Abstract
Background
Burn injury results in increased skeletal muscle protein turnover, where the magnitude of protein breakdown outweighs synthesis resulting in muscle wasting. The impact of increased amino acid (AA) provision on skeletal muscle fractional synthesis rate (FSR) in severely burned patients during their convalescence after discharge from hospital is not known. Subsequently, the purpose of this study was to determine skeletal muscle FSR in response to AA infusion in severely burned pediatric patients at discharge from hospital, and at six and twelve months post injury.
Methods
Stable isotope infusion studies were performed in the postprandial state and during intravenous AA infusion. Skeletal muscle biopsies were obtained and isotope enrichment determined in order to calculate skeletal muscle FSR. Patients were studied at discharge from hospital (n=11), and at six (n=15), and twelve months (n=14) post injury.
Results
The cohorts of patients studied at each time point post injury were not different with regards to age, body mass or burn size. AA infusion failed to stimulate FSR above basal values at discharge from hospital (0.27±0.04 vs. 0.26±0.06 %·hr−1), six months post injury (0.20±0.04 vs. 0.22±0.03 %·hr−1), and twelve months post injury (0.16±0.03 vs. 0.15±0.05 %·hr−1). Daily FSR was numerically lower at six months post burn (5.51±0.79 %·day−1) and significantly (P<0.05) lower at 12 months post burn (3.67±0.65 %·day−1) relative to discharge group (6.32±1.02 %·day−1).
Discussion
The findings of the current study suggest that the deleterious impact of burn injury on skeletal muscle AA metabolism persists for up to one year post injury. In light of these findings, nutritional and pharmacological strategies aimed at attenuating muscle protein breakdown post burn may be a more efficacious approach to maintaining muscle mass in severely burned patients.
Background
Severe burn injury results in a hypercatabolic stress response1, 2which leads to perturbations in whole body3, 4 and skeletal muscle protein metabolism5–7 which persist for several months post injury8, 9. While increased degradation of endogenous proteins stores, principally skeletal muscle, is critical to the adaptive response to severe burn injury, such as increased acute phase protein synthesis and in particular, wound healing6, the concurrent erosion of lean tissue is likely to impede rehabilitation and the patients return to society. In healthy humans, dietary amino acid (AA) provision increases the rate of synthesis of skeletal muscle proteins10. However, over the course of the day protein anabolism and catabolism are well matched, meaning skeletal muscle mass remains fairly constant. In contrast, patients with severe burns are generally in a catabolic state with regards to skeletal muscle, where protein breakdown rates can be more than double those of protein synthesis post injury5, 6. Interestingly, Biolo and colleagues suggested that the chronic elevations in muscle protein breakdown following burn injury leads to a concurrent increase in muscle protein synthesis due to elevated intracellular amino acid availability5. Indeed, numerous studies have reported basal skeletal muscle FSR values in severely burned patients ranging from approximately 0.10 to 0.25%·hr−1 5, 6, 11–15,which is far greater than those typically reported from healthy individuals5.
Skeletal muscle plays critical roles in both functional capacity and metabolic health. Moreover, alterations in muscle mass and/or function are associated with numerous pathologies (for review see 16). Given the increase in protein requirements for wound healing, and prolonged immobilization following severe burn injury, strategies which preserve muscle mass in this patient population would clearly be advantageous with regards to rehabilitation as well as future morbidity and mortality. Despite already elevated rates of protein synthesis, a number of pharmacological interventions have been shown to increase skeletal muscle FSR in burned patients in the acute period post burn (< 4 weeks post injury). Herndon and colleagues demonstrated that skeletal muscle FSR was higher in severely burned children that had received propranolol (0.34±0.06%·hr−1) for two weeks when compared to those who did not (0.24±0.03%·hr−1)11. Furthermore, insulin infusion has also been shown to significantly increase skeletal muscle FSR from basal values in severely burned patients during the acute hospitalization period post injury13, 14.
While pharmacological interventions appear to have the ability to further augment skeletal muscle FSR in severely burned patients11, 13, 14, 17–21, the same cannot be said of AAs. Patterson et al., demonstrated that skeletal muscle FSR was independent of dietary protein intake (ranging from 1 to 4 g·kg−1 day−1) where experimental diets were administered for four days and protein kinetics measured in all patients within the first two weeks post injury15. Interestingly, while increasing dietary protein intake had no impact on skeletal muscle FSR acutely post injury, skin FSR was positively correlated with protein intake, underscoring the need for adequate AA intake in order to facilitate wound healing post burn. While the aforementioned study determined the impact of dietary protein intake on skeletal muscle FSR acutely post burn, little is known regarding the impact of protein provision on skeletal muscle protein metabolism over time following severe burn injury. We have previously shown that amino acid infusion significantly increased phenylalanine rate of disappearance (an indirect marker of protein synthesis) across the leg in pediatric patients with severe burns at six months post injury22. However, direct measures of muscle protein synthesis in response to AA provision following burn injury have only been made within the initial few weeks after injury, where wounds are not closed and the hypermetabolic hypercatabolic stress response to burn injury is at its worst2, 9. With this in mind, the aim of the present study was to determine skeletal muscle FSR in the fasted state and in response to AA infusion in pediatric patients with severe burns at discharge from hospital, and at six and twelve months post injury.
Methods
Patients
Patients admitted to Shriners Hospitals for Children - Galveston with major burns covering ≥ 40 % of their total body surface area (TBSA), were recruited for the present study. The study protocol was approved by the University of Texas Medical Branch Institutional Review Board. Informed consent was obtained from all patients and their guardians. These patients did not receive any experimental drug interventions nor did they participate in a rehabilitative exercise program.
Standard burn care
Upon admission patients were resuscitated with lactated Ringers solution according to the Galveston formula. Burn wounds were excised and closed with donor skin within 48hrsof admission. Grafting was repeated once donor site wounds had healed, until burn wounds were considered to be 95% healed, at which point patients were typically discharged. During their stay in the intensive care unit patients were fed VIVONEX® total enteral nutrition (Nestlé Health Care Nutrition, Minneapolis, MN) which provided macronutrients in the following formulation: 83% carbohydrate, 15% protein, 2% fat. VIVONEX® was administered in order to provide calories which amounted to 1.4 times the patients resting energy expenditure, which was calculated weekly via indirect calorimetry. Once patients had been discharged from intensive care and had returned to consuming solid foods, their diet was supplemented with nutritional drinks (Boost, Nestlé Health Care Nutrition, Minneapolis, MN), to insure that caloric intake amounted to 1.4 times the calculated resting energy expenditure. This supplementation continued until patients were able to achieve sufficient nutrition through diet alone.
Stable isotope infusion trials
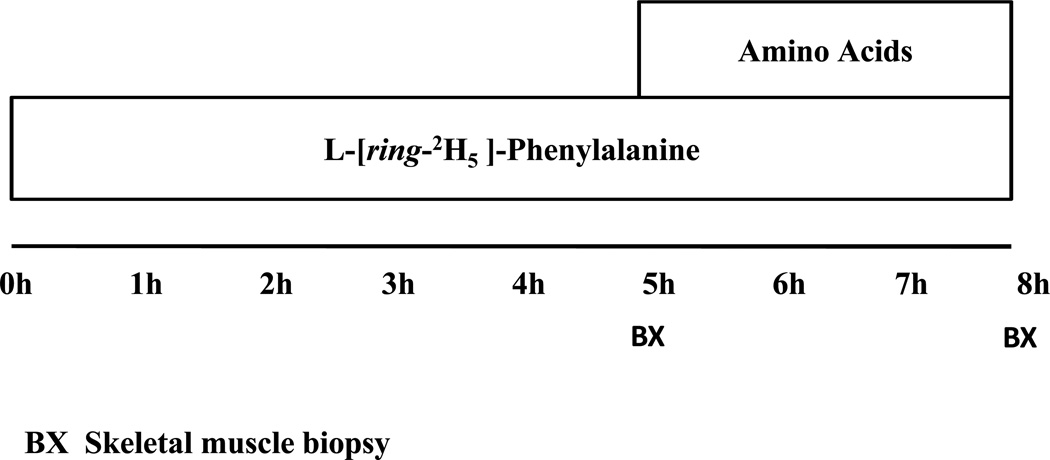
Eight hour infusions of isotopically labeled phenylalanine were performed following an overnight (6–12 hours) fast. Sedated patients (ketamine 4mg·kg−1 intramuscularly) had indwelling catheters placed in an antecubital vein of each arm for the purposes of stable isotope infusions and blood sampling. A baseline blood sample was obtained for background enrichments prior to a primed (3.2 µmol·kg−1) constant (0.08 µmol·kg−1min−1) infusion of L-[ring-2H5]-phenylalanine (Cambridge Isotopes, Andover, MA) being initiated. A schematic overview of this particular stable isotope infusion protocol is depictedin Figure 1. This protocol allows the determination of skeletal muscle FSR in the both the fasted state and during the concurrent infusion of AA. The initial 5 hours of the infusion protocol consisted of a primed constant infusion of L-[ring-2H5]-phenylalanine alone, during the last three hours of the infusion trial a primed (0.45 ml·kg−1) continuous infusion (1.35 ml·kg−1 hr−1) of a 10% Travasol solution (Clintec Nutrition, Deerfield, IL) was also administered to allow the determination of skeletal muscle FSR in response to AA infusion. This 10% Travasol solution contained100 mg·ml−1of AAs in the following composition: leucine (7.3 mg·ml−1); isoleucine (6 mg·ml−1); lysine (5.8 mg·ml−1); valine 5.8 mg·ml−1);phenylalanine (5.6 mg·ml−1);histidine (4.8 mg·ml−1); threonine (4.2 mg·ml−1); methionine (4 mg·ml−1); tryptophan (1.8 mg·ml−1); alanine (20.7 mg·ml−1); arginine(11.5 mg·ml−1); glycine (10.3 mg·ml−1);proline (6.8 mg·ml−1); serine (5 mg·ml−1); and lysine (0.4 mg·ml−1).
Figure 1.
An overview of the stable isotope infusion protocol.
In order to determine skeletal muscle FSR, skeletal muscle biopsies were taken from the vastus laterials muscle at two, five and eight hours using a suction adapted 5 mm Bergstrom needle (Stille, Stockholm Sweden)23. Muscle biopsies were rinsed with cold salineand frozen in liquid nitrogen. Muscle samples were subsequently stored at −80°C and analyzed at a later date. Patients also had full body dual emission X-ray absorptiometry scans at as part of their follow-up hospital visits to determine body composition.
Skeletal muscle analysis
Twenty mg of frozen muscle tissue was weighed before being homogenized by a mechanical tissue grinder in 10% perchloric acid (PCA). The supernatant was subsequently recovered following centrifugation (3000rpm for 10 min at 4°C) and stored at −80°C. This supernatant represents the intracellular (IC) pool of AAs and thus is used to determine IC phenylalanine enrichment. The remaining muscle pellet was washed in 2% PCA, followed by ethanol and finally ethyl ether before being oven dried overnight at 50°C. The next day, dried muscle pellets were hydrolyzed in hydrochloric acid (6M) at 100°C for 12 hours. This hydrolysate, representing the bound protein (BP) pool of AAs was subsequently used to determine the enrichment of bound phenylalanine. At a later date, IC and BP muscle AA fractions were separated in cation exchange columns (200–400 mesh resin with H+ as its counter ion) (BioRad, Hercules CA). Plasma phenylalanine enrichment (tracer/tracee ratio) was determined in tert-butyldimethylsilyl derivatives by Gas Chromatography-Mass Spectrometry (GC-MS) with electron impact ionization (Agilent, Santa Clara CA) as previously described24.
Calculations
Skeletal muscle FSR was calculated using the precursor product method originally described elsewhere24. In this calculation, the precursor is the mean enrichment of the intracellular free AA compartment (EIC), and the product is the difference in enrichment of the bound protein compartment of the two biopsies (EBP) (equation 1). In this study, skeletal muscle FSR is expressed as percent per hour (%·hr−1) and percent per day (%·day−1).
| Equation 1 |
| Equation 2 |
Data and statistical analysis
Data are reported as group means ± SEM unless stated otherwise. Statistically significant differences between basal and AA FSR values were detected by a paired t test (Graphpad Prism version 5, La Jolla CA). To account for overlap among subjects at each time point, a mixed effects model was fit using subject as a random blocking factor. All mixed models were fit in SAS (version 9.2). Statistical significance was accepted when P<0.05.
Results
Patient demographics
Data from a total of 40 stable isotope infusion trials was included for analysis in this current study where patients had been randomized to not receive any investigational pharmacological therapy. In total, 20 patients were studied as part of the current investigation, where six patients were studied at all three time points post injury, seven patients were studied at two of the three time points post injury, and seven patients were studied at one time point post injury. A total of 11 patients were studied at discharge from hospital, 15 patients were studied approximately six months post injury, and 14 patients were studied approximately twelve months post injury. The mean number of days post burn for each group is presented in table 1. The mean age, body mass and body composition were not different between cohorts. In addition, patients in all three groups had large burns, covering >50% of their total body surface area (TBSA) (table 1).
Table 1.
Patient demographics
| Group demographics (mean±SD) | |||
|---|---|---|---|
| Discharge (n=11) |
6 months post burn (n=15) |
12 months post burn (n=14) |
|
| Age (years) | 3.5±2.4 | 3.8±3.2 | 3.6±1.5 |
| Gender (male/female) | 5/6 | 8/8 | 7/8 |
| Height (cm) | 98.7±17.9 | 100.9±18.3 | 98.0±12.4 |
| Body mass (kg) | 15.3±5.5 | 18.1±10.9 | 16.0±4.4 |
| Fat free mass (kg) | 11.8±4.8 | 12.9±6.6 | 11.7±3.8 |
| Fat mass (kg) | 3.7±0.9 | 4.7±4.6 | 4.2±1.3 |
| Burn size (%TBSA) | 59±9 | 58±11 | 59±10 |
| 3rd degree burn (%TBSA) | 51±11 | 51±14 | 39±21 |
| Days post burn | 40±1 | 190±20 | 369±18 |
Skeletal muscle FSR
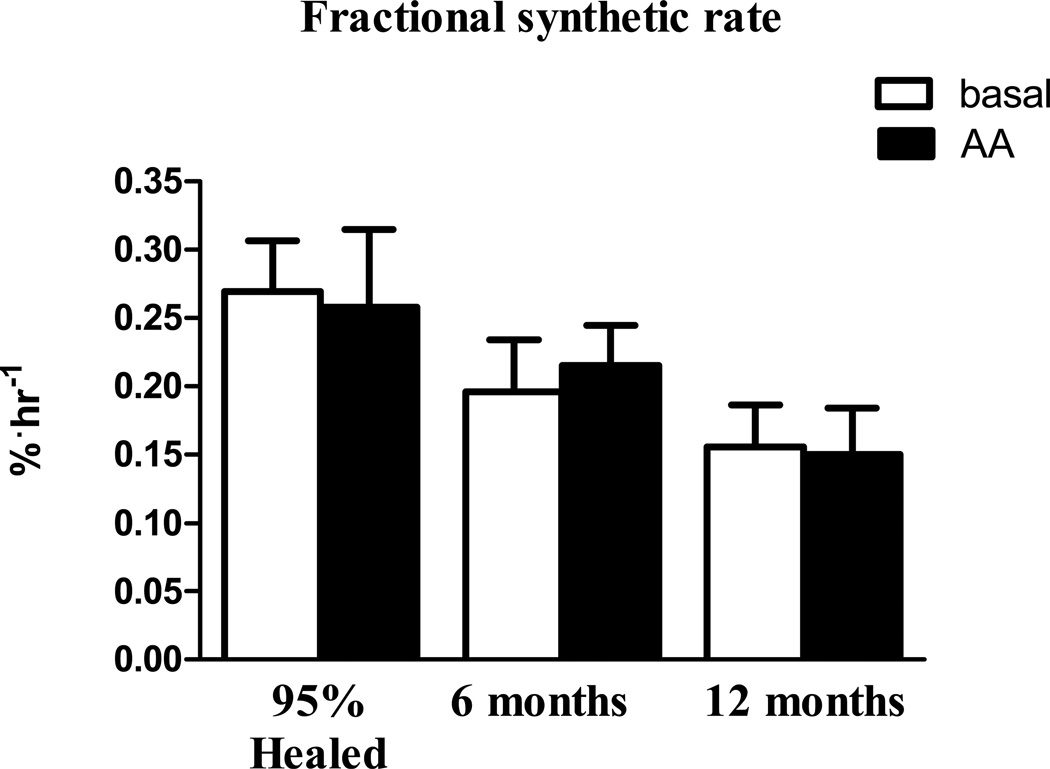
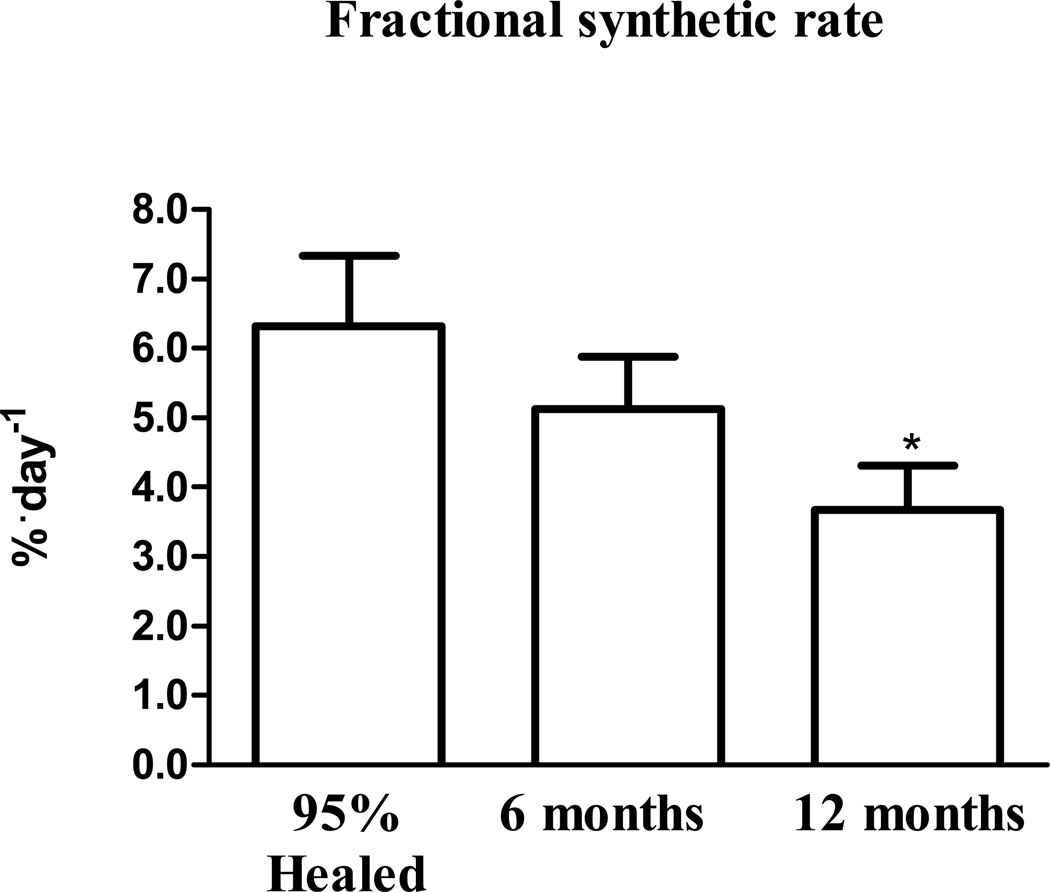
Skeletal muscle FSR in the basal state and in response to AAs in all 3 cohorts of patients (95% healed, six months post burn, and twelve months post burn) are presented in figure 1. Skeletal muscle FSR was unaltered by AA infusion at discharge from hospital (95% healed) (0.27±0.04 vs. 0.26±0.06 %·hr−1), six months post injury (0.20±0.04 vs. 0.22±0.03 %·hr−1), and twelve months post injury (0.16±0.03 vs. 0.15±0.05 %·hr−1). As FSR was not altered by AA infusion a mean of basal + AA FSRs were calculated for each patient and multiplied by 24 for to calculate the mean daily FSR. Mean daily FSR (%·day−1) was highest at discharge (6.32±1.02 %·day−1) and was numerically lower at six months post burn (5.51±0.79 %·day−1) which reached significance (P<0.05) at twelve months post burn when compared to the discharge group (3.67±0.65 %·day−1).
Discussion
Severe burn injury results in profound muscle wasting. It is generally thought that skeletal muscle protein breakdown and synthesis are both elevated post burn, where breakdown outweighs synthesis resulting in net loss of muscle proteins, which in time leads to severe cachexia. In the first few weeks post burn, increasing dietary protein intake does not further increase skeletal muscle FSR15, most probably because intracellular amino acid concentrations are already elevated as a result of muscle protein catabolism5. However, whether this apparent skeletal muscle anabolic resistance to AAs persists for a prolonged period of time post burn is not known. Subsequently, in this current study skeletal muscle FSR was determined in the basal state and during AA infusion in children with severe burns at discharge from hospital as well as six and twelve months post injury. The main finding of this current study was that AA infusion fails to stimulate skeletal muscle FSR in severely burned children for up to one year post injury.
It is generally accepted that skeletal muscle acts as a reservoir of AAs which are mobilized post burn to facilitate wound healing. Indeed, Gore and colleagues reported that skeletal muscle protein breakdown rates were almost identical to wound protein synthesis rates in severely burned patients, where net protein balance in skeletal muscle was profoundly negative whereas net protein balance in the wound was profoundly positive6. Moreover, while increasing protein intake does not appear to increase skeletal muscle FSR in the first two weeks following burn injury, higher dietary protein intake was positively correlated with skin protein FSR15. Taken together, these two studies clearly highlight that endogenous protein stores (skeletal muscle) and exogenous protein intake are both preferentially disposed of by skin for wound healing, at least in the acute period post burn. In addition, our group has previously shown that amino acid infusion increased the rate of phenylalanine disappearance across the leg of severely burned children22. However, the rate of phenylalanine appearance from the leg was also elevated during AA infusion to the extent that protein balance was not altered. This led these researchers to suggest that skeletal muscle was anabolically unresponsive to AAs.
Here we have shown that the apparent anabolic resistance of skeletal muscle to AA infusion persists long after wound closure and discharge from hospital, where AA acids to do not increase skeletal muscle FSR at one year post injury. While the term anabolic resistance is generally ascribed to an impaired synthetic response in skeletal muscle of elderly individuals to amino acids25, it is perhaps incorrect to describe the skeletal muscle of severely burned children as being anabolic resistance to AAs. For example, in elderly individuals described as being anabolically resistant, AAs fail to significantly increase skeletal muscle FSR above basal values, which are typically in the magnitude of 0.05 %·hr−1. Indeed, in healthy individuals, AA intake tends to double skeletal muscle FSR from approximately 0.05 to 0.10 %·hr−110 While AA infusion did not significantly increase skeletal muscle FSR in severely burned patients at all three distinct time point post burn in this current study, basal FSR values ranged from 0.27±0.04 %·hr−1at discharge to 0.16±0.03 %·hr−1at twelve months post injury, which are approximately 5- and 3-fold greater than values typically reported in healthy individuals. If protein breakdown, and the resultant increase in intracellular AA concentration does indeed lead to a concurrent increase muscle protein synthesis, this may offer a mechanistic insight as to why AA infusion fails to further augment skeletal FSR in severely burned children. Indeed, in the acute setting (15 days post burn), muscle proteolysis (83%) and synthesis (50%) determined by the 3 compartment arterio-venous balance method were significantly higher in burned patients compared to healthy volunteers, as was skeletal muscle FSR (49%) determined by the precursor product method5. Furthermore, the intracellular phenylalanine and leucine concentrations were 43% and 74% greater in burned patients’ versus controls, suggesting that acutely post burn, skeletal muscle FSR is stimulated by increased muscle proteolysis and a subsequent rise in intracellular AA concentrations. Indeed, in healthy humans, leucine is known to be a potent stimulator of muscle protein synthesis, further supporting the notion that increased amino acids availability, perhaps most importantly leucine concentrations, in the muscle cell post burn directly stimulate FSR.
While skeletal muscle FSR values appear to be profoundly elevated post burn5, 6, 11−15, our current data suggests that intracellular AA availability may not be limiting to skeletal muscle FSR for up to one year post injury. While our group has previously shown that pharmacological interventions such as propranolol11and oxandrolone17, 19 further increase skeletal muscle protein synthesis while improving muscle protein net balance, the current data suggests that AA availability within skeletal muscle is not the limiting factor to protein synthesis, as AA infusion does not augment FSR. That said, basal FSR declines from discharge to 12 months post injury, suggesting the stimulus for elevated FSR declines with time post injury. Asskeletal muscle FSR remains well above values typically associated with healthy individuals for twelve months post burn suggests that increased muscle protein breakdown is also present. However, a limitation of this current study is that only protein synthesis was measured, and without a measure of breakdown we cannot definitively say that protein breakdown is also elevated for 12 months post burn, and subsequently cannot comment on the effects of AA infusion on muscle protein breakdown or net balance post burn. Indeed, in light of our current data, one can perhaps reasonably assume that if AA infusion does indeed positively impact skeletal muscle mass in burned patients it must do so by attenuating muscle protein breakdown. Subsequently, studies of the effects of AA infusion on indices of protein breakdown and net balance in severely burned patients are warranted.
Assuming that FSR reflects elevated muscle protein breakdown post burn, our data suggests that protein breakdown, to an extent that results in a concurrent increase in FSR is present for up to twelve months post burn. Indeed, when expressing FSR as %·day−1, and assuming that this is a conservative proxy of skeletal muscle protein turnover, it is apparent the skeletal muscle protein turnover is profoundly elevated at discharge from hospital, six and twelve months post burn. For example, assuming FSR values typically range from 0.05–0.10 %·hr−1throughout the day in healthy individuals, this would represent a daily FSR of 1.2–2.4 %·day−1. Taking this as a marker of skeletal muscle protein turnover in healthy individuals, it is clear that protein turnover is profoundly elevated in burned children particularly at discharge (6.32±1.02 %·day−1) and sixmonths post burn (5.51±0.79 %·day−1). This finding is of clinical significance as it suggests that protein requirements of patients recovering from severe burns remain elevated long after discharge form hospital. For example, while chronically elevated FSR, presumably as a result of increased skeletal muscle protein turnover post burn is not augment by AA infusion, it is quite possible that exogenous AA provision may attenuate skeletal muscle catabolism, ultimately preserving muscle mass. However, to accept or refute this aforementioned statement, further detailed studies investigating the impact of AA infusion on skeletal muscle anabolism and catabolism are needed. However, daily FSR was significantly lower at 12 months post burn relative to discharge (95% healed), suggesting that the stimulus for increased protein turnover does decline with time after discharge.
In summary, here we have shown that a 3 hrAA infusion fails to further augment skeletal muscle FSR from basal in severely burned pediatric patients up to one year post injury. Our data suggests that skeletal muscle protein metabolism remains perturbed long after patients are discharge from hospital, which suggest that strategies aimed and preserving existing muscle mass and even increasing muscle mass and function need to be integral parts of patient rehabilitation. In particular, in light of our current findings, the impact of AA acid infusion on skeletal muscle protein breakdown and net balance remains to be determined. Until a concurrently detailing of the effects of AA infusion(alone, or in combination with other macronutrients or pharmacological interventions), on both muscle protein synthesis and breakdown, recommendations concerning the efficacy of increased amino acid provision in severely burned patients cannot be made. However, as protein turnover remains elevated for up to one year post burn it would be reasonable to suggest that these patients require additional dietary protein in order to avoid excessive muscle catabolism.
Figure 2.
Skeletal muscle FSR in the basal state and during AA infusion at three distinct time points post burn. FSR is presented as %·hr−1. Values are group means ± SEM.
Figure 3.
Daily skeletal muscle FSR calculated from the mean of the basal and AA period FSR values from each patient. FSR is presented as %·day−1. Values are group means ± SEM. * different from 95% healed (P<0.05).
Acknowledgements
We would like to thank the patients who participated in this study; the clinical research staff at Shriners Hospitals for Children - Galveston for assisting during stable isotope infusion trials; the research staff of the Metabolism Unit, Shriners Hospitals for Children – Galveston, for performing sample preparation and GC-MS analysis. This study was funded by contributions from the following National Institutes of Health (P50-GM60338, RO1-GM056687 and T32-GM08256) and Shriners of North America (84090, 84080, 71006, 71008 and 79135) grants. CP is partly supported by an Interdisciplinary Rehabilitation Research Postdoctoral Training Grant (H133P110012) from the National Institute of Disability and Rehabilitation Research and Department of Education.
Footnotes
Author contributions
DNH and EB contributed to the study design. CP, MC and ED researched the data. CP, MC, ED, KJ, DNH and EB interpreted the data. CP wrote the manuscript. CP, MC, ED, KJ, DNH and EB critically revised the manuscript.
Contributor Information
Craig Porter, Metabolism Unit, Shriners Hospitals for Children – Galveston, Department of Surgery, University of Texas Medical Branch, Galveston, Texas, cr2porte@utmb.edu.
Matthew Cotter, University of Texas Medical Branch.
Eva C Diaz, University of Texas Medical Branch.
Kristofer Jennings, University of Texas Medical Branch.
David N Herndon, University of Texas Medical Branch.
Elisabet Børsheim, University of Texas Medical Branch.
References
- 1.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Børsheim E, Chinkes DL, McEntire SJ, et al. Whole body protein kinetics measured with a non-invasive method in severely burned children. Burns. 2010;36:1006–1012. doi: 10.1016/j.burns.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prelack K, Yu YM, Dylewski M, et al. The contribution of muscle to whole-body protein turnover throughout the course of burn injury in children. J Burn Care Res. 2010;31:942–948. doi: 10.1097/BCR.0b013e3181f938e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biolo G, Fleming RYM, S P, Nguyen TT, et al. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87:3378–3384. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 6.Gore DC, Chinkes DL, Wolf SE, et al. Quantification of protein metabolism in vivo for skin, wound, and muscle in severe burn patients. JPEN J Parenter Enteral Nutr. 2006;30:331–338. doi: 10.1177/0148607106030004331. [DOI] [PubMed] [Google Scholar]
- 7.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 9.Jahoor F, Desai M, Herndon D, et al. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988;37:330–337. doi: 10.1016/0026-0495(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 10.Tipton KD, Borsheim E, Wolf SE, et al. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab. 2002;284:76–89. doi: 10.1152/ajpendo.00234.2002. [DOI] [PubMed] [Google Scholar]
- 11.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 12.Tuvdendorj D, Chinkes DL, Zhang XJ, et al. Adult patients are more catabolic than children during acute phase after burn injury: a retrospective analysis on muscle protein kinetics. Intensive Care Med. 2011;37:1317–1322. doi: 10.1007/s00134-011-2223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gore DC, Wolf SE, Sanford AP, et al. Extremity hyperinsulinemia stimulates muscle protein synthesis in severely injured patients. Am J Physiol Endocrinol Metab. 2004;286:529–534. doi: 10.1152/ajpendo.00258.2003. [DOI] [PubMed] [Google Scholar]
- 14.Ferrando AA, Chinkes DL, Wolf SE, et al. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229:11–18. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson BW, Nguyen T, Pierre E, et al. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism. 1997;46:573–578. doi: 10.1016/s0026-0495(97)90196-7. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 17.Ferrando AA, Sheffield-Moore M, Wolf SE, et al. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001;29:1936–1942. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Gore DC, Wolf SE, Sanford A, et al. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Ann Surg. 2005;241:334–342. doi: 10.1097/01.sla.0000152013.23032.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–564. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–720. doi: 10.1097/00000658-199905000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai Y, Aarsland A, Herndon DN, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222:283–294. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuvdendorj D, Chinkes DL, Zhang XJ, et al. Skeletal muscle is anabolically unresponsive to an amino acid infusion in pediatric burn patients 6 months postinjury. Ann Surg. 2011;253:592–597. doi: 10.1097/SLA.0b013e31820d9a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. (Bergström J) [PubMed] [Google Scholar]
- 24.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research. New Jersey: John Wiley & Sons, Inc., publication; 2005. [Google Scholar]
- 25.Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl Physiol Nutr Metab. 2009:377–381. doi: 10.1139/H09-012. [DOI] [PubMed] [Google Scholar]