Abstract
Lymphangiomatosis is a rare disease of lymphatic proliferation for which no adequate treatment is known. We report the first successful case of bilateral lung transplantation for the treatment of end-stage pulmonary lymphangiomatosis. A successful outcome was achieved with continued survival beyond four years posttransplant and stable lung function. The primary obstacle to significant gains in pulmonary function were thoracic, skeletal, and abdominal lymphangiomatosis that led to pulmonary restriction. Our report demonstrates that pulmonary lymphangiomatosis should be included among those diseases for which lung transplantation is considered potentially beneficial treatment but also emphasizes the importance of screening patients carefully for chest wall and abdominal lymphangiomas that may impede recovery.
Keywords: lymphoproliferative disease, pulmonary disease/lung transplantation, pretransplant morbidity, transplant outcome
Introduction
Lymphangiomatosis is a progressive and generally untreatable disease caused by the abnormal and extensive proliferation of lymphatic tissue. The lymphatic proliferation can be either diffuse or discrete in nature and can be found throughout the body, the most common sites being within the skeleton, the abdomen, and the thorax (1). Lymphangiomatosis and lymphangioleiomyomatosis (LAM) are distinct entities; though the two disorders share certain clinical features and both involve lymphatic vasculature, LAM is a disregulated proliferation of smooth muscle cells associated with lymphatic spaces, while lymphangiomatosis involves proliferation of the lymphatics themselves. Patients with advanced lymphangiomatosis develop a variety of symptoms depending on the location of their lymphangiomas; these symptoms may include recurrent chylous ascites and gastrointestinal obstruction from abdominal disease, dyspnea and restrictive lung disease from thoracic involvement, and fractures from skeletal lesions (2). While lymphangiomas of the skeleton are often debilitating, disfiguring and painful, visceral involvement carries an especially grim prognosis. Specifically, pleural and lung disease are associated with especially high mortality rates due to chronic hypercarbia and respiratory failure (3, 4). This pulmonary decline is caused by local compression of bronchioles and restrictive pleural disease and typically presents as either a restrictive pattern or a mixed restrictive and obstructive pattern on pulmonary function testing. Lung function can be further limited by recurrent chylous pleural effusions (1, 2). Unfortunately surgery, radiation, and chemotherapy have all been tried with only limited success, and these rarely result in any long-term improvement of symptoms. We describe the first case of successful bilateral lung transplantation for the treatment of end-stage thoracic lymphangiomatosis, and we discuss the effects of the patient's residual skeletal disease on her allograft function.
Case Report
A 29-year-old woman with a history of lymphangiomatosis presented to the pulmonary clinic requesting an evaluation for pulmonary transplantation. The patient was first diagnosed with lymphangiomatosis at eleven years of age following an evaluation for multiple long bone fractures. Her disease affected only her bones until the age of 14 when she developed recurrent chylous pleural effusions. These were managed successfully with bilateral pleurodeses and a right pleurectomy, and thereafter the patient's effusions did not recur for over ten years. In the fall of 2003 the patient began to develop shortness of breath with exertion, then recurrent headaches and heart palpitations. Evaluation eventually showed a peripheral oxygen saturation of 80% and progressive thoracic lymphangiomatosis. She was placed on 2-3 L of oxygen and referred to our institution for consideration of lung transplantation. Over the course of the next 6 months she was admitted to her local hospital 4 times with dyspnea and hypercarbia. During one admission her PCO2, which peaked at 112 mmHg, only improved after BiPAP. When she presented to our transplant clinic in December 2003, the patient required 2-3 L of oxygen at baseline and was unable to work due to her pulmonary deterioration. Her PCO2 was 66 mmHg, and her PO2 was 48 mmHg. Pulmonary function testing showed that her forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were 0.57 L (19% of expected) and 0.63 L (18% of expected), respectively. A chest radiograph (Figure 1) demonstrated dense bilateral lower lung opacities and multiple lytic skeletal lesions, both consistent with lymphangiomatosis. In addition to her pulmonary and continuing skeletal disease, the patient had also developed ascites and splenomegaly secondary to her lymphangiomatosis. Despite these complications, the patient had never been ventilator dependent and was able to meet all institutional conditioning requirements for transplantation. After carefully considering her risk of death from pulmonary disease and the current transplantation guidelines for sarcoidosis and LAM, diseases similarly complicated by extrapulmonary disease and prior pulmonary procedures (5, 6), the patient was listed for lung transplantation and underwent a bilateral lung transplant approximately 5 months later. Bilateral antero-transternal thoracotomies were performed and the chest was entered through the fourth intercostal space. The procedure was performed and the chest was entered through the fourth intercostal space. The procedure was performed without cardiopulmonary bypass and proceeded without complication, although extensive pleural adhesions from her prior pleurectomy and pleurodeses prolonged resection of the native lungs. Pathology from the patient's native lungs confirmed diffuse pleuropulmonary and perivascular lymphangiomatosis (Figure 2). Total ischemic times for the right and left donor lungs were 6 hours and 20 minutes and 8 hours and 50 minutes, respectively.
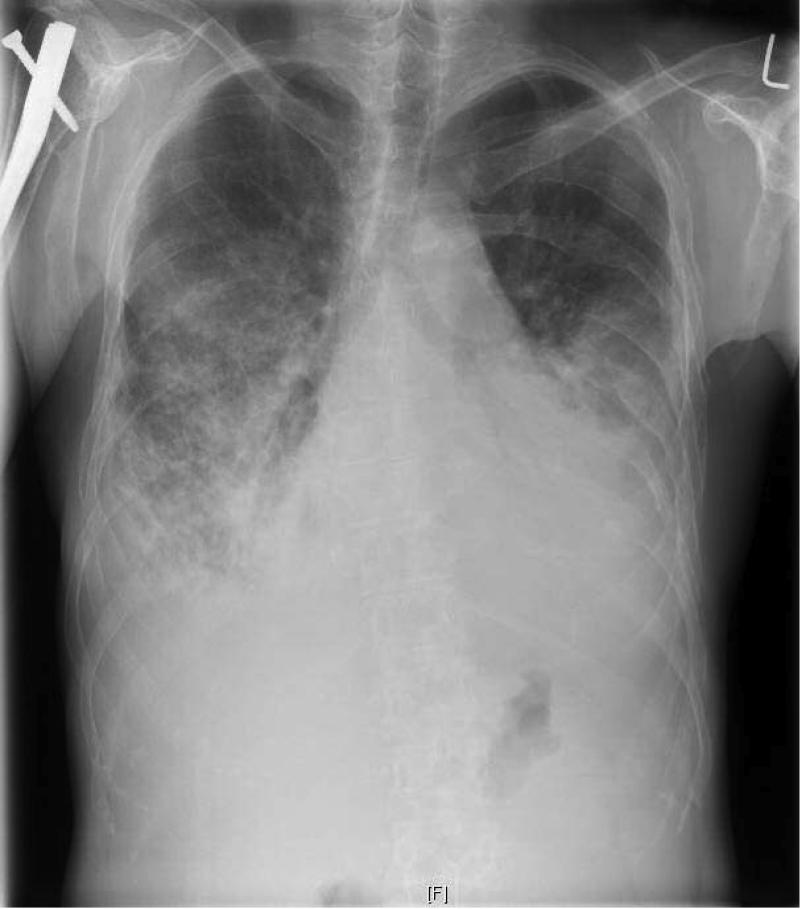
Figure 1.
Chest radiograph prior to transplantation demonstrating dense bilateral lower lung heterogeneous opacities consistent with patient's diagnosis of lymphangiomatosis. Diffuse osteopenia with innumerable lytic and expansile lesions can also be appreciated. Orthopedic hardware in the right humerus is visible.
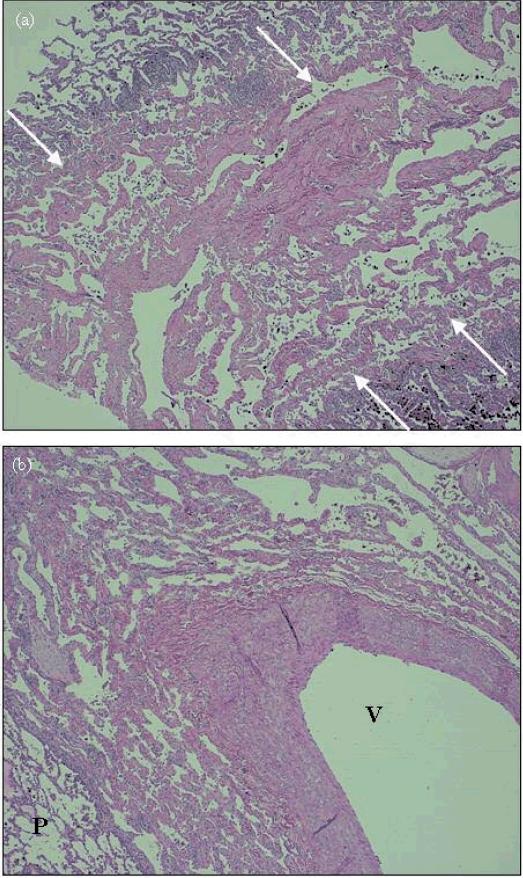
Figure 2.
H&E stain of native lung at 40x magnification. (a) right lung, peripheral upper lobe – septal lymphangiomatosis (between arrows) is seen in the center of the field, bordered on both sides by normal lung parenchyma. (b) left lung, central upper lobe – perivascular lymphangiomatosis, extensive lymphatic channels surround this arteriole (V indicates vessel, P indicated normal parenchyma).
The patient's post-operative course was complicated by respiratory insufficiency. She experienced recurrent chylous effusions that eventually caused pleural thickening and restriction. This restriction was further exacerbated by residual skeletal lymphangiomatosis and recurrent intra-abdominal chylous ascites. As a result the patient temporarily required a tracheostomy to achieve adequate ventilation. In addition, she underwent a paracentesis and retroperitoneal catheterization in order to treat her recurrent intra-abdominal chylous ascites and to further relieve her pulmonary restriction. The patient was ultimately discharged home four months following her admission for transplant and has been maintained at home for four years on an immunosuppression regimen of azathioprine, prednisone, and tacrolimus. Despite disease-free lung parenchyma, the patient continues to suffer from restrictive lung disease due to pleural thickening, persistent ascites, and multiple rib fractures (Figure 3). Her FEV1 and FVC following transplant stabilized at 0.95 L (33% of expected) and 1.04 L (31% of expected), respectively; at that time her PCO2 was 50 mmHg and her PO2 was 65 mmHg. Since transplantation she has been hospitalized only once for management of her chylous ascites. Although the patient experienced only a small improvement in her lung capacity, the patient's progressive downhill course prior transplantation has stabilized, in part reflected by her improved oxygenation, reduced hypercarbia and markedly reduced hospital admissions.
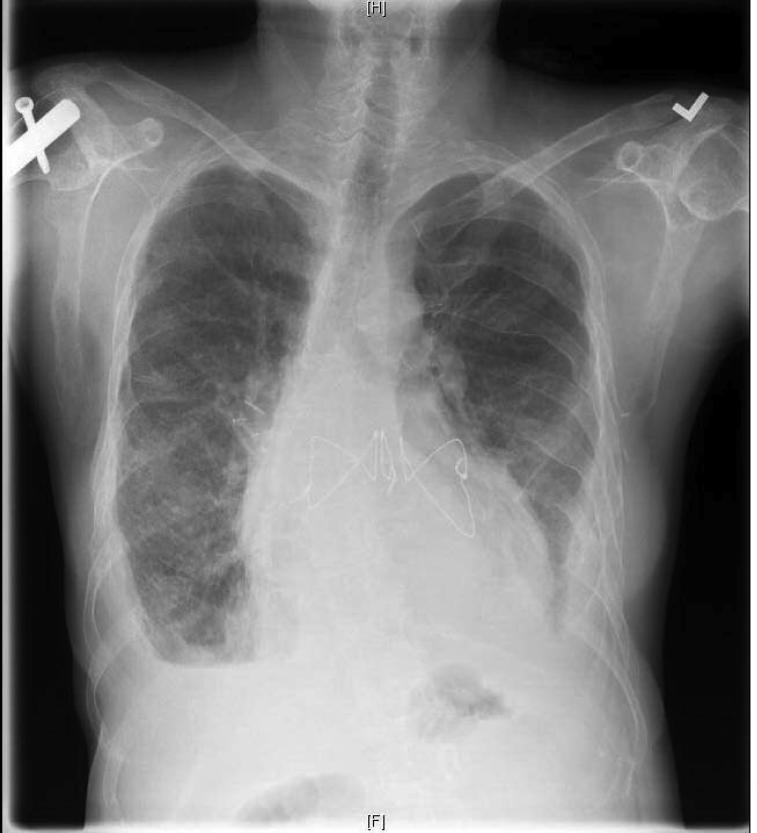
Figure 3.
Chest radiograph following transplantation demonstrates reduction of parenchymal opacity. Bilateral pleural thickening, orthopedic hardware, and skeleton lesions are still visible.
Discussion
Lymphangiomatosis is a rare disease of multifocal and diffuse lymphatic proliferation for which there is no adequate treatment. The pathogenesis of the disease itself is poorly understood making treatment design difficult, and the population of patients is so small that clinical research in this area is severely limited. As such, most information on lymphangiomatosis and its treatment is found in the form of isolated case reports and very small case series. A review of these reports reveals that multiple strategies for cure and management of this disease have been attempted; they range from surgery and radiation for localized lymphangiomas to chemotherapy for widespread disease.
Surgical resection is often considered for patients with a small number of isolated lymphangiomas and can result in rapid improvement of localized symptoms. Unfortunately, lymphangiomas are difficult to resect entirely since the borders between normal and abnormal tissue are vague and abnormal tissue is often immediately adjacent to critical organs. As a result, many patients are left with residual disease that eventually proliferates and is accompanied by a return of symptoms (7). In addition to resection, surgical approaches have also included pleurectomy and thoracic duct ligation for palliation of recurrent chylous effusions (8-10). Patients with a small number of discrete lymphangiomas have also been treated with percutaneous doxycycline. In a small case series all five patients experienced improvement in their symptoms but the treatment was always intended for palliation only (11). Radiation therapy has been an additional option for the treatment of isolated but inoperable disease. Lesions treated with radiation have regressed for months or even years at a time, but this therapy has only been attempted in patients with a discrete field of abnormality and patients are at risk of radiation pneumonitis (12). For widespread disease, many providers have turned to chemotherapy with some success. Lesions appear to resolve or show slowed growth in response to systemic interferon (IFN), but patients must continue infusions indefinitely, IFN has mild side effects that often limit dosage, and there are no publications of complete resolution of symptoms with this treatment (13-15).
To our knowledge, the case described here is the first successful bilateral lung transplant for the treatment of lymphangiomatosis. An earlier case series refers to an attempted heart-bilateral lung transplant for lymphangiomatosis in a 7 year old boy, but the patient died from complications of his surgery and no primary description was reported (2). Lung transplantation has an obvious benefit over isolated surgical resection because pleural and parenchymal disease is entirely replaced with healthy tissue. This eliminates the possibility that resection will leave behind occult lymphangiomatosis in the lung. Furthermore, pulmonary failure is a late manifestation of lymphangiomatosis and usually signals end-stage disease thereby justifying the additional risk of life-long immunosuppression that is conferred by transplantation. Given the extent of this particular patient's pulmonary disease, transplantation was considered a reasonable risk. The patient in this case did not recover easily or return to entirely normal lung function, but her pulmonary function did increase overall and she has not reentered the downward spiral in lung function that she experienced in the year preceding her transplant. Additionally, since transplantation she has never required hospitalization for management of her pulmonary disease.
Given the systemic nature of lymphangiomatosis, the possibility of lymph-specific complications, and the ethical considerations of transplanting valuable donor lungs into a patient with a heretofore unknown risk, we carefully reviewed the experience of lung transplantation for the proliferative disorder lymphangioleiomyomatosis (LAM) (16) before proceeding with listing. While lymphangiomatosis is quite distinct from LAM, both diseases present similar challenges to successful transplantation because they involve lymphatic obstruction, extensive pleural adhesions, and extrapulmonary disease (17). But despite the increased technical difficulty and post-operative complications caused by the aforementioned obstacles, LAM recipients ultimately have short-term mortality rates, intensive care unit times, and long-term survivial rates that are comparable to the general lung transplant population (6). This suggested that our patient with lymphangiomatosis would also benefit from transplantation despite her pleural adhesions and extrapulmonary disease and that the donor lungs provided to her would be used appropriately.
Patients with both LAM and lymphangiomatosis are also at risk of chylous effusions, both preceding and following transplantation. This is presumably related to obstruction of the thoracic duct and other routes of lymph drainage. Despite our aggressive attempts to manage the chylous effusion in our patient with lymphangiomatosis the recurrent effusions likely contributed to the pleural thickening seen on chest radiograph and eventually to her suboptimal lung outcomes.
An additional concern when transplanting patients with lymphangiomatosis is the potential for exacerbation of residual disease. Given how little we know of lymphangiomatosis, it is possible that a functional immune system normally restrains the disease; therefore transplant immunosuppression regimens could potentially aggravate existing disease. While the patient here has had continuing symptoms from her extrapulmonary disease, her symptoms did not increase in severity, location, or quality after beginning immunosuppression. Neither has there been any response of her disease to immunosuppression suggesting that other therapies are needed to prevent the progression of the lesions. This observation is consistent with reports of liver transplantation in this population that found that growth of lymphangiomas is not reduced or exacerbated by immunosuppresion (18).
As might have been expected based on the extent of her pretransplant extrapulmonary disease, our patient's recovery has been limited by pulmonary restriction secondary to pleural thickening, skeletal disease and ascites. And while lung transplantation for the pulmonary manifestations of lymphangiomatosis has not been curative in this particular case, the patient's pulmonary function has now stabilized and reflects a modest improvement over her pretransplant level of function. In addition, her oxygenation, degree of hypercarbia, and number of hospital admissions have all improved following transplantation. Our experience suggests that lymphangiomatosis should not be an absolute contraindication to pulmonary transplantation, especially when a patient is experiencing respiratory failure due to a high burden of parenchymal disease. Future patients presenting for transplantation for lymphangiomatosis, however, should be carefully evaluated on an individual basis for the extent of thoracic skeletal involvement and abdominal disease since this has been the primary obstacle for significant gains in pulmonary function in our experience. While the presence of extrapulmonary disease should not be viewed as an absolute contraindication to pulmonary transplantation, the location and severity of that disease requires careful and individual assessment before subjecting a patient to the risks of transplantation. In summary, we demonstrate for the first time that lung transplantation can be an appropriate and potentially beneficial therapy for selected patients with pulmonary lymphangiomatosis.
Acknowledgments
Funding: National Heart Lung and Blood Institute (HL69978) and Duke Stead Scholars Program Running Title “Lung Transplantation for Lymphangiomatosis”
Abbreviations
- LAM
lymphangioleiomyomatosis
- PFT
pulmonary function testing
- IFN
interferon
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
References
- 1.Faul JL, Berry GJ, Raffin TA, Colby TV, Ruoss SJ, Walter MB, et al. Thoracic Lymphangiomas, Lymphangiectasis, Lymphangiomatosis, and Lymphatic Dysplasia Syndrome. Americal Journal of Respiratory and Critical Care Medicine. 2000;161:10371046. doi: 10.1164/ajrccm.161.3.9904056. [DOI] [PubMed] [Google Scholar]
- 2.Tazelaar HHD, Kerr DD, Yousem SSA, Saldana MMJ, Langston CC, Colby TTV. Diffuse pulmonary lymphangiomatosis. Human Pathology. 1993;24(12):1313–1322. doi: 10.1016/0046-8177(93)90265-i. [DOI] [PubMed] [Google Scholar]
- 3.Ramani PP, Shah AA. Lymphangiomatosis. Histologic and immunohistochemical analysis of four cases. The American journal of surgical pathology. 1993;17(4):329–335. [PubMed] [Google Scholar]
- 4.Case Records of the Massachusetts General Hospital (case 30-1980). New England Journal of Medicine. 1980;303:270–276. doi: 10.1056/NEJM198007313030508. [DOI] [PubMed] [Google Scholar]
- 5.Shah L. Lung transplantation in sarcoidosis. Seminars in respiratory and critical care medicine. 2007;28(1):134–140. doi: 10.1055/s-2007-970339. [DOI] [PubMed] [Google Scholar]
- 6.Pechet TT, Meyers BF, Guthrie TJ, Battafarano RJ, Trulock EP, Cooper JD, et al. Lung transplantation for lymphangioleiomyomatosis. The Journal of Heart and Lung Transplantation. 2004;23(3):301–308. doi: 10.1016/S1053-2498(03)00195-5. [DOI] [PubMed] [Google Scholar]
- 7.Rostom AY. Current topic: Treatment of thoracic lymphangiomatosis. Arch Dis Child. 2000;83:138–139. doi: 10.1136/adc.83.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamada K, Ishii Y, Nakaya M, Sawabata N, Fukuda K, Suzuki H. Solitary lymphangioma of the lung. Histopathology. 1995;27(5):482–483. doi: 10.1111/j.1365-2559.1995.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 9.Browse NL, Allen DR, Wilson NM. Management of chylothorax. Br J Surg. 1997;84(12):1711–1716. [PubMed] [Google Scholar]
- 10.Bhatti MAK, Ferrante JW, Gielchinsky I, Norman JC. Pleuropulmonary and Skeletal Lymphangiomatosis with Chylothorax and Chylopericardium. Annals of Thoracic Surgery. 1985;40:398–401. doi: 10.1016/s0003-4975(10)60078-1. [DOI] [PubMed] [Google Scholar]
- 11.Molitch HI, Unger EC, Witte CL, vanSonnenberg E. Percutaneous Sclerotherapy of Lymphangiomas. Radiology. 1995;194:343–347. doi: 10.1148/radiology.194.2.7529933. [DOI] [PubMed] [Google Scholar]
- 12.Kandil A, Rostom AY, Mourad WA, Khafaga Y, Gershuny AR, el-Hosseiny G. Successful control of extensive thoracic lymphangiomatosis by irradiation. Clin Oncol (R Coll Radiol. 1997;9(6):407–411. doi: 10.1016/s0936-6555(97)80140-9. [DOI] [PubMed] [Google Scholar]
- 13.Laverdiere C, et al. Improvement of disseminated lymphangiomatosis with recombinant interferon therapy. Pediatric pulmonology. 2000;29(4):321–324. doi: 10.1002/(sici)1099-0496(200004)29:4<321::aid-ppul13>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt MA, Nelson SC, Sencer SF, Bostrom BC, Kurachek SC, Nesbit ME. Treatment of Childhood Lymphangiomas with Interferon-a. Journal of Pediatric Hematology/Oncology. 1997;19(3):232–236. doi: 10.1097/00043426-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Ozeki M, Funato M, Kanda K, Ito M, Teramoto T, Kaneko H, et al. Clinical Improvement of Diffuse Lymphangiomatosis with Pegylated Interferon Alfa-2B Therapy: Case Report and Review of the Literature. 2007;24(7):513–524. doi: 10.1080/08880010701533603. [DOI] [PubMed] [Google Scholar]
- 16.Zisis C, Spiliotopoulos K, Patronis M, Filippakis G, Stratakos G, Tzelepis G, et al. Diffuse lymphangiomatosis: Are there any clinical or therapeutic standards? The Journal of Thoracic and Cardiovascular Surgery. 2007;133(6):1664–1665. doi: 10.1016/j.jtcvs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006;13(4):276–285. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 18.Ra SH, Bradley RF, Fishbein MC, Busuttil RW, Lu DSK, Lassman CR. Recurrent hepatic lymphangiomatosis after orthotopic liver transplantation. Liver Transplantation. 2007;13:1593–1597. doi: 10.1002/lt.21306. [DOI] [PubMed] [Google Scholar]