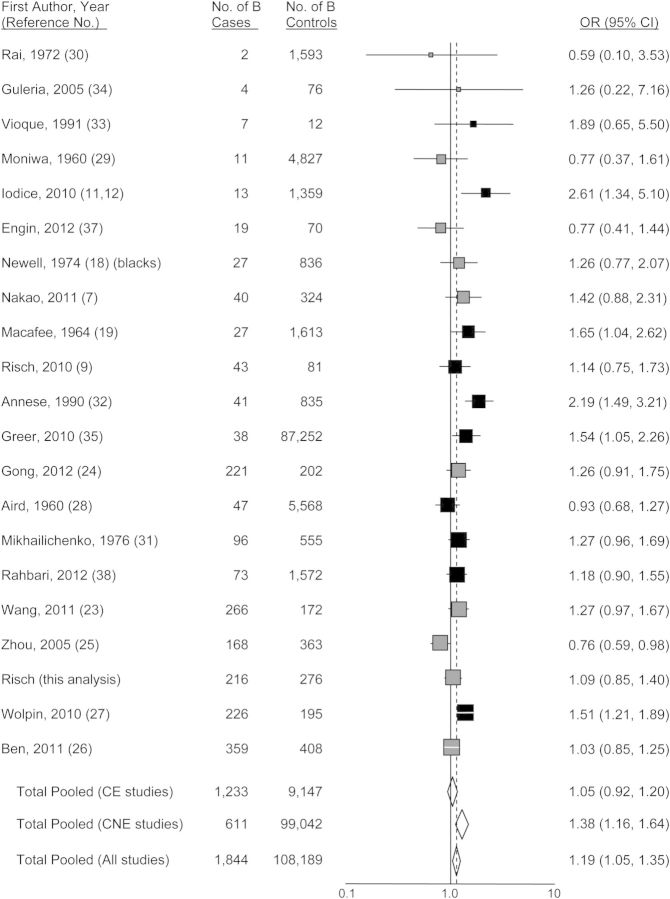
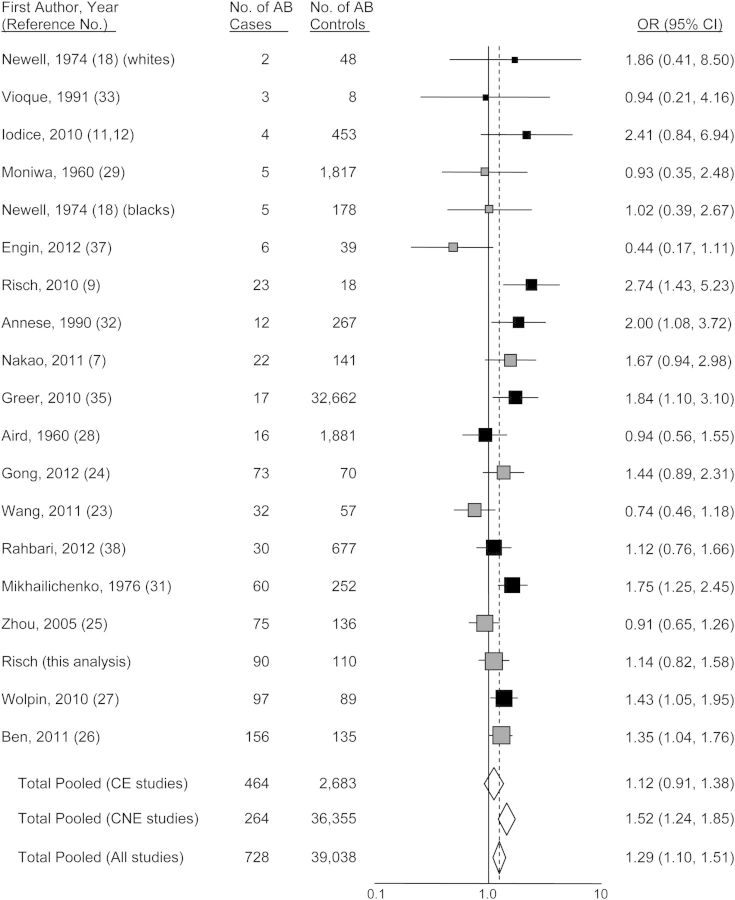
Figures 1–4 show funnel plots of the random-effects meta-analyses of pancreatic cancer risk according to blood groups A, B, AB, and all non-O, respectively, versus group O. No evidence of publication bias appears in any of the figures. Overall, all 3 non-O phenotypes are associated with significantly increased risk compared with group O. Exclusion of individual studies showed that no study affected the magnitudes of association by more than 4.5% (Web Table 3). Even though all 3 non-O blood groups have significant associations, the blood group–specific risks differ from each other in magnitude; in combined analysis of 20 studies directly examining risk for blood group A compared with blood group B, the odds ratio was 1.16 (95% CI: 1.01, 1.34; P = 0.033). Further, this difference is present in heterogeneity of group B risk between CagA-positive H. pylori–endemic populations (largely East Asian) versus nonendemic (Western) populations. For the studies of CagA-positive strain-endemic populations (identified in Web Table 2), the summary group B versus group O odds ratio was 1.05 (95% CI: 0.92, 1.20), whereas for Western studies, the group B versus O odds ratio was 1.38 (95% CI: 1.16, 1.64) (Figure 2), which was very similar to the odds ratio of 1.42 for group A versus group O in the Western studies. The odds ratios for group B versus O between the CagA-endemic and nonendemic studies were significantly different (P = 0.014). Within the endemic populations, the group B odds ratios were similar between the Chinese and Japanese studies (P = 0.68). For group AB versus group O, a similar significant difference of odds ratios between CagA-endemic and nonendemic populations was also seen (P = 0.040) (Figure 3). In the analyses of studies of endemic and nonendemic populations, no individual study affected the magnitude of the odds ratio by more than 8.0% (Web Table 3).
Figure 1.
Random-effects meta-analysis forest plot of the odds ratio of pancreatic cancer according to ABO blood group A with respect to group O. The studies are arranged in descending order of their variances, as a funnel plot. The solid squares are centered on the odds ratio (OR) point estimate from each study, and the horizontal line through each square indicates the 95% confidence interval (CI) for the study estimate. The area of each square represents the relative weight of the study in the meta-analysis. The center of each diamond indicates the inverse variance-weighted summary estimate of the magnitude of association, and the horizontal tips of the diamond represent the 95% CI. Summary odds ratios are shown for all studies combined and separately for studies of CagA-positive Helicobacter pylori–endemic populations (“CE,” shown in gray) and CagA-nonendemic populations (“CNE,” shown in black).
Figure 2.
Random-effects meta-analysis forest plot of the odds ratio of pancreatic cancer according to ABO blood group B with respect to group O. The studies are arranged in descending order of their variances, as a funnel plot. The solid squares are centered on the odds ratio (OR) point estimate from each study, and the horizontal line through each square indicates the 95% confidence interval (CI) for the study estimate. The area of each square represents the relative weight of the study in the meta-analysis. The center of each diamond indicates the inverse variance-weighted summary estimate of the magnitude of association, and the horizontal tips of the diamond represent the 95% CI. Summary odds ratios are shown for all studies combined and separately for studies of CagA-positive Helicobacter pylori–endemic populations (“CE,” shown in gray) and CagA-nonendemic populations (“CNE,” shown in black).
Figure 3.
Random-effects meta-analysis forest plot of the odds ratio of pancreatic cancer according to ABO blood group AB with respect to group O. The studies are arranged in descending order of their variances, as a funnel plot. The solid squares are centered on the odds ratio (OR) point estimate from each study, and the horizontal line through each square indicates the 95% confidence interval (CI) for the study estimate. The area of each square represents the relative weight of the study in the meta-analysis. The center of each diamond indicates the inverse variance-weighted summary estimate of the magnitude of association, and the horizontal tips of the diamond represent the 95% CI. Summary odds ratios are shown for all studies combined and separately for studies of CagA-positive Helicobacter pylori–endemic populations (“CE,” shown in gray) and CagA-nonendemic populations (“CNE,” shown in black).
Figure 4.
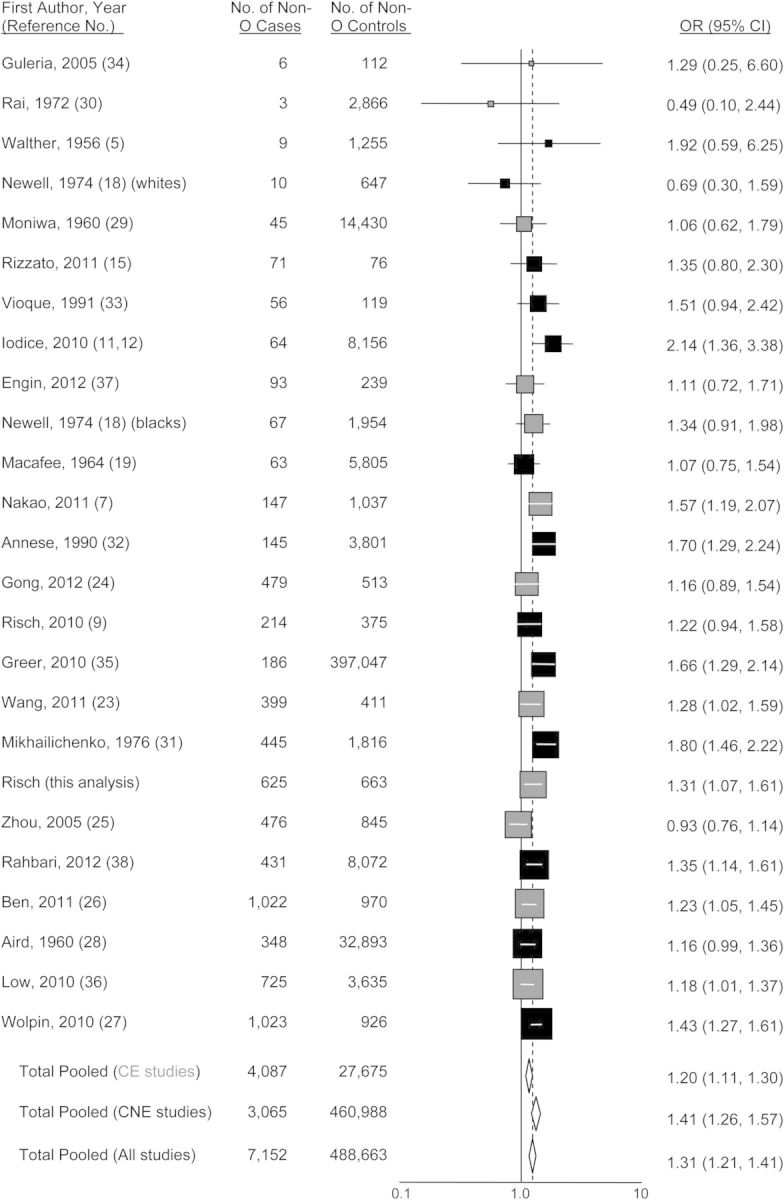
Random-effects meta-analysis forest plot of the odds ratio of pancreatic cancer according to non-O ABO blood groups taken as a whole, with respect to group O. The studies are arranged in descending order of their variances, as a funnel plot. The solid squares are centered on the odds ratio (OR) point estimate from each study, and the horizontal line through each square indicates the 95% confidence interval (CI) for the study estimate. The area of each square represents the relative weight of the study in the meta-analysis. The center of each diamond indicates the inverse variance-weighted summary estimate of the magnitude of association, and the horizontal tips of the diamond represent the 95% CI. Summary odds ratios are shown for all studies combined and separately for studies of CagA-positive Helicobacter pylori–endemic populations (“CE,” shown in gray) and CagA-nonendemic populations (“CNE,” shown in black).
Three studies that provided ABO genotype frequency details (Nakao et al. (7), Wolpin et al. (27), and the present study) allowed calculation of pooled pancreatic cancer odds ratio estimates for AA, AO, BB, and BO compared with genotype OO (group O), as follows: 1.59 (95% CI: 1.27, 1.98); 1.43 (95% CI: 1.25, 1.65); 1.62 (95% CI: 0.77, 3.38); and 1.28 (95% CI: 1.07, 1.53), respectively. No difference was apparent between the summary odds ratios for AA and AO (P = 0.42) or BB and BO (P = 0.37). This lack of difference held in the 1 study of Western populations (27) (P = 0.19 for AA vs. AO and P = 0.17 for BB vs. BO) and in the 2 studies of East Asian populations (Nakao et al. (7) and the present study) (P = 0.75 and P = 0.77, respectively), though the numbers of subjects who had homozygous B alleles were small.
Table 3 presents the odds ratios in inverse direction for groups B, AB, and O with respect to group A for CagA-endemic and -nonendemic population studies separately. In the CagA-endemic population studies, all 3 blood groups had lower risk than group A, whereas in the CagA-nonendemic population studies, only group O differed from group A.
Table 3.
Pancreatic Cancer Odds Ratio According to ABO Blood Group With Respect to Group A, in Meta-Analysis of CagA-Endemic and -Nonendemic Populations
| Blood Group | Studies of Populations Endemic for CagA-Positive Helicobacter pylori |
Studies of Populations Not Endemic for CagA-Positive Helicobacter pylori |
||
|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | |
| B versus A | 0.76 | 0.66, 0.86 | 0.99 | 0.79, 1.22 |
| AB versus A | 0.82 | 0.67, 1.00 | 1.07 | 0.89, 1.28 |
| O versus A | 0.73 | 0.64, 0.85 | 0.74 | 0.62, 0.88 |
DISCUSSION
In our population-based case-control study, we found significantly increased risk of pancreatic cancer in Han Chinese individuals with ABO blood group A compared with group O, but little evidence of increased risk associated with groups B or AB. In a meta-analysis including our investigation and 23 published studies, we found significantly increased pancreatic cancer risk with all 3 non-O blood types in studies of individuals in populations not endemic for CagA-positive strains of H. pylori. However, in studies of endemic populations (largely Chinese or Japanese), we found increased risk associated only with group A and no increased risk with groups B or AB. The presence of 1 or 2 A alleles (genotypes AA and AO) or 1 or 2 B alleles (genotypes BB and BO) did not affect these findings.
Previous investigators of ABO blood groups and risk of pancreatic cancer have hypothesized that the effects of non-O blood type on risk may be mediated by a small variety of physiological differences, including inflammation alterations through ABO-related changes in tumor necrosis factor-α, E-selectin, or soluble intercellular adhesion molecule 1 (40–44). Paré et al. (42) found significantly decreased levels of soluble intercellular adhesion molecule 1 for blood group subtype A101 (A1) versus O, but not for A201 (A2), B, or AB versus O. Both A101 and A201 alleles are associated with expression of blood group A antigens, though the A201 glycosyltransferase is 30–50 times weaker than the A101 enzyme (45). Perhaps more importantly, compared with A101, A antigens in A201 individuals are more narrowly expressed on a restricted range of antigen core-glycoprotein structures and with appreciably lesser abundance (46). Interestingly, Wolpin et al. (13) examined pancreatic cancer risk according to subtypes A101 and A201, showing significantly increased risk with A101 alleles but not with A201 alleles. Wolpin et al. (13) attributed the lack of increased risk to the functionally lower α1,3 N-acetylgalactosamine A-determinant attachment ability of the A201 transferase. Although this enzyme difference may be true, we suggest that the appreciably reduced expression of A antigen determinants in individuals carrying A201 alleles makes them more like carriers of O alleles in terms of pancreatic cancer risk. We note that the strongest ABO-associated SNP, rs507666, in the genome-wide association study study of Paré et al. (42) had an r2 of only 1.5% with soluble intercellular adhesion molecule 1 levels, whereas 3 SNPs in the ICAM-1 locus itself had together an r2 of 6.9%, and tobacco smoking, body mass index, and age had r2 values of 13.5%, 3.5%, and 1.2%, respectively. The ICAM-1 locus was not seen in the top 100 findings of the PanScan genome-wide association study (6, 47). Thus, at least for effects on soluble intercellular adhesion molecule 1 levels, it seems doubtful that this mechanism conveys the ABO association with risk of pancreatic cancer.
In addition, it is difficult to envision how such host physiological effects of the ABO blood groups would differ between East Asian and Western populations for group B but not for group A. Among Western individuals of group A, approximately one-quarter carry 1 or 2 A201 alleles (35, 48–50) (Table 4), and 18% of group A carry A201 alleles without any A101 alleles, whereas the A201 allele is not seen among Chinese or Japanese individuals (who carry A101 or A102, which have equal transferase activity) (48, 51–53) (Table 4). On the basis of A201 allele frequency, pancreatic cancer risk for blood group A individuals as a whole should be greater in studies of Chinese and Japanese populations (54, 55) than in Western ones; however, in our meta-analysis, the risks appear to be similar (Figure 1). The same problem applies to blood group AB: 17% of Western AB persons carry A201 compared with none among Chinese or Japanese AB individuals, but the risk of pancreatic cancer is seen for AB only among studies of Western individuals (Figure 3) (P = 0.054) compared with studies of East Asian individuals. Finally, the majority of B blood group genotypes (B101 O01, B101 O02, and B101 B101) comprise the same alleles in both Western and East Asian populations, with similar relative frequencies between the 2 regions (Table 4), yet our meta-analysis (Figure 2) demonstrates increased risk with group B only in Western studies (P = 0.014). According to our results (Table 3), all non-O blood groups convey increased risk in Western populations, whereas only blood group A conveys increased risk in East Asian populations. Perhaps this explains why the 2 genome-wide association studies of pancreatic cancer in China (56) and Japan (36), that examined only rs505922 genotype and thus only non-O blood groups combined versus group O, have not found significant associations; they combine blood groups B and AB with A, diluting the A association.
Table 4.
Published Frequencies of ABO Alleles in Chinese, Japanese, and White European Populations
| Allele Group | Chinese Population |
Japanese Population |
White European Population |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Allele Group Frequencya, % | Allele Type | Allele Type Frequencyb, % | Allele Group Frequencyc, % | Allele Type | Allele Type Frequencyd, % | Allele Group Frequencye, % | Allele Type | Allele Type Frequencyf, % | |
| A Alleles | 20.2 | A101 | 7.0 | 28.3 | A101 | 25.1 | 25.0 | A101 | 77.5 |
| A102 | 92.1 | A102 | 74.3 | A102 | 1.0 | ||||
| A201 | A201 | A201 | 21.2 | ||||||
| A(var)g | 0.9 | A(var)g | 0.6 | A(var)g | 0.3 | ||||
| B Alleles | 20.0 | B101 | 99.1 | 17.6 | B101 | 99.7 | 8.8 | B101 | 99.4 |
| B(var)g | 0.9 | B(var)g | 0.3 | B(var)g | 0.6 | ||||
| O Alleles | 59.8 | O01 | 59.0 | 54.1 | O01 | 52.3 | 66.2 | O01 | 60.0 |
| O02 | 38.8 | O02 | 47.7 | O02 | 35.2 | ||||
| O(var)g | 2.2 | O(var)g | O(var)g | 4.8 | |||||
a Based on data from 285,481 Han individuals across China (54).
b Based on genotyping Han individuals in Hong Kong (n = 125) (48) and Zhejiang Province (n = 417) (51).
c Based on data from 4,464,349 Japanese individuals (55).
d Based on genotyping Japanese individuals in Osaka (n = 520) (52) and in the Tokyo area (n = 340) (53).
e Based on data from 708,842 Pittsburgh, Pennsylvania, blood donors (35).
f Based on genotyping white European individuals in London (n = 98) (48); Germany (n = 169) (49); Italy (n = 232) (50); and Israel (n = 180) (50).
g A(var), B(var), and O(var) denote collected minor and rare variants.
We suggest that this apparent difference among populations in the ABO blood group associations with risk of pancreatic cancer may result from differences in H. pylori colonization among the populations. In our Connecticut study (9), increased risk associated with H. pylori seropositivity occurred only for individuals colonized by CagA-negative bacterial strains. In that study, seropositivity for CagA-positive strains was associated with a nonsignificant decreased risk. Both CagA-positive and CagA-negative H. pylori seropositivities were prevalent among individuals in the study. In contrast, in Chinese and Japanese populations, much larger fractions of the populations are colonized by H. pylori than are those in the United States, and the majority of colonized individuals carry CagA-positive bacterial strains, which in those populations may not be associated with increased risk of pancreatic cancer (57). CagA-positive H. pylori strains bind to gastric mucosal epithelial Lewis b and H type 1 antigens (58). This binding is sterically adjacent to the ABO A and B determinants on terminal glycoprotein chains and likely interacts with those antigens (59). Thus, the differential risks of the B and AB blood groups between East Asian and Western populations may involve organism strain frequency differences between CagA-positive endemic versus -nonendemic areas.
Our Shanghai case-control study has a number of strengths. Patients were recruited through an “instant case reporting system” in 37 major hospitals in urban Shanghai, and normal controls were randomly selected from the entire nontransient population of the urban area, greatly enhancing study representativeness. Our case ascertainment was thorough, with physician report as the initial ascertainment criterion and final review and confirmation of case eligibility by a dedicated panel of clinical and pathology experts. Subject response and availability of biosamples were as high as or higher than in any population-based pancreatic cancer case-control study of which we are aware. More than 99% of study subjects were of Han ethnicity, avoiding population stratification differences.
With regard to our meta-analysis, some points are worth considering. The validity of our results rests largely on 3 factors: accuracy of the measurement of interest, representativeness of the controls, and representativeness of the cases. For ABO phenotype determination, most of the included studies used standard hemagglutination methods that have been in clinical use for the major blood types for a century (60, 61) and on which blood banking relies. Only 3 of our included studies examined ABO genotype; thus, even though our analysis involved more than 10,000 cases in total and a great excess of controls, in some of the genotype categories subdivided by ethnicity there were still insufficient numbers of subjects to yield definitive conclusions. Also, not all published studies fully evaluated the ABO phenotype, which is a particular issue for Chinese and Japanese genome-wide association studies, where risks associated with blood groups B and AB appear to be different from the risk for group A. With respect to controls, demonstrating representativeness is typically done by comparing distributions of subject characteristics with those of known or established groups. For the major ethnic groups in our meta-analysis (white European, Chinese, and Japanese), the control ABO phenotype group frequencies are within a few percentage points of known distributions (62, 63) (Table 4 and Web Table 4), and all of the phenotype frequencies are in adequate Hardy-Weinberg equilibrium (Web Table 4). Some of the studies included in our meta-analysis involved controls sampled from large groups of blood donors. In self-selecting to provide blood, such donors may have other characteristics associated with altered risk of pancreatic cancer, even if they are generally considered to be representative of their studies’ ethnic compositions. Limiting analyses to studies not involving blood-donor controls did not change any of our results appreciably (data not shown). Finally, the representativeness of cases included in case-control studies of pancreatic cancer could potentially be compromised because of factors related to disease survival and the tendency of case-control studies to miss subjects with shorter survival. For ABO, none of the phenotype groups has been definitively associated with survival differences. The studies by Dandona et al. (14) and Rizzato et al. (15) do not show survival advantages according to blood group. Three other studies suggest that patients with blood group O may have somewhat longer survival than do non-O patients (26, 37, 38). If this survival difference applied to patient participation in case-control studies, such studies would be relatively enriched for group O cases compared with controls, creating an artificial association between group O and increased risk. However, our meta-analysis demonstrated increased risks for the non-O blood groups. Therefore, if anything, a survival benefit conferred by group O would attenuate the odds ratios observed here. There is no evidence that patient survival differs between blood groups A and B, so potential survival issues in case-control studies would not account for differences in risk between blood groups A and B in Western compared with Asian and other populations.
In summary, in a population-based case-control study and in meta-analysis, ABO blood group A was associated with increased risk of pancreatic cancer, and in Western populations, blood groups B and AB were also associated with increased risk, though not in Chinese or Japanese populations. These findings are provocative and compelling for further research to explore mechanisms of how ABO blood group determinants are involved in the etiology of this disease.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Chronic Disease Epidemiology, Yale School of Public Health, New Haven, Connecticut (Harvey A. Risch, Lingeng Lu, Herbert Yu); Department of Epidemiology, Shanghai Cancer Institute, Jiao Tong University, Shanghai, People's Republic of China (Jing Wang, Wei Zhang, Yu-Tang Gao); Department of Pancreas and Hepatobiliary Surgery, Pancreatic Cancer Center/Institute, Cancer Hospital, Shanghai Medical College, Fudan University, Shanghai, People's Republic of China (Quanxing Ni); and Epidemiology Program, University of Hawaii Cancer Center, Honolulu, Hawaii (Herbert Yu).
This work was supported by the US National Cancer Institute (grant 5R01CA114421); the Science and Technology Commission of Shanghai Municipality (grant 08411954100); and the Shanghai Cancer Institute (grant SB10-06).
We thank the staff of the 37 hospitals for their unfailing support in case reporting and recruitment, the clinicians and pathologists of the case-subject eligibility review panel for their careful decisions, and Lu Sun and the other project staff of the case-control study for their invaluable dedication to the study. We also thank Thomas Cain, Na Ni, and Frank Lee for their technical support in carrying out the laboratory assays.
The funding sources for this study did not participate in the design or conduct of the study, the collection, management, analysis, or interpretation of data, or the preparation, review, or approval of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Gao Y-T, Lu W. Cancer Incidence, Mortality and Survival Rates in Urban Shanghai (1973–2000) Shanghai, China: Second Military Medical University Press; 2007. [Google Scholar]
- 2.Gong Z, Holly EA, Bracci PM. Survival in population-based pancreatic cancer patients: San Francisco Bay area, 1995–1999. Am J Epidemiol. 2011;174(12):1373–1381. doi: 10.1093/aje/kwr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012;23(7):1880–1888. doi: 10.1093/annonc/mdr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowenfels AB, Maisonneuve P. Pancreatic cancer: development of a unifying etiologic concept. Ann N Y Acad Sci. 1999;880:191–200. doi: 10.1111/j.1749-6632.1999.tb09523.x. [DOI] [PubMed] [Google Scholar]
- 5.Case J, Raeburn C, Walther WW. Blood groups in relation to malignant diseases. Lancet. 1956;271(6950):970–972. doi: 10.1016/s0140-6736(56)90324-5. [DOI] [PubMed] [Google Scholar]
- 6.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies ABO blood group susceptibility variants for pancreatic cancer. Nat Genet. 2009;41(9):986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakao M, Matsuo K, Hosono S, et al. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 2011;102(5):1076–1080. doi: 10.1111/j.1349-7006.2011.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patnaik SK, Helmberg W, Blumenfeld OO. BGMUT: NCBI dbRBC database of allelic variations of genes encoding antigens of blood group systems. Nucleic Acids Res. 2012;40(1):D1023–D1029. doi: 10.1093/nar/gkr958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risch HA, Yu H, Lu L, et al. ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case-control study. J Natl Cancer Inst. 2010;102(7):502–505. doi: 10.1093/jnci/djq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AVIS. The blood groups [in Italian] http://www.avismi.it/download/pdf/aem/2009_01-04.pdf. Accessed March 5, 2012.
- 12.Iodice S, Maisonneuve P, Botteri E, et al. ABO blood group and cancer. Eur J Cancer. 2010;46(18):3345–3350. doi: 10.1016/j.ejca.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Wolpin BM, Kraft P, Xu M, et al. Variant ABO blood group alleles, secretor status, and risk of pancreatic cancer: results from the pancreatic cancer cohort consortium. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3140–3149. doi: 10.1158/1055-9965.EPI-10-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dandona M, Gao F, Lineham DC, et al. Re: “ABO blood group and the risk of pancreatic cancer” [letter] J Natl Cancer Inst. 2010;102(2):135–137. doi: 10.1093/jnci/djp447. [DOI] [PubMed] [Google Scholar]
- 15.Rizzato C, Campa D, Giese N, et al. Pancreatic cancer susceptibility loci and their role in survival. PLoS One. 2011;6(11):e27921. doi: 10.1371/journal.pone.0027921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokic NZ, Adanja JB, Vlajinac DH, et al. Case-control study of pancreatic cancer in Serbia, Yugoslavia. Neoplasma. 1996;43(5):353–356. [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Newell GR, Gordon JE, Monlezun AP, et al. ABO blood groups and cancer. J Natl Cancer Inst. 1974;52(5):1425–1430. doi: 10.1093/jnci/52.5.1425. [DOI] [PubMed] [Google Scholar]
- 19.Macafee AL. ABO blood groups and carcinoma of pancreas. Ulster Med J. 1964;33(2):129–131. [PMC free article] [PubMed] [Google Scholar]
- 20.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9(2 suppl):33S–39S. [PubMed] [Google Scholar]
- 21.Perez-Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2004;9(1 suppl):1S–6S. doi: 10.1111/j.1083-4389.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 22.Epplein M, Signorello LB, Zheng W, et al. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer Epidemiol Biomarkers Prev. 2011;20(5):826–834. doi: 10.1158/1055-9965.EPI-10-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D-S, Chen D-L, Ren C, et al. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer. 2012;131(2):461–468. doi: 10.1002/ijc.26376. [DOI] [PubMed] [Google Scholar]
- 24.Gong Y, Yang Y-S, Zhang X-M, et al. ABO blood type, diabetes and risk of gastrointestinal cancer in northern China. World J Gastroenterol. 2012;18(6):563–569. doi: 10.3748/wjg.v18.i6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou G-Z, Li Z-S. Clinical epidemiological research on pancreatic cancer: an analysis of 1027 cases. World Chin J Digestol. 2005;13(1):55–60. [Google Scholar]
- 26.Ben Q, Wang K, Yuan Y, et al. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case-control study. Int J Cancer. 2011;128(5):1179–1186. doi: 10.1002/ijc.25426. [DOI] [PubMed] [Google Scholar]
- 27.Wolpin BM, Kraft P, Gross M, et al. Pancreatic cancer risk and ABO blood group alleles: results from the pancreatic cancer cohort consortium. Cancer Res. 2010;70(3):1015–1023. doi: 10.1158/0008-5472.CAN-09-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aird I, Lee DR, Roberts JAF. ABO blood groups and cancer of oesophagus, cancer of pancreas, and pituitary adenoma. BMJ. 1960;1(5180):1163–1166. doi: 10.1136/bmj.1.5180.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moniwa H. Statistical studies on the correlation between the ABO-blood groups and some diseases. Tohoku J Exp Med. 1960;72:275–289. doi: 10.1620/tjem.72.275. [DOI] [PubMed] [Google Scholar]
- 30.Rai S, Saronwala KC, Singh R. ABO blood groups in cancer of the gastro-intestinal tract. Indian J Cancer. 1972;9(1):97–100. [PubMed] [Google Scholar]
- 31.Mikhailichenko VA. ABO blood-group system in patients with pancreatic cancer [in Russian] Vopr Onkol. 1976;22(12):72–73. [PubMed] [Google Scholar]
- 32.Annese V, Minervini M, Gabbrielli A, et al. ABO blood groups and cancer of the pancreas. Int J Pancreatol. 1990;6(2):81–88. doi: 10.1007/BF02933042. [DOI] [PubMed] [Google Scholar]
- 33.Vioque J, Walker AM. Pancreatic cancer and ABO blood type: a case-control study [in Spanish] Med Clin (Barc) 1991;96(20):761–764. [PubMed] [Google Scholar]
- 34.Guleria K, Singh HP, Kaur H, et al. ABO blood groups in gastrointestinal tract (GIT) and breast carcinoma patients. Anthropologist. 2005;7(3):189–192. [Google Scholar]
- 35.Greer JB, Yazer MH, Raval JS, et al. Significant association between ABO blood group and pancreatic cancer. World J Gastroenterol. 2010;16(44):5588–5591. doi: 10.3748/wjg.v16.i44.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Low SK, Kuchiba A, Zembutsu H, et al. Genome-wide association study of pancreatic cancer in Japanese population. PLoS One. 2010;5(7):e11824. doi: 10.1371/journal.pone.0011824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engin H, Bilir C, Üstün H, et al. ABO blood group and risk of pancreatic cancer in a Turkish population in Western Black Sea region. Asian Pac J Cancer Prev. 2012;13(1):131–133. [PubMed] [Google Scholar]
- 38.Rahbari NN, Bork U, Hinz U, et al. ABO blood group and prognosis in patients with pancreatic cancer. BMC Cancer. 2012;12:319. doi: 10.1186/1471-2407-12-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogel F, Krüger J. Statistical relationships between the ABO blood groups and diseases with the exception of the infectious diseases [in German] Blut. 1968;16(6):351–376. doi: 10.1007/BF01632080. [DOI] [PubMed] [Google Scholar]
- 40.Barbalic M, Dupuis J, Dehghan A, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19(9):1863–1872. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melzer D, Perry JRB, Hernandez D, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs) PLoS Genet. 2008;4(5):e1000072. doi: 10.1371/journal.pgen.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paré G, Chasman DI, Kellogg M, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6578 women. PLoS Genet. 2008;4(7):e1000118. doi: 10.1371/journal.pgen.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paterson AD, Lopes-Virella MF, Waggott D, et al. Genomewide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Arterioscler Thromb Vasc Biol. 2009;29(11):1958–1967. doi: 10.1161/ATVBAHA.109.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi L, Cornelis MC, Kraft P, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet. 2010;19(9):1856–1862. doi: 10.1093/hmg/ddq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto F, McNeill PD, Hakomori S. Human histo-blood group A2 transferase coded by A2 allele, one of the A subtypes, is characterized by a single base deletion in the coding sequence, which results in an additional domain at the carboxyl terminal. Biochem Biophys Res Commun. 1992;187(1):366–374. doi: 10.1016/s0006-291x(05)81502-5. [DOI] [PubMed] [Google Scholar]
- 46.Clausen H, Levery SB, Nudelman E, et al. Repetitive A epitope (type 3 chain A) defined by blood group A1-specific monoclonal antibody TH-1: chemical basis of qualitative A1 and A2 distinction. Proc Natl Acad Sci U S A. 1985;82(4):1199–1203. doi: 10.1073/pnas.82.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42(3):224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yip SP. Single-tube multiplex PCR-SSCP analysis distinguishes 7 common ABO alleles and readily identifies new alleles. Blood. 2000;95(4):1487–1492. [PubMed] [Google Scholar]
- 49.Nishimukai H, Fukumori Y, Okiura T, et al. Genotyping of the ABO blood group system: analysis of nucleotide position 802 by PCR-RFLP and the distribution of ABO genotypes in a German population. Int J Legal Med. 1996;109(2):90–93. doi: 10.1007/BF01355523. [DOI] [PubMed] [Google Scholar]
- 50.Nishimukai H, Fukumori Y, Tsujimura R, et al. Rare alleles of the ABO blood group system in two European populations. Legal Med. 2009;11(1 suppl):479S–481S. doi: 10.1016/j.legalmed.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 51.Zhu F, Tao S, Xu X, et al. Distribution of ABO blood group allele and identification of three novel alleles in the Chinese Han population. Vox Sang. 2010;98(4):554–559. doi: 10.1111/j.1423-0410.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 52.Fukumori Y, Ohnoki S, Shibata H, et al. Suballeles of the ABO blood group system in a Japanese population. Hum Hered. 1996;46(2):85–91. doi: 10.1159/000154331. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi K, Iwasaki M, Anan K, et al. An analysis of polymorphism for the ABO blood group genes in a Japanese population based on polymerase chain reaction. Anthropol Sci. 1999;107(2):109–121. [Google Scholar]
- 54.Chen Z, Zhao T, Zhang G. ABO blood group distribution in the Chinese population [in Chinese] Hereditas (Beijing) 1982;4(2):4–7. [Google Scholar]
- 55.Fujita Y, Tanimura M, Tanaka K. The distribution of the ABO blood groups in Japan. Jpn J Hum Genet. 1978;23(2):63–109. doi: 10.1007/BF02001790. [DOI] [PubMed] [Google Scholar]
- 56.Wu C, Miao X, Huang L, et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat Genet. 2012;44(1):62–66. doi: 10.1038/ng.1020. [DOI] [PubMed] [Google Scholar]
- 57.Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95:948–960. doi: 10.1093/jnci/95.13.948. [DOI] [PubMed] [Google Scholar]
- 58.Alkout AM, Blackwell CC, Weir DM, et al. Isolation of a cell surface component of Helicobacter pylori that binds H type 2, Lewis a, and Lewis b antigens. Gastroenterology. 1997;112(4):1179–1187. doi: 10.1016/s0016-5085(97)70129-x. [DOI] [PubMed] [Google Scholar]
- 59.Risch HA. Pancreatic cancer: Helicobacter pylori colonization, N-nitrosamine exposures, and ABO blood group. Mol Carcinog. 2012;51(1):109–118. doi: 10.1002/mc.20826. [DOI] [PubMed] [Google Scholar]
- 60.Bond CJ. On auto-haemagglutination: a contribution to the physiology and pathology of the blood. Br Med J. 1920;2(3129):925–927. doi: 10.1136/bmj.2.3129.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bond CJ. On auto-haemagglutination: a contribution to the physiology and pathology of the blood. Br Med J. 1920;2(3130):973–976. doi: 10.1136/bmj.2.3130.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beckman L. A Contribution to the Physical Anthropology and Population Genetics of Sweden. Lund, Sweden: Berlingska Boktryck; 1959. p. 21. [Google Scholar]
- 63.Bloodbook.com. Racial and ethnic distribution of ABO blood types. http://www.bloodbook.com/world-abo.html. ). (Accessed July 23, 2012)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.