Abstract
Amelogenins are the most abundant extracellular matrix proteins secreted by ameloblasts during tooth development and are important for enamel formation. Recently, amelogenins have been detected not only in ameloblasts, which are differentiated from the epithelial cell lineage, but also in other tissues, including mesenchymal tissues at low levels, suggesting that amelogenins possess other functions in these tissues. The therapeutic application of an enamel matrix derivative rich in amelogenins resulted in the regeneration of cementum, alveolar bone, and periodontal ligament (PDL) in the treatment of experimental or human periodontitis, indicating the attractive potential of amelogenin in hard tissue formation. In addition, a full-length amelogenin (M180) and leucine-rich amelogenin peptide (LRAP) regulate cementoblast/PDL cell proliferation and migration in vitro. Interestingly, amelogenin null mice show increased osteoclastogenesis and root resorption in periodontal tissues. Recombinant amelogenin proteins suppress osteoclastogenesis in vivo and in vitro, suggesting that amelogenin is involved in preventing idiopathic root resorption. Amelogenins are implicated in tissue-specific epithelial-mesenchymal or mesenchymal-mesenchymal signaling; however, the precise molecular mechanism has not been characterized.
In this review, we first discuss the emerging evidence for the additional roles of M180 and LRAP as signaling molecules in mesenchymal cells. Next, we show the results of a yeast two-hybrid assay aimed at identifying protein-binding partners for LRAP. We believe that gaining further insights into the signaling pathway modulated by the multifunctional amelogenin proteins will lead to the development of new therapeutic approaches for treating dental diseases and disorders.
Keywords: amelogenin, yeast two-hybrid, M180, leucine-rich amelogenin peptide(LRAP), signaling molecules
Amelogenin Gene Structure, Splice Isoform, and Proteins
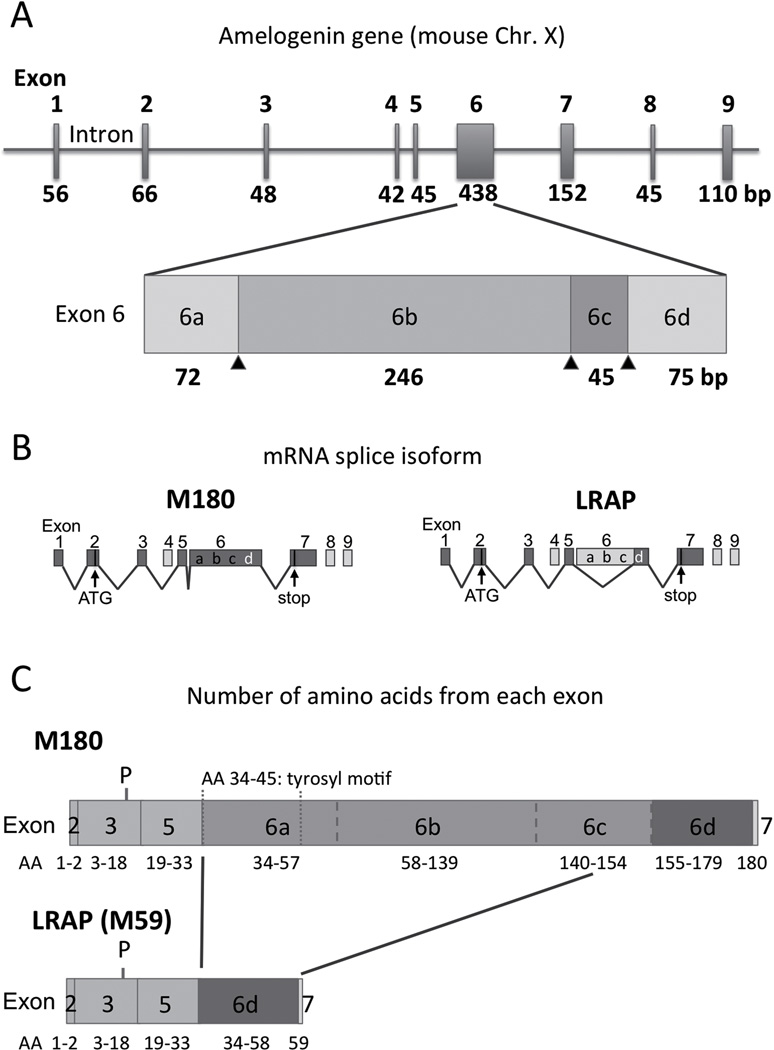
Amelogenins are the most abundant extracellular matrix proteins secreted by ameloblasts1), and are believed to play critical roles in enamel formation and mineralization. Human chromosome 4 contains a cluster of enamel matrix genes that are located immediately proximal to a cluster belonging to the dentin-or bone-related SIBLING genes. By contrast, amelogenin genes are located on the sex chromosomes, and are highly conserved in at least 26 mammalian species2). Amelogenin genes probably migrated from another locus to preserve their function in an ancient evolutionary process. Amelogenin genes in some species, including bovine and human, are located on both X (Amelx) and Y (Amely) chromosomes. In humans, the number of protein transcripts from AMELY is about 10% of the amount of AMELX3). Mice have only a single Amelx gene and no corresponding gene on the Y chromosome4). The Amelx gene contains seven exons, although exon 4 is not found in some species. In humans and rodents, additional exons 8 and 9 were recently identified (Fig. 1) 5). At least 16 mRNAs are generated from the mouse Amelx gene in ameloblasts via alternate splicing6–8). Exon 6 is the largest and it contains three internal splice acceptor sites (Fig. 1A; a–c in exon 6). The most abundant amelogenin isoform in mice is the product of exons 2, 3, 5, 6, and 7, which is named M180 and consists of 180 amino acids9). Leucine- rich amelogenin peptide (LRAP), also known as M59 or A-49), is the second most abundant isoform, which results from a major cryptic splice deletion at the 5’-end of exon 6 (Fig. 1B and C) 10). Amelogenin proteins undergo sequential proteolysis by matrix metalloproteinase 20 (MMP20) and kallikrein 4 (KLK4) during enamel secretion and maturation11–13), resulting in highly heterogeneous proteins in the enamel matrix. The N-terminal region includes a phosphorylation site at serine 1614), and a tyrosyl motif that binds N-acetylglucosamine and keratins15–17), which may alter nanosphere formation and the biological functions of amelogenin as a matrix or enamel mineralization (Fig. 1C) 18).
Fig. 1.
Amelogenin gene architecture, splice isoforms, and proteins in the mouse. (A) The amelogenin gene (Amelx) consists of nine exons. There are three alternative acceptor sites (arrowheads) in exon 6. (B) The two major splicing isoforms of the amelogenin gene: M180 and LRAP. Most transcripts are transcribed from exons 1 to 7 and skip exons 4, 8, and 9. Exon 2 consists of a Kozak consensus sequence in addition to an ATG start codon. A stop codon is located as the second codon of exon 7. (C) M180 and LRAP (M59) amelogenin proteins. The initial 16 amino acids (AAs) at the N-terminus are signal peptides and are removed before secretion.
Mature amelogenins retain only two AAs from exon 2 and one AA from exon 7. The serine at position 16 from the N terminus is phosphorylated. LRAP lacks a tyrosyl motif and the central hydrophobic part in M180, which are translated from exon 6a, b, and c. The terminology for exon 6 splicing has been published previously. 6,9)
The Role of Amelogenin in Periodontal Tissues: Emerging Evidence for the Additional Roles of M180 and LRAP
Although many reports have suggested that amelogenins play critical roles in proper enamel formation and are responsible for human amelogenesis imperfecta (AI), the functions of each amelogenin isoform have not been clearly delineated because of their heterogeneity. In order to elucidate the functions of amelogenin, Gibson et al. generated amelogenin null mice with a targeted deletion in the Amelx gene19). The teeth from these null mice show a chalky-white discoloration, resembling human hypoplastic AI. To understand the role of individual amelogenin isoforms in enamel formation, transgenic mouse lines were generated that expressed a single amelogenin, e.g., M180 or LRAP, under the control of the bovine amelogenin promoter. These were subsequently mated with amelogenin null mice to reintroduce the specific isoform into ameloblasts. The report indicated that M180 could partially rescue the hypoplastic phenotype seen in amelogenin null mice, but that LRAP could not20) (see Gibson’s review in this volume). The amelogenin splicing isoforms may play distinct roles in enamel formation. Therapeutic applications of an enamel matrix derivative (Emdogain; Straumann, Switzerland) rich in amelogenins have been reported to result in the regeneration of cementum, alveolar bone, and periodontal ligament (PDL) in the treatment of experimental or human periodontitis21–24), indicating the attractive potential of amelogenin in hard tissue formation. Interestingly, amelogenin expression outside of enamel tissues, such as in periodontal tissues, cementoblasts, PDL cells, or Hertwig’s epithelial root sheath (HERS), has been reported in studies utilizing immunohistochemistry or in situ hybridization25–28). Using reverse transcription-polymerase chain reaction (RTPCR), we also detected two amelogenin alternative splice isoforms, M180 and LRAP, in molar cementoblast/PDL (CM/PDL) cells from wild-type mice29). These expression patterns imply that amelogenin is involved in cementum formation and/or in the homeostasis of periodontal tissue through unknown mechanisms. Therefore, we further investigated periodontal tissues in Amelx null mice and observed the increased formation of cementicles, irregular calcified globules on the root surface, as compared to the wild type. Most surprisingly, the area of root resorption and number of osteoclasts (odontoclasts) on the root surface were increased significantly in 6–12-monthold mice in the Amelx null mouse tooth root. The loss of amelogenin from the cementoblasts or epithelial cell rests of Malassez in periodontal tissues may lead to cementum defects and increased numbers of osteoclasts by changing the RANK-RANKL pathway in Amelx null mice29). Moreover, the co-culture of wildtype osteoclast progenitors with Amelx null CM/PDL cells resulted in increased tartrate-resistant acid phosphatase (TRAP)-positive cell formation30). This was abolished by adding recombinant porcine LRAP to the medium, but not by adding P172 (porcine analogue of M180). In a rat root resorption model, the application of P173 (P172 plus an initial methionine), LRAP, and enamel matrix derivative to tooth roots markedly reduced the number of odontoclasts on root surfaces and inhibited cementum and root dentin resorption31). In addition, LRAP mediated the induction of the migration and proliferation of CM/PDL cells30). Although P172 also induced migration and proliferation, it had a less significant effect than LRAP. These findings suggest that amelogenin protects the cementum from abnormal osteoclastogenesis, and that LRAP, and to some extent full-length amelogenin, is important for the development and maintenance of periodontal tissues.
Screening Potential Protein-Binding Partners for LRAP
As reviewed above, amelogenins, especially LRAP, may possess cell signaling roles in several types of cell; however, it remains unclear how amelogenins affect cell signaling. To determine whether LRAP has unknown receptors or binding proteins that are involved in the regulation of osteoclastogenesis and bone mineralization as potential signaling transducers, we performed large-scale screening using a yeast two-hybrid system (Matchmaker GAL4 Two- Hybrid System 3; Clontech, CA, USA).
In experiments, two different cDNA libraries were screened as potential sources: a human bone marrow (hBone marrow) premade cDNA library (Clontech) and a mouse CM/PDL cell custom cDNA library. For the hBone marrow library screening, the AH109 yeast strain transformed by a pGBKT7-mLRAP bait vector, which expresses mouse LRAP protein in yeast, was mated with a Y187 yeast strain that had been transformed with the hBone marrow cDNA library. The CM/PDL cell cDNA library was constructed from cultured primary mouse CM/PDL cells according to the manufacturer’s protocol (Matchmaker SMART cDNA Library Construction & Screening Kits; Clontech). The synthesized first-strand cDNA was amplified by long-distance PCR in order to generate sufficient double-stranded cDNA. Then, the double-stranded cDNA, pGADT7-Rec prey vector, and pGBKT7-mLRAP bait vector were co-transformed into the AH109 yeast strain so that in vivo homologous recombination generated pGADT7-Rec-cDNA library clones in the yeast and the screening could be performed simultaneously in one step. The mated mixture for hBone marrow or the cotransfected mixture for mCM/PDL was plated on 50 150-mm ager plates with nutritional selection medium (SD/ -Ade/ -His/ -Leu/ -Trp), respectively, followed by stringent screening for transformants expressing interacting proteins. After 7 days, the growing colonies were inoculated on fresh SD/-Ade/-His/-Leu/-Trp/X-α -Gal master plates and cultured for an additional 4–7 days to identify the positive interacting protein pairs that activated the MEL1 reporter gene, which encodes α-galactosidase.
In hBone marrow and mCM/PDL screening, 500 and 213 blue colonies were picked up from each master plate, respectively. We amplified the library inserts by PCR and analyzed the PCR products and their fragment sizes when digested with the four-cutter restriction enzyme AluI. Agarose gel electrophoresis revealed that 240 and 180 clones were unique clones in each respective library. The plasmids containing cDNA from the library were recovered and sequenced. Among the unique clones in each library, ~40 and ~50 colonies, respectively, contained an open reading frame fused to the activation domain sequence, indicating that the proper fusion proteins were made in these clones. The number of clones screened was estimated by counting the number of growing colonies on an SD/-Leu/-Trp test plate. We screened 7.0×106 and 4.2×106 clones from two rounds of hBone marrow library and mCM/PDL screenings, respectively, which should be sufficient to represent every gene for most organisms.
LRAP Interacting Proteins in the Bone Marrow and CM/PDL
Based on the results of the yeast two-hybrid screening, potential candidates for binding partners of LRAP were identified, including cell membrane proteins, enzymes, and extracellular matrix. The interacting proteins that fit our search criteria are listed in Tables 1 and 2. From hBone marrow library screening (Table 1), we found that LRAP bound to an immunoglobulin (Ig)-like domain in several different molecules, which may be involved in the immune system, oxidase-like family were identified as common candidates in both the hBone and mCM/PDL libraries. Here, we will discuss a few promising candidates.
Table 1.
LRAP interacting proteins in human bone marrow.
| Gene accession no. |
Abbreviation | Details |
|---|---|---|
| NM_002483 | CEACAM6 | Carcinoembryonic antigen-related cell adhesion molecule 6. Seven clones: AA135–344 (stop), 157–344 (stop) and full clones incl. immunoglobulin domains and asparagine-linked glycosylation sites. |
| NM_006864 | LILRB3 | Leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 3 (LILRB3), transcript variant 2 (mouse homologue of PIR-B). Two clones: AA 256–631 (stop) incl. immunoglobulin domains. |
| NM_032603 | LOXL3 | Lysyl oxidase like-3. AA 365–3121 (stop) incl. scavenger receptor cysteine-rich (a family reminiscent of the immunoglobulin superfamily) domain. |
| NM_004867 | ITM2A | Integral membrane protein 2A. Full clone incl. BRICHOS domain. |
| NM_002778 | PSAP | Prosaposin. AA 422–526 (stop) incl. saposin-like type A and B region. |
| NM_002205 | ITGA5 | Integrin alpha 5 (fibronectin receptor, alpha polypeptide). Full clone. |
| NM_001909 | CTSD | Cathepsin D. AA 295–412(stop). |
List of LRAP interacting proteins in human bone marrow.
NCBI (http//www.ncbi.nlm.nih.gov/nuccore) gene accession number, abbreviations of the gene name, and clone details including the protein portion isolated in yeast two-hybrid screening are listed. The clones in bold are discussed in this manuscript. A full clone includes an entire open reading frame. stop, stop codon: AA, amino acid.
Table 2.
LRAP interacting proteins in mouse CM/PDL.
| Gene accession no. |
Abbreviation | Details |
|---|---|---|
| NM_010729 | Loxl1 | Lysyl oxidase-like 1. Seven clones: AA 259–607 (stop), 430–607 (stop), and 496–607 (stop) incl. lysyl-oxidase superfamily domain. |
| NM_007743 | Col1a2 | Collagen, type I, alpha 2. Two clones: AA 576–1372 (stop) 1178–1372 (stop) incl. fibrillar collagen C-terminal domain. |
| NM_007737 | Col5a2 | Collagen, type V, alpha 2. Two clones: AA 935–1497 (stop) and full clone. |
| NM_019986 | Habp4 | Hyaluronic acid binding protein 4. Full clone. Also known as Ki-1/57, probably involved in cellular events, such as pre-mRNA splicing. |
| NM_008410 | Itm2b | Integral membrane protein 2B. AA 105–206 (stop) incl. BRICHOS domain. |
| NM_010227 | Flna | Filamin alpha. AA 2560–2639 (stop) of filamin alpha transcript variant 1 incl. filamin tandem sequence repeats that are predicted to have immunoglobulin-like folds. |
| NM_010860 | Myl6 | Myosin light polypeptide 6. Full clone incl. EF-hand, calcium binding motif. |
| NM_001164352 | Efemp2 | EGF-containing fibulin-like extracellular matrix protein 2 isoform 2. AA 140–370 (stop) incl. calcium-binding EGF-like domain and Von Willebrand factor type A (vWA) domain. |
List of LRAP interacting proteins in mouse CM/PDL.
NCBI (http://www.ncbi.nlm.nih.gov/nuccore) gene accession number, abbreviations of gene name, and clone details including the protein portion isolated in the yeast two-hybrid screening are listed. The clones in bold are discussed in this manuscript. Full clone includes an entire open reading frame. stop, stop codon: AA, amino acid
The carcinoembryonic antigen (CEA)-related cell adhesion molecule 6 (CEACAM6, also known as CD66c), a human homologue of mouse CEACAM1 isoform 2, is a gene located on human chromosome 19q13.2. The glycosylphosphatidylinositol (GPI)- anchored membrane protein CEACAM6 belongs to the Ig superfamily and appears as a highly glycosylated protein with the typical N-terminal variable Ig like domain followed by 0 to 6 constant Ig-like domains. CEACAMs are expressed in normal epithelium, angiogenically activated endothelium, and hematopoietic cells32,33) The cytoplasmic domain of CEACAM1-3/4 L possesses an immunoreceptor tyrosine-based inhibition motif (ITIM), which contains two tyrosine residues that can recruit and activate SH2 domain-containing tyrosine kinases and tyrosine phosphatases34). Interestingly, there are now convincing data showing that CEACAMs and several of the human GPI-linked molecules participate in signal transduction; however, no GPI-linked proteins belonging to the CEA family are expressed in rodents32,35), leading to difficulty in determining the functions of GPI-linked CEA family members. Possible functions of CEACAM family members are cell adhesion, tumor suppression, regulation of lymphocyte and dendritic cell activation, and acting as a receptor of bacteria, including Neisseria species.
Leukocyte immunoglobulin-like receptor, subfamily B (LILR-B) is a mouse homologue of paired immunoglobulin-like receptor B (Pir-b), located on human chromosome 19q13.4. PIR-B and four isoforms of LILR-B, harboring ITIM inhibitory domains, constitutively recruit SHP-1 in the presence of RANKL and M-CSF on cultured osteoclast precursor cells, suggesting that some can suppress osteoclast development in vivo and in vitro36). Indeed, co-stimulatory signals mediated by the ITAM (immunoreceptor tyrosine-based activation motif) cooperate with RANKL to achieve bone homeostasis. FcRγ and DAP12, both of which harbor an ITAM, regulate the development of functional osteoclasts via Syk tyrosine kinase37). Consequently, dual regulation of intracellular signals through the ITAM and ITIM may be crucial for maintaining a balance of osteoclastogenesis. The binding of LRAP and Ig receptors should be investigated to elucidate whether amelogenin can regulate osteoclastogenesis by changing the kinase activities in osteoclast precursors, although reports have suggested that regulating the RANK-RANKL signaling pathway reduced osteoclastogenesis in mesenchymal cells.
ITM2A and Itm2b, members of the integral membrane protein 2 (ITM2) family (also referred to as the BRI2 family), were identified from each cDNA library independently. This gene family encodes a type II membrane protein, possesses a BRICHOS domain, which is a conserved motif common to family members, and is thought to play a role in the targeting of proteins to the secretory pathway or to intracellular processing38). Proteins sharing the BRICHOS motif are associated with diverse phenotypes, such as dementia, cancer, and respiratory distress. In particular, recent studies have focused on the involvement of ITM2B, which shows high homology to ITM2A, in dementia observed in a patient with ITM2B gene mutations39,40). In the early stages of differentiation, ITM2A may be associated with the inhibition of chondrogenesis initiation, and elevated expression of ITM2A in adipose tissues may be linked to the poorer chondrogenic differentiation potential of these cells41). Several reports suggest that LRAP influences chondrocytic differentiation and proliferation in mesenchymal cells42–44). We are currently investigating the effects of LRAP on chondrocytic cell differentiation in the context of ITM2A involvement.
Lysyl oxidase-like 3 and 1 were independently identified from the library screening of hBone marrow and mouse CM/PDL cells, respectively. The lysyl oxidase (LOX) family is an extracellular copper-dependent amine oxidase that is critical for the biogenesis of connective tissue matrices through the crosslinking of collagens and elastin. A highly conserved amino acid sequence at the C-terminal end of the family members appears to be sufficient to retain enzymatic function45). In addition to its enzymatic role, the phosphorylation of MAPK, ERK1, and ERK2 induced by fibroblast growth factor (FGF)-2 was significantly reduced by the addition of LOX in vitro46). A recent report indicated that LOX propeptide in proLOX inhibited FGF-induced signaling and osteoblast proliferation47). Moreover, fibronectin, which is critical for the proteolytic activation of proLOX48), was downregulated via unknown mechanisms on adding amelogenin (M180) to osteoblasts49). Collectively, these experiments suggest that LRAP is also involved in the MAPK-ERK pathway, possibly by regulating LOX function directly or indirectly. LOX activity and proLOX cleavage in the presence or absence of LRAP are worthy of further investigation. Two splice products of amelogenin [M73 (LRAP+exon4) and M59 (LRAP)] have been shown to bind to a 95-kDa transmembrane protein identified as lysosomal membrane protein-1 (LAMP-1)50,51). Furthermore, the amelogenins also bound to LAMP-2 and LAMP-3/CD63 using a yeast two-hybrid system50,52), although no LAMPs were identified in our screening. LAMPs are highly glycosylated proteins, associated with vesicular structures of the endocytic pathway, and are implicated as binding proteins that function in a scavenger or protein uptake process after the degradation of amelogenin protein in tooth development53); however, a recent study suggested that H174 (human homologue of M180) increased the proliferation of mesenchymal stem cells by interacting with LAMP-1 via the MAPK-ERK signaling pathway54). Although the exact mechanisms mediated by LAMPs remain to be elucidated, LAMPs are the most thoroughly investigated binding proteins of amelogenins to date.
Concluding Remarks
Emerging evidence suggests that amelogenins possess multiple functions, not only as an extracellular matrix for enamel mineralization, but also as signaling molecules in mesenchymal cells including periodontal tissues and in osteoclastogenesis. In addition to the MEK-ERK pathway, recent publications suggested that amelogenins including full-length amelogenin and LRAP also regulate the Wnt/β-catenin pathway55,56). Further comprehensive insights into the signaling pathway modulated by the amelogenin proteins will lead to the development of new therapeutic approaches for treating dental diseases and disorders such as idiopathic root resorption and periodontitis.
Acknowledgments
We would like to acknowledge the great help provided by the NIDCR DNA Sequencing Core. We would also like to thank Dr. Shin-ichi Harashima and Kiyoyuki Torigoe for their useful discussion and technical advice regarding yeast screening.
We would like to thank Taduru Sreenath for helpful discussions and Shelagh Powers for expert editorial assistance. These studies were supported by the Division of Intramural Research, NIDCR, NIH. We are also grateful for the grant given to us by the Japanese Ministry of Education, Global Center of Excellence (GCOE) Program, which supported our research.
References
- 1.Termine JD, Belcourt AB, Christner PJ, Conn KM, Nylen MU. Properties of dissociatively extracted fetal tooth matrix proteins. I. Principal molecular species in developing bovine enamel. J. Biol. Chem. 1980;255:9760–9768. [PubMed] [Google Scholar]
- 2.Delgado S, Girondot M, Sire JY. Molecular evolution of amelogenin in mammals. J. Mol. Evol. 2005;60:12–30. doi: 10.1007/s00239-003-0070-8. [DOI] [PubMed] [Google Scholar]
- 3.Salido EC, Yen PH, Koprivnikar K, Yu LC, Shapiro LJ. The human enamel protein gene amelogenin is expressed from both the X and the Y chromosomes. Am. J. Hum. Genet. 1992;50:303–316. [PMC free article] [PubMed] [Google Scholar]
- 4.Lau EC, Mohandas TK, Shapiro LJ, Slavkin HC, Snead ML. Human and mouse amelogenin gene loci are on the sex chromosomes. Genomics. 1989;4:162–168. doi: 10.1016/0888-7543(89)90295-4. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Mathews C, Gao C, DenBesten PK. Identification of two additional exons at the 3’ end of the amelogenin gene. Arch. Oral. Biol. 1998;43:497–504. doi: 10.1016/s0003-9969(98)00013-2. [DOI] [PubMed] [Google Scholar]
- 6.Hu CC, Ryu OH, Qian Q, Zhang CH, Simmer JP. Cloning, characterization, and heterologous expression of exon-4-containing amelogenin mRNAs. J. Dent. Res. 1997;76:641–647. doi: 10.1177/00220345970760020401. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JD, Ball RL, Kawai T, Tye CE, Tsuchiya M, Simmer JP. Origin, splicing, and expression of rodent amelogenin exon 8. J. Dent. Res. 2006;85:894–899. doi: 10.1177/154405910608501004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Yuan ZA, Aragon MA, Kulkarni AB, Gibson CW. Comparison of body weight and gene expression in amelogenin null and wild-type mice. Eur. J. Oral. Sci. 2006;114(Suppl 1):190–193. doi: 10.1111/j.1600-0722.2006.00286.x. discussion 201–192, 381. [DOI] [PubMed] [Google Scholar]
- 9.Veis A. Amelogenin gene splice products : potential signaling molecules. Cell. Mol. Life. Sci. 2003;60:38–55. doi: 10.1007/s000180300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fincham AG, Belcourt AB, Termine JD, Butler WT, Cothran WC. Amelogenins. Sequence homologies in enamel-matrix proteins from three mammalian species. Biochem. J. 1983;211:149–154. doi: 10.1042/bj2110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett JD, Simmer JP. Proteinases in developing dental enamel. Crit. Rev. Oral. Biol. Med. 1999;10:425–441. doi: 10.1177/10454411990100040101. [DOI] [PubMed] [Google Scholar]
- 12.Simmer JP, Hu JC. Expression, structure, and function of enamel proteinases. Connect. Tissue. Res. 2002;43:441–449. doi: 10.1080/03008200290001159. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y, Papagerakis P, Yamakoshi Y, Hu JC, Bartlett JD, Simmer JP. Functions of KLK4 and MMP-20 in dental enamel formation. Biol. Chem. 2008;389:695–700. doi: 10.1515/BC.2008.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fincham AG, Hu Y, Lau EC, Slavkin HC, Snead ML. Amelogenin post-secretory processing during biomineralization in the postnatal mouse molar tooth. Arch. Oral. Biol. 1991;36:305–317. doi: 10.1016/0003-9969(91)90101-y. [DOI] [PubMed] [Google Scholar]
- 15.Ravindranath RM, Moradian-Oldak J, Fincham AG. Tyrosyl motif in amelogenins binds Nacetyl-D-glucosamine. J. Biol. Chem. 1999;274:2464–2471. doi: 10.1074/jbc.274.4.2464. [DOI] [PubMed] [Google Scholar]
- 16.Ravindranath RM, Tam WY, Bringas P, Jr, Santos V, Fincham AG. Amelogenin-cytokeratin 14 interaction in ameloblasts during enamel formation. J. Biol. Chem. 2001;276:36586–36597. doi: 10.1074/jbc.M104656200. [DOI] [PubMed] [Google Scholar]
- 17.Ravindranath RM, Tam WY, Nguyen P, Fincham AG. The enamel protein amelogenin binds to the N-acetyl-D-glucosamine-mimicking peptide motif of cytokeratins. J. Biol. Chem. 2000;275:39654–39661. doi: 10.1074/jbc.M006471200. [DOI] [PubMed] [Google Scholar]
- 18.Wiedemann-Bidlack FB, Kwak SY, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. Effects of phosphorylation on the self-assembly of native full-length porcine amelogenin and its regulation of calcium phosphate formation in vitro. J. Struct. Biol. 2011;173:250–260. doi: 10.1016/j.jsb.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson CW, Yuan ZA, Hall B, Longenecker G, Chen E, Thyagarajan T, Sreenath T, Wright JT, Decker S, Piddington R, Harrison G, Kulkarni AB. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J. Biol. Chem. 2001;276:31871–31875. doi: 10.1074/jbc.M104624200. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Suggs C, Wright JT, Yuan ZA, Aragon M, Fong H, Simmons D, Daly B, Golub EE, Harrison G, Kulkarni AB, Gibson CW. Partial rescue of the amelogenin null dental enamel phenotype. J. Biol. Chem. 2008;283:15056–15062. doi: 10.1074/jbc.M707992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammarstrom L, Heijl L, Gestrelius S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J. Clin. Periodontol. 1997;24:669–677. doi: 10.1111/j.1600-051x.1997.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 22.Gestrelius S, Andersson C, Lidstrom D, Hammarstrom L, Somerman M. In vitro studies on periodontal ligament cells and enamel matrix derivative. J. Clin. Periodontol. 1997;24:685–692. doi: 10.1111/j.1600-051x.1997.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 23.Cardaropoli G, Leonhardt AS. Enamel matrix proteins in the treatment of deep intrabony defects. J. Periodontol. 2002;73:501–504. doi: 10.1902/jop.2002.73.5.501. [DOI] [PubMed] [Google Scholar]
- 24.Velasquez-Plata D, Scheyer ET, Mellonig JT. Clinical comparison of an enamel matrix derivative used alone or in combination with a bovinederived xenograft for the treatment of periodontal osseous defects in humans. J. Periodontol. 2002;73:433–440. doi: 10.1902/jop.2002.73.4.433. [DOI] [PubMed] [Google Scholar]
- 25.Slavkin HC, Bessem C, Fincham AG, Bringas P, Jr, Santos V, Snead ML, Zeichner-David M. Human and mouse cementum proteins immunologically related to enamel proteins. Biochim. Biophys. Acta. 1989;991:12–18. doi: 10.1016/0304-4165(89)90021-4. [DOI] [PubMed] [Google Scholar]
- 26.Hammarstrom L. The role of enamel matrix proteins in the development of cementum and periodontal tissues. Ciba. Found. Symp. 1997;205:246–255. doi: 10.1002/9780470515303.ch17. discussion 255–260. [DOI] [PubMed] [Google Scholar]
- 27.Fong CD, Hammarstrom L. Expression of amelin and amelogenin in epithelial root sheath remnants of fully formed rat molars. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2000;90:218–223. doi: 10.1067/moe.2000.107052. [DOI] [PubMed] [Google Scholar]
- 28.Sonoyama W, Seo BM, Yamaza T, Shi S. Human Hertwig’s epithelial root sheath cells play crucial roles in cementum formation. J. Dent. Res. 2007;86:594–599. doi: 10.1177/154405910708600703. [DOI] [PubMed] [Google Scholar]
- 29.Hatakeyama J, Sreenath T, Hatakeyama Y, Thyagarajan T, Shum L, Gibson CW, Wright JT, Kulkarni AB. The receptor activator of nuclear factor-kappa B ligand-mediated osteoclastogenic pathway is elevated in amelogenin-null mice. J. Biol. Chem. 2003;278:35743–35748. doi: 10.1074/jbc.M306284200. [DOI] [PubMed] [Google Scholar]
- 30.Hatakeyama J, Philp D, Hatakeyama Y, Haruyama N, Shum L, Aragon MA, Yuan Z, Gibson CW, Sreenath T, Kleinman HK, Kulkarni AB. Amelogenin-mediated regulation of osteoclastogenesis,and periodontal cell proliferation and migration. J. Dent. Res. 2006;85:144–149. doi: 10.1177/154405910608500206. [DOI] [PubMed] [Google Scholar]
- 31.Yagi Y, Suda N, Yamakoshi Y, Baba O, Moriyama K. In vivo application of amelogenin suppresses root resorption. J. Dent. Res. 2009;88:176–181. doi: 10.1177/0022034508329451. [DOI] [PubMed] [Google Scholar]
- 32.Obrink B. CEA adhesion molecules : multifunctional proteins with signal-regulatory properties. Curr. Opin. Cell. Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obrink B. On the role of CEACAM1 in cancer. Lung. Cancer. 2008;60:309–312. doi: 10.1016/j.lungcan.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N. The carboxylterminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J. Biol. Chem. 1999;274:335–344. doi: 10.1074/jbc.274.1.335. [DOI] [PubMed] [Google Scholar]
- 35.Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes KV, Karlsson A, Kuroki M, Lin SH, Lucka L, Najjar SM, Neumaier M, Obrink B, Shively JE, Skubitz KM, Stanners CP, Thomas P, Thompson JA, Virji M, von Kleist S, Wagener C, Watt S, Zimmermann W. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell. Res. 1999;252:243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 36.Mori Y, Tsuji S, Inui M, Sakamoto Y, Endo S, Ito Y, Fujimura S, Koga T, Nakamura A, Takayanagi H, Itoi E, Takai T. Inhibitory immunoglobulin- like receptors LILRB and PIR-B negatively regulate osteoclast development. J. Immunol. 2008;181:4742–4751. doi: 10.4049/jimmunol.181.7.4742. [DOI] [PubMed] [Google Scholar]
- 37.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Pulido L, Devos D, Valencia A. BRICHOS : a conserved domain in proteins associated with dementia, respiratory distress and cancer. Trends. Biochem. Sci. 2002;27:329–332. doi: 10.1016/s0968-0004(02)02134-5. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Miller VM, Levites Y, West KJ, Zwizinski CW, Moore BD, Troendle FJ, Bann M, Verbeeck C, Price RW, Smithson L, Sonoda L, Wagg K, Rangachari V, Zou F, Younkin SG, Graff-Radford N, Dickson D, Rosenberry T, Golde TE. BRI2(ITM2b)inhibits Abeta deposition in vivo. J. Neurosci. 2008;28:6030–6036. doi: 10.1523/JNEUROSCI.0891-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsachaki M, Ghiso J, Efthimiopoulos S. BRI2 as a central protein involved in neurodegeneration. Biotechnol. J. 2008;3:1548–1554. doi: 10.1002/biot.200800247. [DOI] [PubMed] [Google Scholar]
- 41.Boeuf S, Börger M, Hennig T, Winter A, Kasten P, Richter W. Enhanced ITM2A expression inhibits chondrogenic differentiation of mesenchymal stem cells. Differentiation. 2009;78:108–115. doi: 10.1016/j.diff.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Nebgen D, Inoue H, Sabsay B, Wei K, Ho C, Veis A. Identification of the Chondrogenic-inducing Activity from Bovine Dentin : bCIA) as a Lowmolecular-mass Amelogenin Polypeptide. J. Dent. Res. 1999;78:1484–1494. doi: 10.1177/00220345990780090201. [DOI] [PubMed] [Google Scholar]
- 43.Veis A, Tompkins K, Alvares K, Wei K, Wang L, Wang XS, Brownell AG, Jengh SM, Healy KE. Specific amelogenin gene splice products have signaling effects on cells in culture and in implants in vivo. J. Biol. Chem. 2000;275:41263–41272. doi: 10.1074/jbc.M002308200. [DOI] [PubMed] [Google Scholar]
- 44.Lacerda-Pinheiro S, Septier D, Tompkins K, Veis A, Goldberg M, Chardin H. Amelogenin gene splice products A + 4 and A−4 implanted in soft tissue determine the reorientation of CD45-positive cells to an osteo-chondrogenic lineage. J. Biomed. Mater. Res. A. 2006;79:1015–1022. doi: 10.1002/jbm.a.30912. [DOI] [PubMed] [Google Scholar]
- 45.Lucero HA, Kagan HM. Lysyl oxidase : an oxidative enzyme and effector of cell function. Cell. Mol. Life. Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Nugent MA, Zhao Y, Chau AN, Li SJ, Chou IN, Liu G, Kagan HM. Lysyl oxidase oxidizes basic fibroblast growth factor and inactivates its mitogenic potential. J. Cell. Biochem. 2003;88:152–164. doi: 10.1002/jcb.10304. [DOI] [PubMed] [Google Scholar]
- 47.Vora SR, Palamakumbura AH, Mitsi M, Guo Y, Pischon N, Nugent MA, Trackman PC. Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts. J. Biol. Chem. 2010;285:7384–7393. doi: 10.1074/jbc.M109.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fogelgren B, Polgár N, Szauter KM, Ujfaludi Z, Laczkó R, Fong KS, Csiszar K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J. Biol. Chem. 2005;280:24690–24697. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- 49.Nishiguchi M, Yuasa K, Saito K, Fukumoto E, Yamada A, Hasegawa T, Yoshizaki K, Kamasaki Y, Nonaka K, Fujiwara T, Fukumoto S. Amelogenin is a negative regulator of osteoclastogenesis via downregulation of RANKL, M-CSF and fibronectin expression in osteoblasts. Arch. Oral. Biol. 2007;52:237–243. doi: 10.1016/j.archoralbio.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Zou Y, Wang H, Shapiro JL, Okamoto CT, Brookes SJ, Lyngstadaas SP, Snead ML, Paine ML. Determination of protein regions responsible for interactions of amelogenin with CD63 and LAMP1. Biochem. J. 2007;408:347–354. doi: 10.1042/BJ20070881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tompkins K, George A, Veis A. Characterization of a mouse amelogenin [A−4] /M59 cell surface receptor. Bone. 2006;38:172–180. doi: 10.1016/j.bone.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Tannukit S, Zhu D, Snead ML, Paine ML. Enamel matrix protein interactions. J.Bone. Miner. Res. 2005;20:1032–1040. doi: 10.1359/JBMR.050111. [DOI] [PubMed] [Google Scholar]
- 53.Shapiro JL, Wen X, Okamoto CT, Wang HJ, Lyngstadaas SP, Goldberg M, Snead ML, Paine ML. Cellular uptake of amelogenin, and its localization to CD63, and Lamp1-positive vesicles. Cell. Mol. Life. Sci. 2007;64:244–256. doi: 10.1007/s00018-006-6429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang YC, Tanimoto K, Tanne Y, Kamiya T, Kunimatsu R, Michida M, Yoshioka M, Yoshimi Y, Kato Y, Tanne K. Effects of human fulllength amelogenin on the proliferation of human mesenchymal stem cells derived from bone marrow. Cell. Tissue. Res. 2010;342:205–212. doi: 10.1007/s00441-010-1064-7. [DOI] [PubMed] [Google Scholar]
- 55.Matsuzawa M, Sheu TJ, Lee YJ, Chen M, Li TF, Huang CT, Holz JD, Puzas JE. Putative signaling action of amelogenin utilizes the Wnt/beta-catenin pathway. J. Periodontal. Res. 2009;44:289–296. doi: 10.1111/j.1600-0765.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 56.Warotayanont R, Frenkel B, Snead ML, Zhou Y. Leucine-rich amelogenin peptide induces osteogenesis by activation of the Wnt pathway. Biochem. Biophys. Res. Commun. 2009;387:558–563. doi: 10.1016/j.bbrc.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]